Effect of Surface Composition and Structure of the Mesoporous Ni/KIT-6 Catalyst on Catalytic Hydrodeoxygenation Performance
Abstract
1. Introduction
2. Results and Discussion
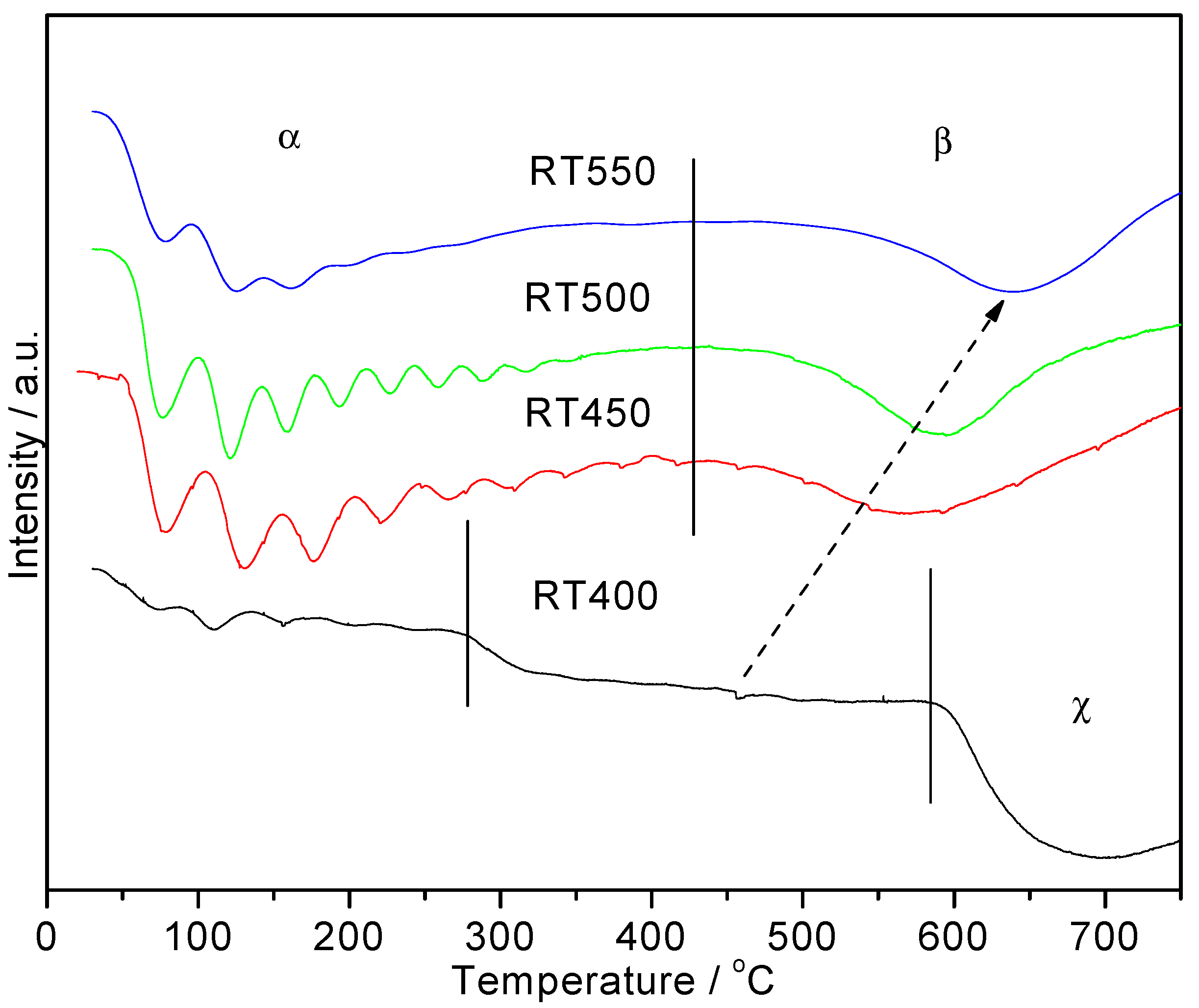
2.1. H2-TPR Studies
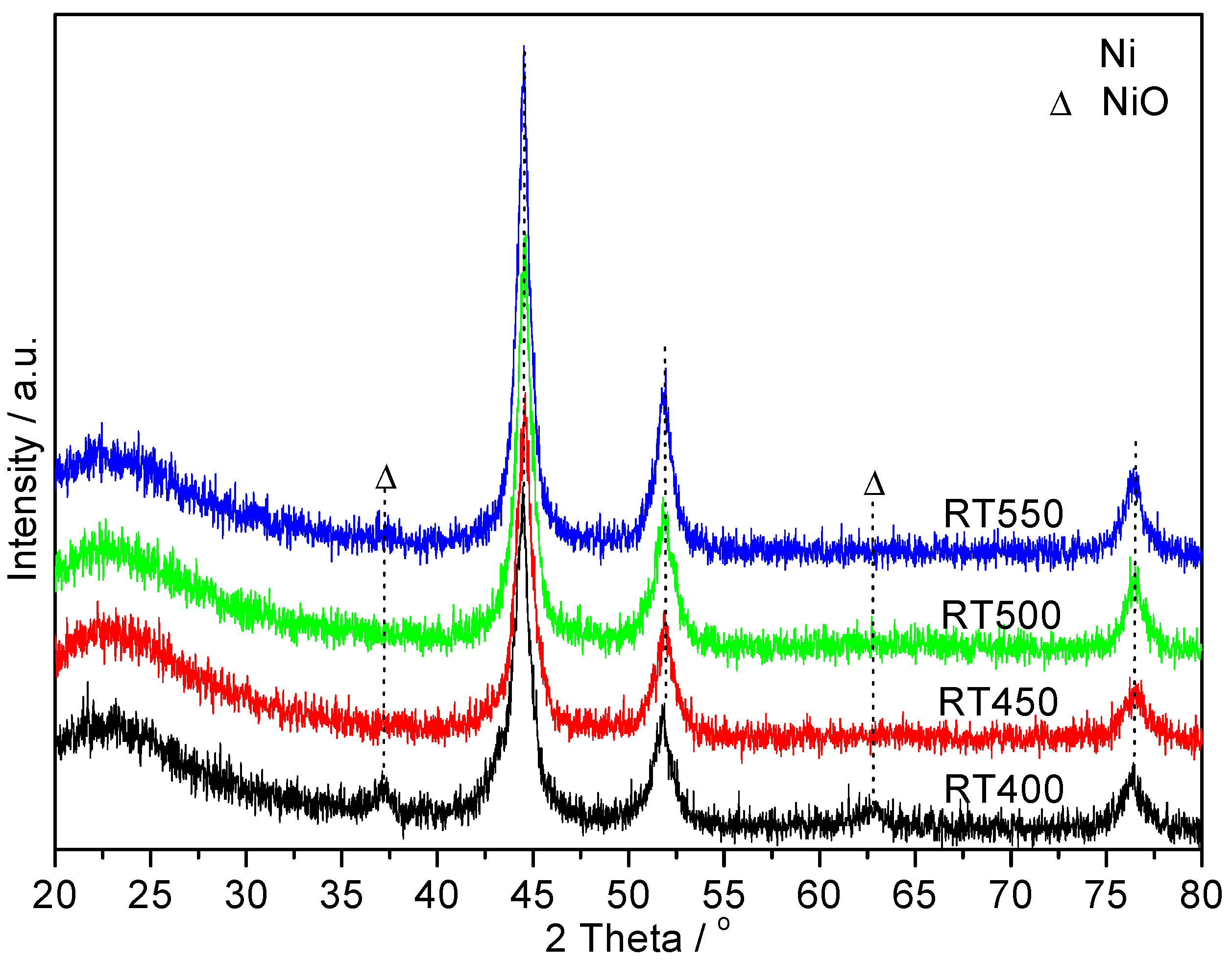
2.2. XRD Characterization
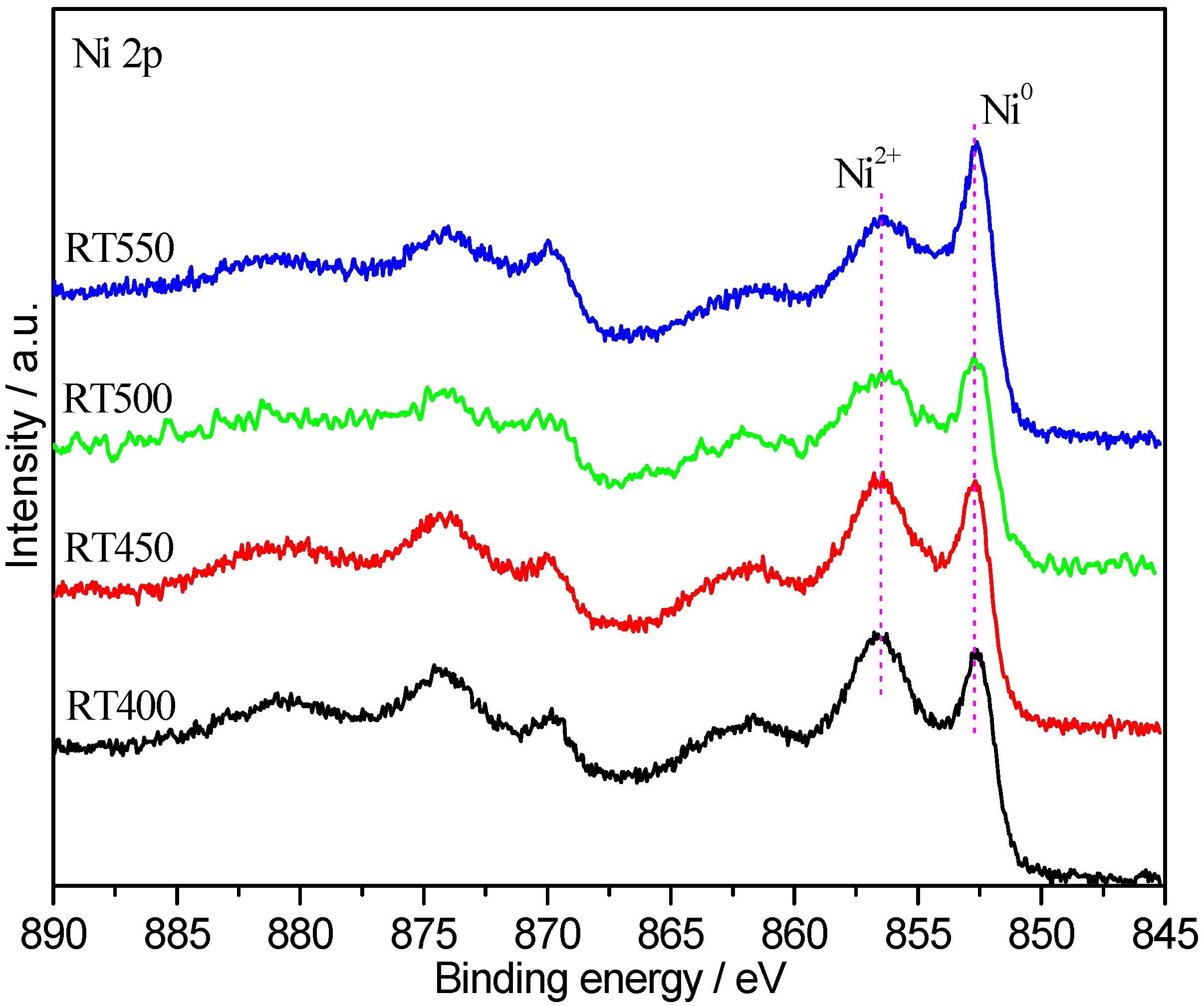
2.3. Quasi In-Situ XPS Studies
2.4. H2-TPD Studies
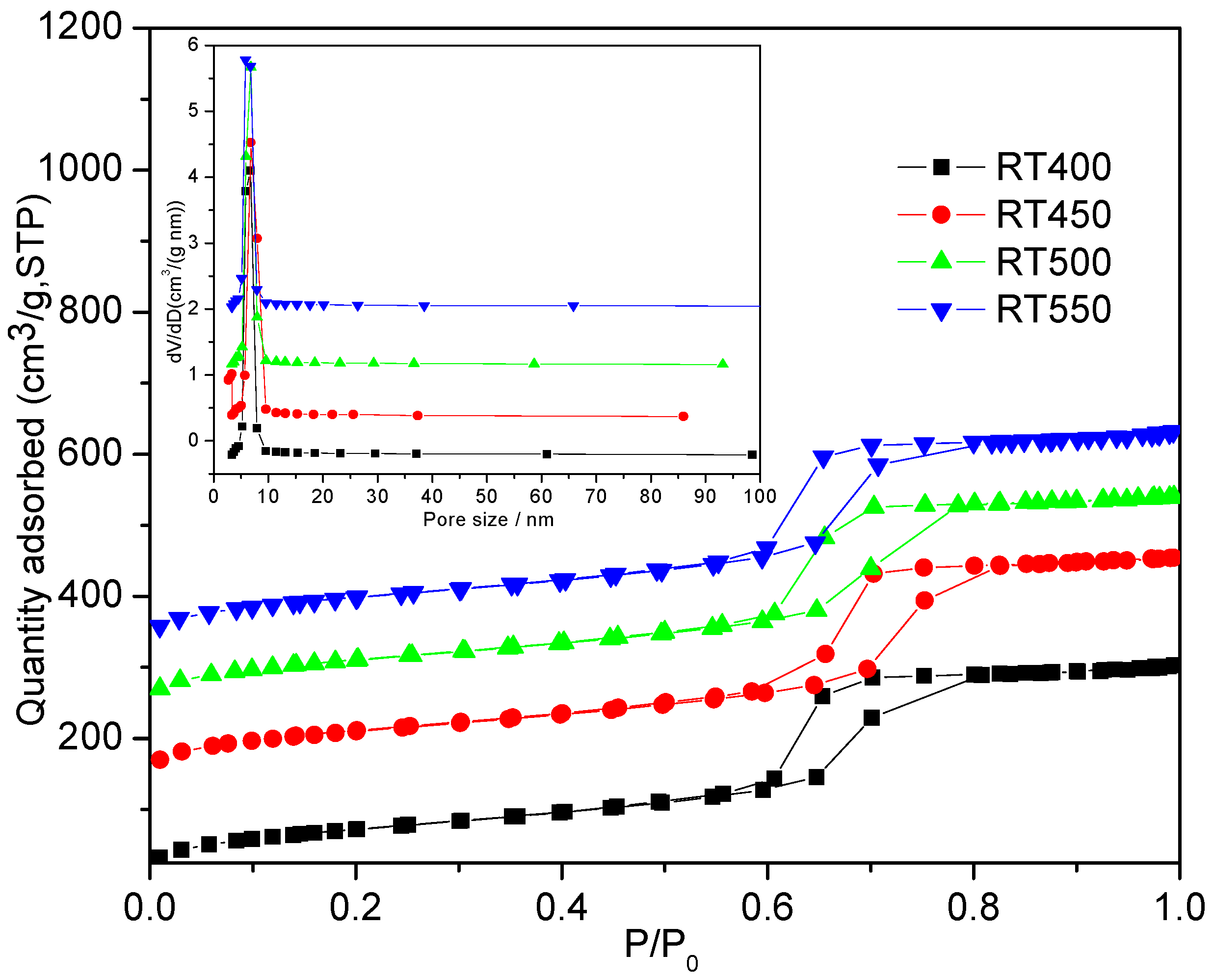
2.5. BET Characteristics
2.6. TEM Characteristics
2.7. Catalytic Activity Test
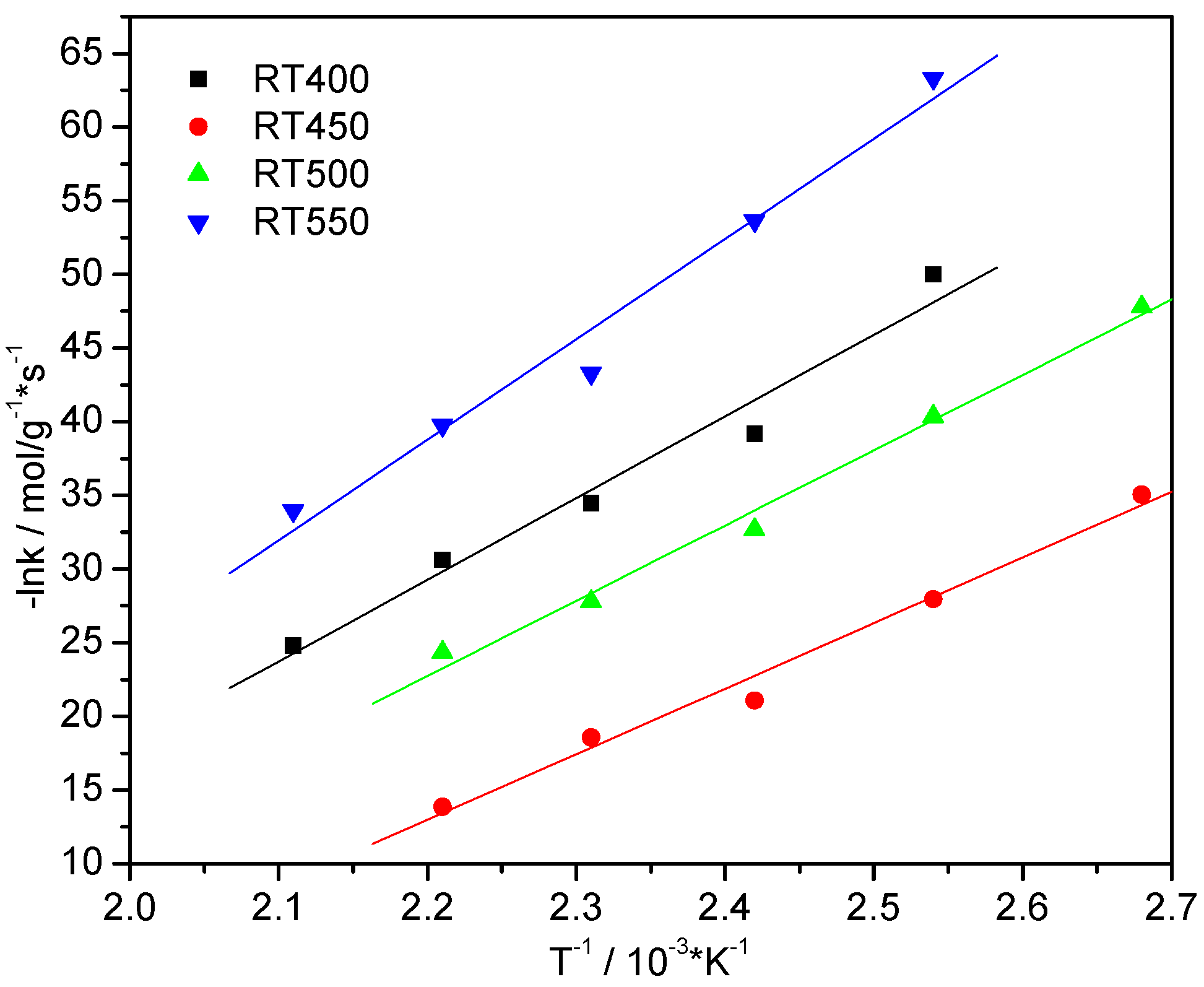
2.8. Activation Energy Studies
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.2.1. XRD Characterization
3.2.2. Quasi In-Situ XPS Studies
3.2.3. BET Characterization
3.2.4. H2-TPD/R Studies
3.2.5. TEM Characterization
3.2.6. Catalytic Activity Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Li, G.L.; Rogers, K.; Lin, H.F.; Zheng, Y. Hydrotreating of waste cooking oil over supported CoMoS catalyst—Catalyst deactivation mechanism study. Mol. Catal. 2017, 443, 228–240. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic hydrodeoxygenation of triglycerides: An approach to clean diesel fuel production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green diesel synthesis by hydrodeoxygenation of bio-based feedstocks: Strategies for catalyst design and development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Man, Z.; Khan, A.S.; Muhammad, N.; Sarwono, A. Preparation and kinetics study of biodiesel production from waste cooking oil using new functionalized ionic liquids as catalysts. Renew. Energy 2017, 114, 755–765. [Google Scholar] [CrossRef]
- Farid, M.A.A.; Hassan, M.A.; Taufiq-Yap, Y.H.; Shirai, Y.; Hasan, M.Y.; Zakaria, M.R. Waterless purification using oil palm biomass-derived bioadsorbent improved the quality of biodiesel from waste cooking oil. J. Clean. Prod. 2017, 165, 262–272. [Google Scholar] [CrossRef]
- Jung, J.M.; Lee, S.R.; Lee, J.; Lee, T.; Tsang, D.C.W.; Kwon, E.E. Biodiesel synthesis using chicken manure biochar and waste cooking oil. Bioresour. Technol. 2017, 244, 810–815. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Zauzi, N.S.A. Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew. Energy 2017, 114, 437–447. [Google Scholar] [CrossRef]
- Ling, T.R.; Chang, J.S.; Chiou, Y.J.; Chern, J.M.; Chou, T.C. Characterization of high acid value waste cottonseed oil by temperature programmed pyrolysis in a batch reactor. J. Anal. Appl. Pyrolysis 2016, 120, 222–230. [Google Scholar] [CrossRef]
- Wang, X.M.; Qin, X.L.; Li, D.M.; Yang, B.; Wang, Y.H. One-step synthesis of high-yield biodiesel from waste cooking oils by a novel and highly methanol-tolerant immobilized lipase. Bioresour. Technol. 2017, 235, 18–24. [Google Scholar] [CrossRef]
- Horácek, J.; St’ávová, G.; Kelbichová, V.; Kubicka, D. Zeolite-Beta-supported platinum catalysts for hydrogenation/hydrodeoxygenation of pyrolysis oil model compounds. Catal. Today 2013, 204, 38–45. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Z.; Lin, W.G.; Song, W.L.; Christensen, J.M.; Jensen, A.D. Hydrodeoxygenation of phenol over Pd catalysts by in-situ generated hydrogen from aqueous reforming of formic acid. Catal. Commun. 2016, 82, 46–49. [Google Scholar] [CrossRef]
- Lu, J.M.; Faheem, B.M.S.; Heyden, A. Theoretical investigation of the decarboxylation and decarbonylation mechanism of propanoic acid over a Ru(0001) model surface. J. Catal. 2015, 324, 14–24. [Google Scholar] [CrossRef]
- Kubičková, I.; Kubička, D. Utilization of triglycerides and related feedstocks for production of clean hydrocarbon fuels and petrochemicals: A review. Waste Biomass Valorization 2010, 1, 293–308. [Google Scholar] [CrossRef]
- Yang, L.Q.; Carreon, M.A. Effect of reaction parameters on the decarboxylation of oleic acid over Pt/ZIF-67membrane/zeolite 5A bead catalysts. J. Chem. Technol. Biotechnol. 2016, 92, 52–58. [Google Scholar] [CrossRef]
- Di, L.; Yao, S.K.; Guang, S.S.; Wu, J.; Dai, W.L.; Guan, N.J.; Li, L.D. Robust ruthenium catalysts for the selective conversion of stearic acid to diesel-range alkanes. Appl. Catal. B 2016, 201, 137–149. [Google Scholar] [CrossRef]
- Chen, J.Z.; Xu, Q.Y. Hydrodeoxygenation of biodiesel-related fatty acid methyl esters to diesel-range alkanes over zeolite-supported ruthenium catalysts. Catal. Sci. Technol. 2016, 6, 7239–7251. [Google Scholar] [CrossRef]
- Kubicka, D.; Bejblov, M.; Vlk, J. Conversion of vegetable oils into hydrocarbons over CoMo/MCM-41 catalysts. Top. Catal. 2010, 53, 168–178. [Google Scholar] [CrossRef]
- Kovács, S.; Kasza, T.; Thernesz, A.; Horváth, I.W.; Hancsók, J. Fuel production by hydrotreating of triglycerides on NiMo/Al2O3/F catalyst. Chem. Eng. J. 2011, 176, 237–243. [Google Scholar] [CrossRef]
- Hachemi, I.; Kumar, N.; Mäki-Arvela, P.; Roine, J.; Peurla, M.; Hemming, J.; Salonen, J.; Murzin, D.Y. Sulfur-free Ni catalyst for production of green diesel by hydrodeoxygenation. J. Catal. 2017, 347, 205–221. [Google Scholar] [CrossRef]
- Hachemi, I.; Jeništová, K.; Mäki-Arvela, P.; Kumar, N.; Eränen, K.; Hemming, J.; Murzin, D.Y. Comparative study of sulfur-free nickel and palladium catalysts in hydrodeoxygenation of different fatty acid feedstocks for production of biofuels. Catal. Sci. Technol. 2016, 6, 1476–1487. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Saad, M.W.A. CO2 methanation over Ni-promoted mesostructured silica nanoparticles: Influence of Ni loading and water vapor on activity and response surface methodology studies. Chem. Eng. J. 2015, 260, 757–764. [Google Scholar] [CrossRef]
- Zhou, G.L.; Wu, T.; Xie, H.M.; Zheng, X.X. Effects of structure on the carbon dioxide methanation performance of Co-based catalysts. Int. J. Hydrogen Energy 2013, 38, 10012–10018. [Google Scholar] [CrossRef]
- Zhou, G.L.; Wu, T.; Zhang, H.B.; Xie, H.M.; Feng, Y.C. Carbon dioxide methanation on ordered mesoporous Co/KIT-6 catalyst. Chem. Eng. Commun. 2014, 201, 233–240. [Google Scholar] [CrossRef]
- Chen, S.; Pan, X.Y.; Miao, C.X.; Xie, H.M.; Zhou, G.L.; Jiao, Z.J.; Zhang, X.M. Study of catalytic hydrodeoxygenation performance for the Ni/KIT-6 catalysts. J. Saudi Chem. Soc. 2018, 22, 614–627. [Google Scholar] [CrossRef]
- Chen, S.; Miao, C.X.; Luo, Y.; Zhou, G.L.; Xiong, K.; Jiao, Z.J.; Zhang, X.M. Study of catalytic hydrodeoxygenation performance of Ni catalysts: Effects of prepared method. Renew. Energy 2018, 115, 1109–1117. [Google Scholar] [CrossRef]
- Ferencz, Z.; Varga, E.; Puskás, R.; Kónya, Z.; Baán, K.; Oszkó, A.; Erdohelyi, A. Reforming of ethanol on Co/Al2O3 catalysts reduced at different temperatures. J. Catal. 2018, 358, 118–130. [Google Scholar] [CrossRef]
- Zhou, G.L.; Liu, H.R.; Cui, K.K.; Xie, H.M.; Jiao, Z.J.; Zhang, G.Z.; Xiong, K.; Zheng, X.X. Methanation of carbon dioxide over Ni/CeO2 catalysts: Effects of support CeO2 structure. Int. J. Hydrogen Energy 2017, 42, 16108–16117. [Google Scholar] [CrossRef]
- Zhou, G.L.; Xie, H.M.; Gui, B.G.; Zhang, G.Z.; Zheng, X.X. Influence of NiO on the performance of CoO-based catalysts for the selective oxidation of CO in H2-rich gas. Catal. Commun. 2012, 19, 42–45. [Google Scholar] [CrossRef]
- Zhou, G.L.; Liu, H.R.; Cui, K.K.; Jia, A.P.; Hu, G.S.; Jiao, Z.J.; Liu, Y.Q.; Zhang, X.M. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation. Appl. Surf. Sci. 2016, 383, 248–252. [Google Scholar] [CrossRef]
- Arslan, A.; Dogu, T. Effect of calcination/reduction temperature of Ni impregnated CeO2-ZrO2 catalysts on hydrogen yield and coke minimization in low temperature reforming of ethanol. Int. J. Hydrogen Energy 2013, 38, 7268–7279. [Google Scholar] [CrossRef]
- Pauly, N.; Yubero, F.; García-García, F.J.; Tougaard, S. Quantitative analysis of Ni2p photoemission in NiO and Ni diluted in a SiO2 matrix. Surf. Sci. 2016, 644, 46–52. [Google Scholar] [CrossRef]
- Boudjahem, A.G.; Bettahar, M.M. Effect of oxidative pre-treatment on hydrogen spillover for a Ni/SiO2 catalyst. J. Mol. Catal. A 2017, 426, 190–197. [Google Scholar] [CrossRef]
- Zhou, G.L.; Gui, B.G.; Xie, H.M.; Yang, F.; Chen, Y.; Chen, S.M.; Zheng, X.X. Influence of CeO2 morphology on the catalytic oxidation of ethanol in air. J. Indus. Eng. Chem. 2014, 20, 160–165. [Google Scholar] [CrossRef]
- Bie, Y.; Lehtonen, J.; Kanervo, J. Hydrodeoxygenation (HDO) of methyl palmitate over bifunctional Rh/ZrO2 catalyst: Insights into reaction mechanism via kinetic modeling. Appl. Catal. A 2016, 526, 183–190. [Google Scholar] [CrossRef]
- Kellenberger, A.; Jahne, E.; Adler, H.J.; Khandelwal, T.; Dunscha, L. In situ FTIR spectroelectrochemistry of poly [2-(3-thienyl) ethyl acetate] and its hydrolyzed derivatives. Electrochim. Acta 2008, 53, 7054–7060. [Google Scholar] [CrossRef]
- Tan, X.; Lan, H.; Xie, H.M.; Zhou, G.L.; Jiang, Y. Role of surface oxygen species of mesoporous CeCu oxide catalyst in OVOCs catalytic combustion. J. Environ. Chem. Eng. 2017, 5, 2068–2076. [Google Scholar] [CrossRef]
- Zhou, G.L.; Dai, B.C.; Xie, H.M.; Zhang, G.Z.; Xiong, K.; Zheng, X.X. CeCu composite catalyst for CO synthesis by reverse water–gas shift reaction: Effect of Ce/Cu mole ratio. J. CO2 Util. 2017, 21, 292–301. [Google Scholar] [CrossRef]

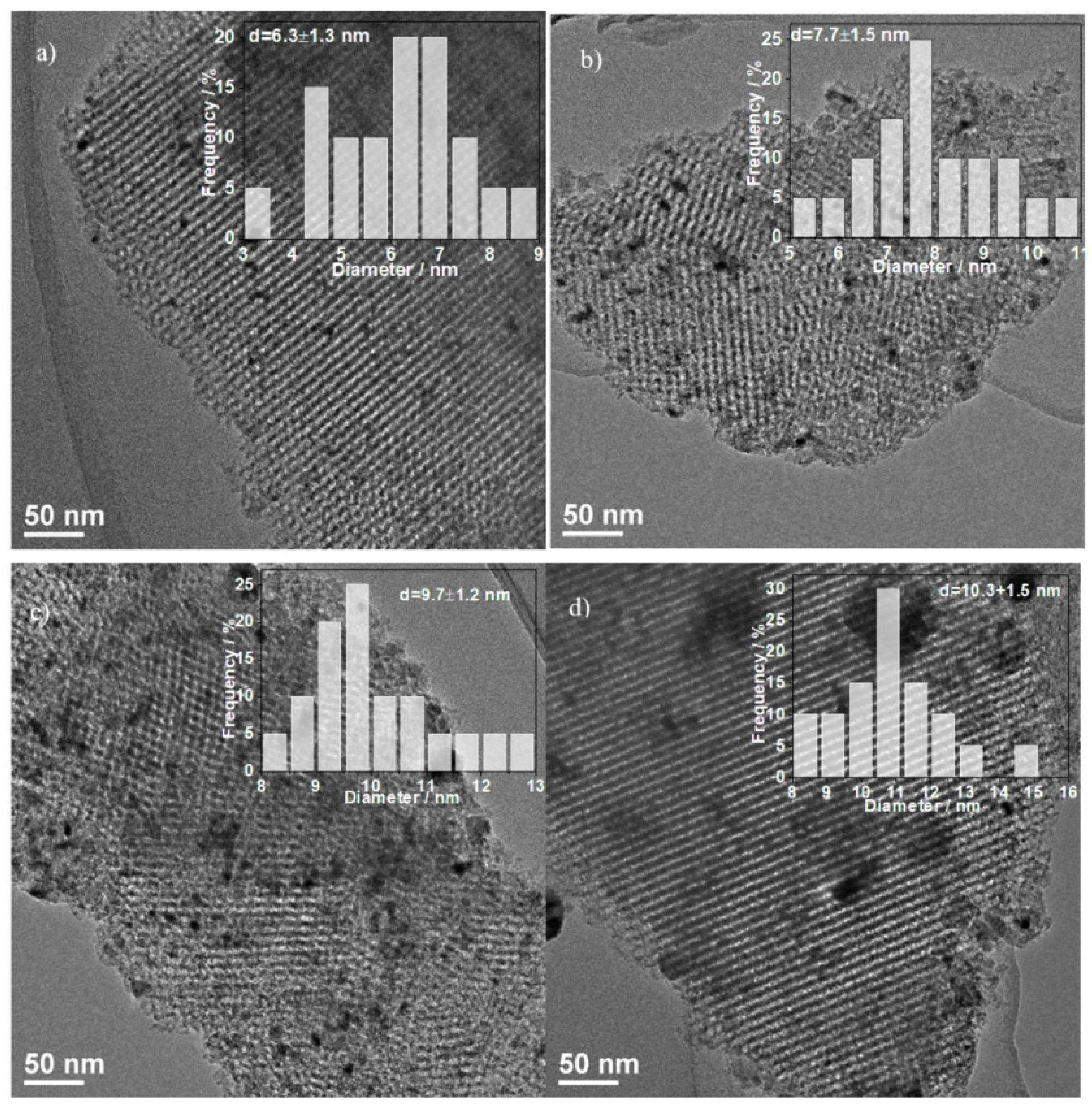
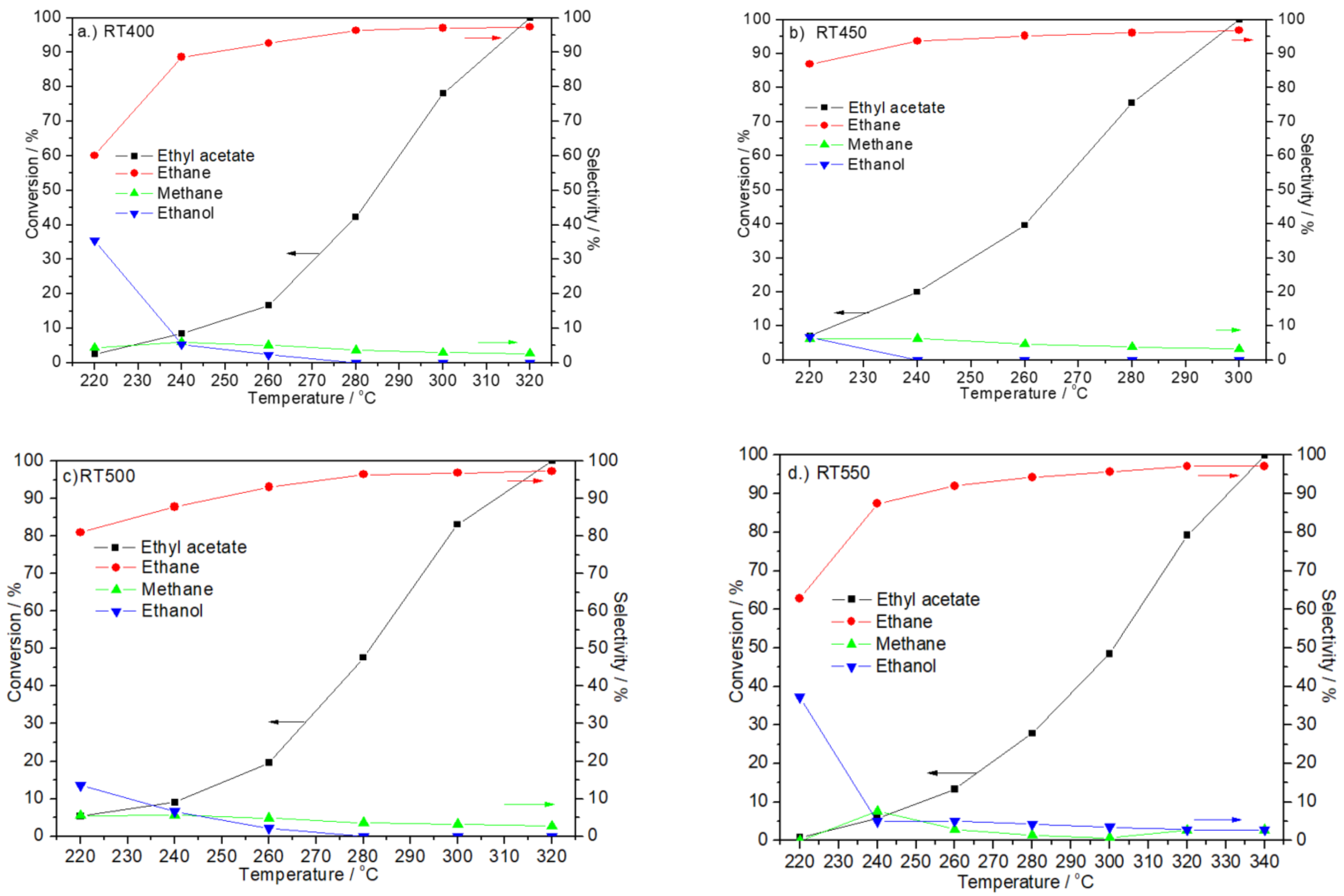
| Catalyst | Crystal Size from XRD (nm) a | H2-TPD (α Region) Quantity (µmol/g) | Surface Area (m2/g) b | Average Pore Diameter (nm) c | Total Pore Volume (cm3/g) d | Particle Size from TEM (nm) e |
|---|---|---|---|---|---|---|
| RT400 | 5.9 | 27.6 | 349.2 | 6.08 | 0.55 | 6.3 |
| RT450 | 7.4 | 65.8 | 348.9 | 6.35 | 0.56 | 7.7 |
| RT500 | 9.1 | 52.4 | 347.7 | 6.17 | 0.54 | 9.7 |
| RT550 | 10 | 49.9 | 343.4 | 6.03 | 0.56 | 10.3 |
| KIT-6 | — | — | 562.5 | 6.56 | 1.22 | — |
| Catalysts | Equation | Activation Energy (kJ/mol) |
|---|---|---|
| RT400 | Y = 9.208X − 12.44 | 81.0 |
| RT450 | Y = 8.066X − 23.17 | 67.7 |
| RT500 | Y = 9.264X − 16.14 | 79.5 |
| RT550 | Y = 11.33X − 9.65 | 90.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, S.; Wang, F.; Deng, L.; Ren, J.; Jiao, Z.; Zhou, G. Effect of Surface Composition and Structure of the Mesoporous Ni/KIT-6 Catalyst on Catalytic Hydrodeoxygenation Performance. Catalysts 2019, 9, 889. https://doi.org/10.3390/catal9110889
Zhang X, Chen S, Wang F, Deng L, Ren J, Jiao Z, Zhou G. Effect of Surface Composition and Structure of the Mesoporous Ni/KIT-6 Catalyst on Catalytic Hydrodeoxygenation Performance. Catalysts. 2019; 9(11):889. https://doi.org/10.3390/catal9110889
Chicago/Turabian StyleZhang, Xianming, Shuang Chen, Fengjiao Wang, Lidan Deng, Jianmin Ren, Zhaojie Jiao, and Guilin Zhou. 2019. "Effect of Surface Composition and Structure of the Mesoporous Ni/KIT-6 Catalyst on Catalytic Hydrodeoxygenation Performance" Catalysts 9, no. 11: 889. https://doi.org/10.3390/catal9110889
APA StyleZhang, X., Chen, S., Wang, F., Deng, L., Ren, J., Jiao, Z., & Zhou, G. (2019). Effect of Surface Composition and Structure of the Mesoporous Ni/KIT-6 Catalyst on Catalytic Hydrodeoxygenation Performance. Catalysts, 9(11), 889. https://doi.org/10.3390/catal9110889




