Ion Exchange of One-Pot Synthesized Cu-SAPO-44 with NH4NO3 to Promote Cu Dispersion and Activity for Selective Catalytic Reduction of NOx with NH3
Abstract
:1. Introduction
2. Results and Discussion
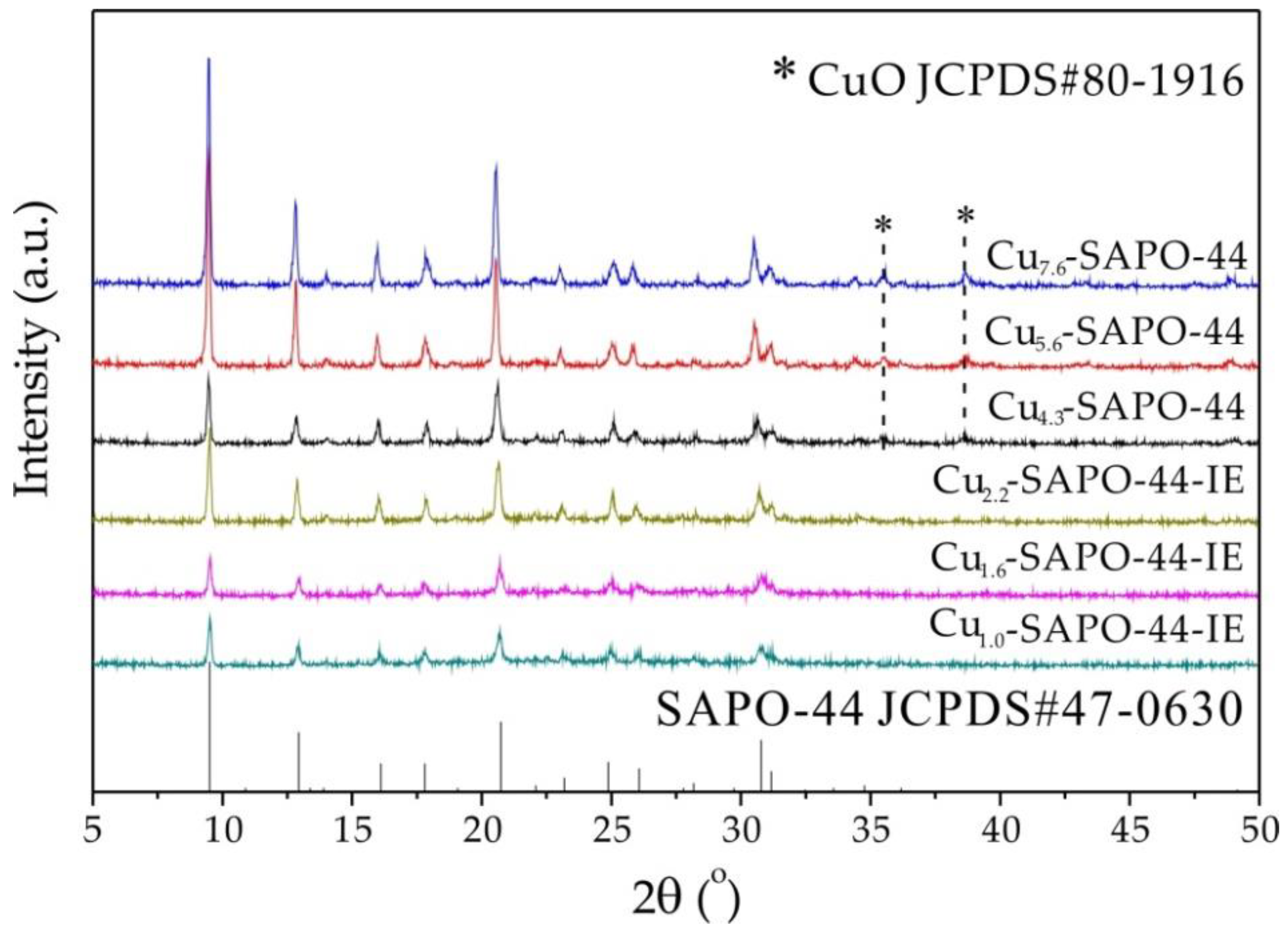
2.1. XRD
2.2. ICP
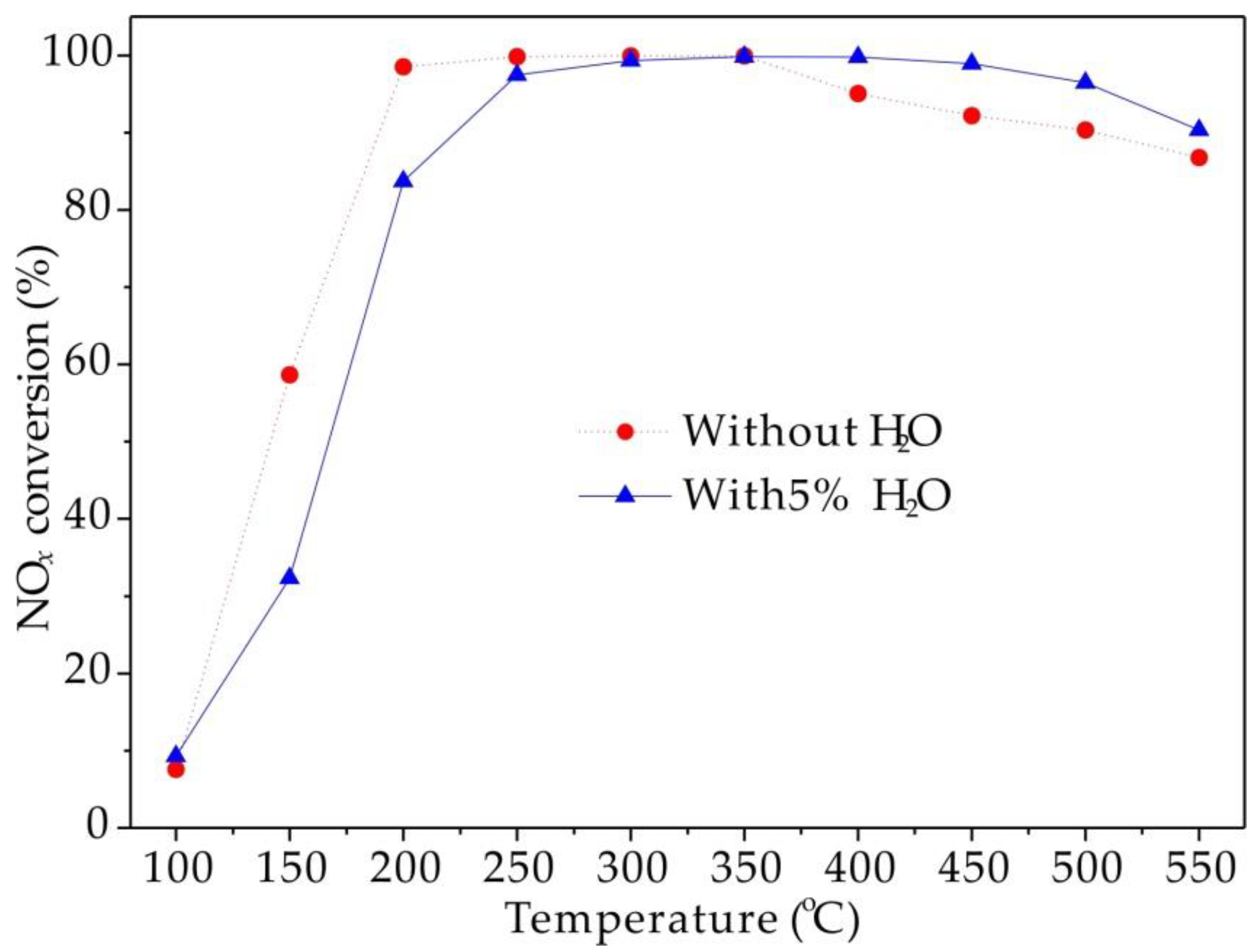
2.3. Catalytic Activity
2.4. N2 Adsorption/Desorption
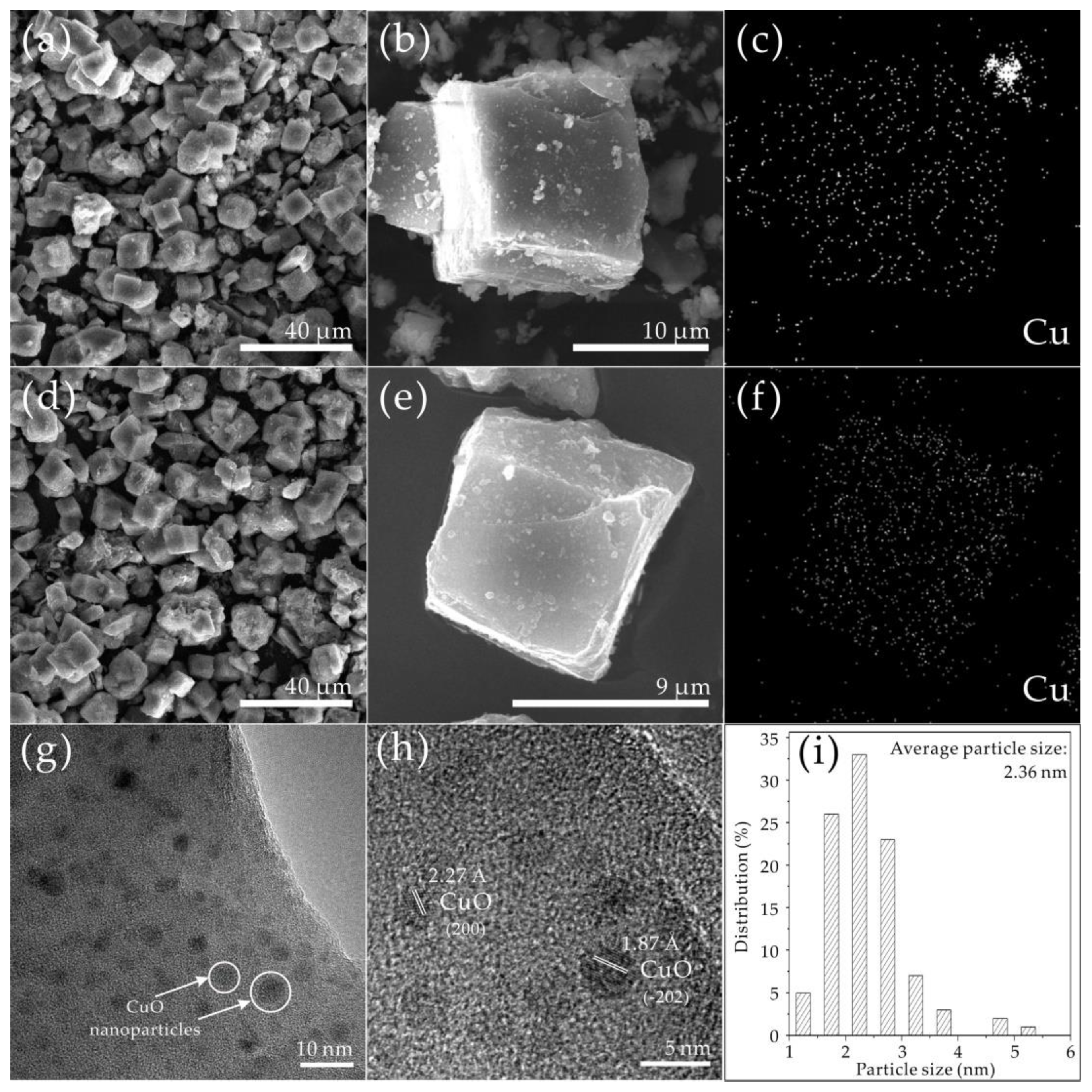
2.5. Electron Micrograph
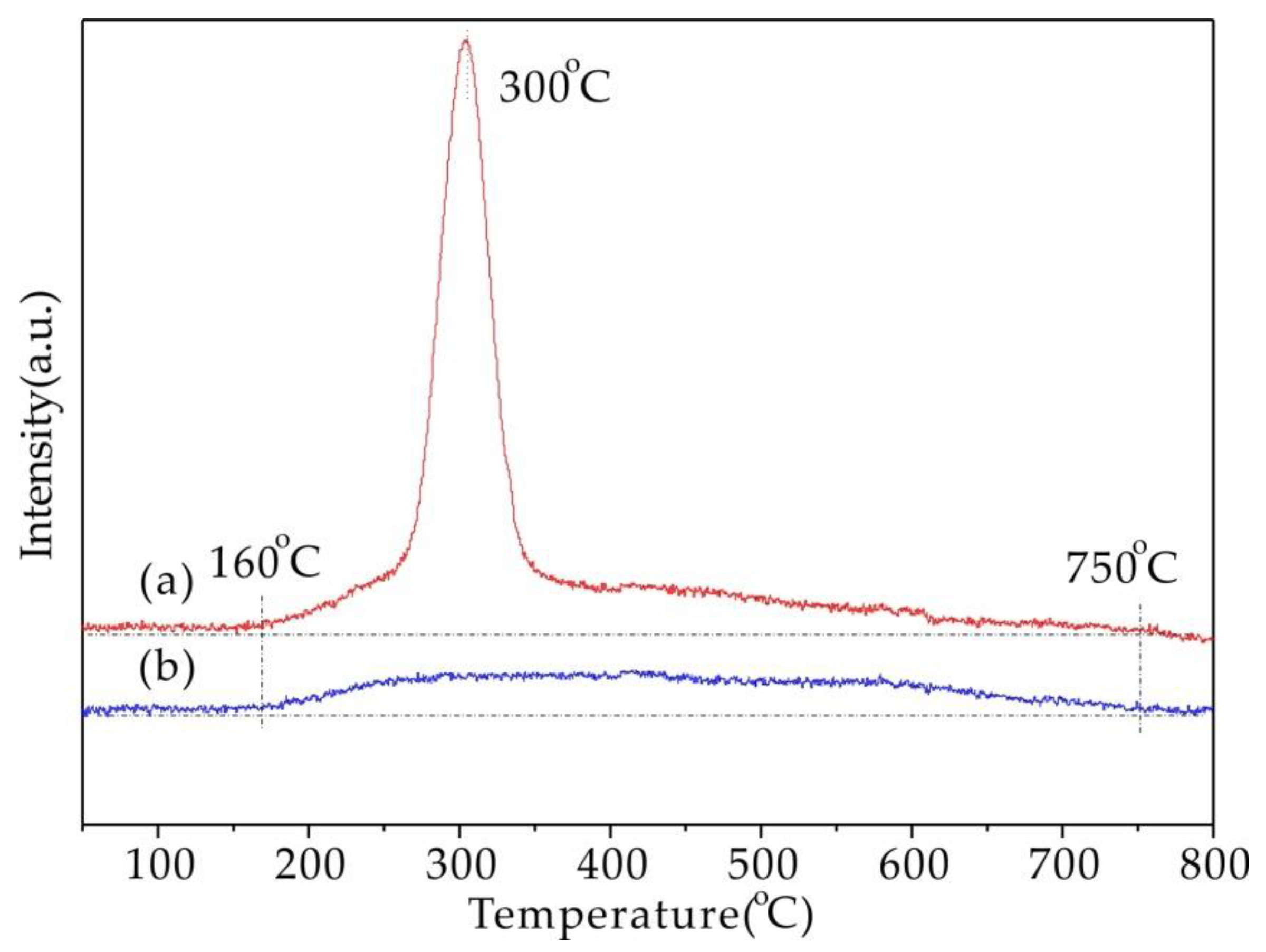
2.6. H2-TPR
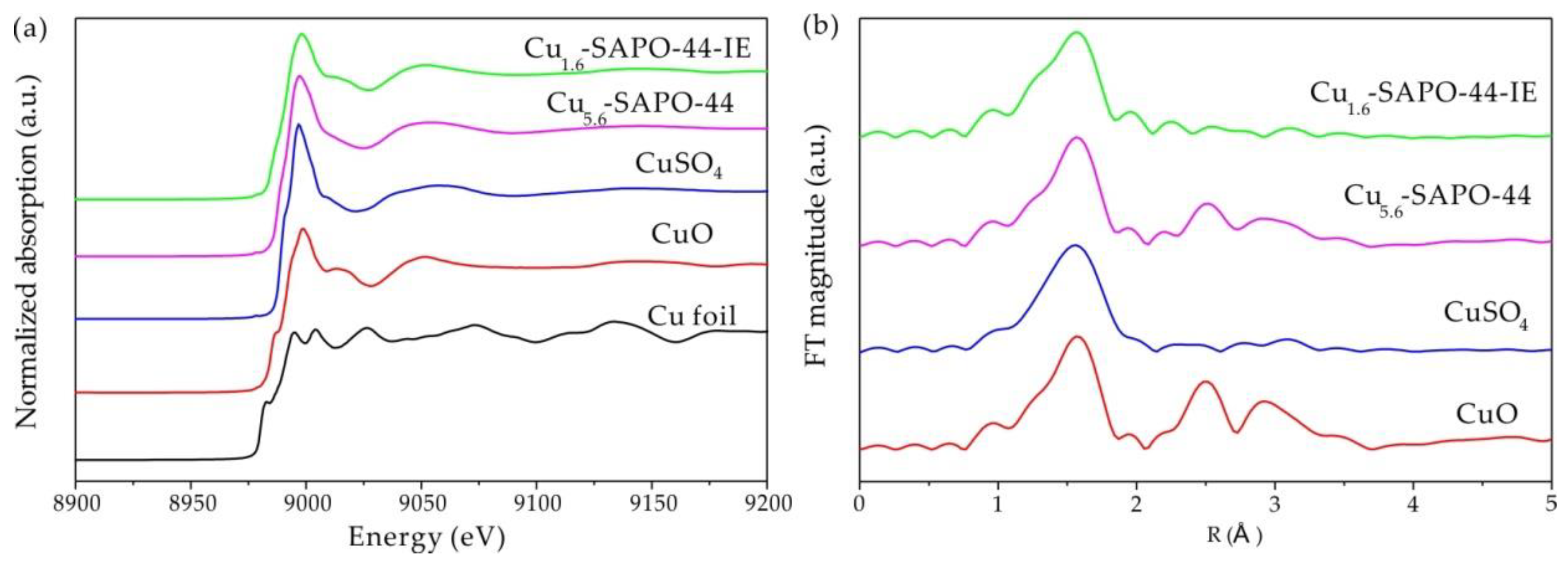
2.7. XAFS
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterizations
3.3. Catalyst Activity Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and evaluation of Cu-SAPO-34 catalysts for ammonia selective catalytic reduction. 1. Aqueous solution ion exchange. ACS Catal. 2013, 3, 2083–2093. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhao, H.W.; Haller, G.; Li, Y.D. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Turrina, A.; Eschenroeder, E.C.V.; Bode, B.E.; Collier, J.E.; Apperley, D.C.; Cox, P.A.; Casci, J.L.; Wright, P.A. Understanding the structure directing action of copper-polyamine complexes in the direct synthesis of Cu-SAPO-34 and Cu-SAPO-18 catalysts for the selective catalytic reduction of NO with NH3. Microporous Mesoporous Mater. 2015, 215, 154–167. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z.L. Zeolitic materials for deNOx selective catalytic reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Shan, W.P.; Song, H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Liu, F.D.; Yu, Y.B.; He, H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines: Structure-activity relationship and reaction mechanism aspects. Chem. Commun. 2014, 50, 8445–8463. [Google Scholar] [CrossRef]
- Ren, L.M.; Zhu, L.F.; Yang, C.G.; Chen, Y.M.; Sun, Q.; Zhang, H.Y.; Li, C.J.; Nawaz, F.; Meng, X.J.; Xiao, F.S. Designed copper-amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Moliner, M.; Franch, C.; Kustov, A.; Corma, A. Rational direct synthesis methodology of very active and hydrothermally stable Cu-SAPO-34 molecular sieves for the SCR of NOx. Appl. Catal. B Environ. 2012, 127, 273–280. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Warrender, S.J.; Picone, A.L.; Wright, P.A.; Weckhuysen, B.M.; Beale, A.M. Changing active sites in Cu-CHA catalysts: deNOx selectivity as a function of the preparation method. Microporous Mesoporous Mater. 2013, 166, 144–152. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and evaluation of Cu/SAPO-34 catalysts for NH3-SCR 2: Solid-state ion exchange and one-pot synthesis. Appl. Catal. B Environ. 2015, 162, 501–514. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, N.N.; Wang, X.; Li, Q.; Ma, X.C.; Qi, Y.X.; Zheng, L.R.; Anderson, J.A.; Zhang, Z.L. Efficient synthesis of the Cu-SAPO-44 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Catal. Today 2019, 332, 35–41. [Google Scholar] [CrossRef]
- Xie, L.J.; Liu, F.D.; Ren, L.M.; Shi, X.L.; Xiao, F.S.; He, H. Excellent performance of one-pot synthesized Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.M.; Zhang, Y.B.; Zeng, S.J.; Zhu, L.F.; Sun, Q.; Zhang, H.Y.; Yang, C.G.; Meng, X.J.; Yang, X.G.; Xiao, F.S. Design and synthesis of a catalytically active Cu-SSZ-13 zeolite from a copper-amine complex template. Chin. J. Catal. 2012, 33, 92–105. [Google Scholar] [CrossRef]
- Guo, Q.; Fan, F.; Ligthart, D.A.J.M.; Li, G.; Feng, Z.; Hensen, E.J.M.; Li, C. Effect of the nature and location of copper species on the catalytic nitric oxide selective catalytic reduction performance of the copper/SSZ-13 zeolite. ChemCatChem 2014, 6, 634–639. [Google Scholar] [CrossRef]
- Li, H.; Xin, Y.; Wang, X.; Zhou, Y.H.; Li, Q.; Zhang, Z.L. A novel dual-template method for synthesis of SAPO-44 zeolite. RSC Adv. 2016, 6, 35910–35913. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, X.; Li, Q.; Ma, X.C.; Qi, Y.X.; Zheng, L.R.; Anderson, J.A.; Zhang, Z.L. The potential of Cu-SAPO-44 in selective catalytic reduction of NOx with NH3. ChemCatChem 2016, 8, 3740–3745. [Google Scholar] [CrossRef]
- Xue, J.J.; Wang, X.Q.; Qi, G.; Wang, J.; Shen, M.Q.; Li, W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 2013, 297, 56–64. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Qi, G.; Weng, D. Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J. Catal. 2012, 289, 21–29. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure-activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Richter, M.; Fait, M.J.G.; Eckelt, R.; Schneider, M.; Radnik, J.; Heidemann, D.; Fricke, R. Gas-phase carbonylation of methanol to dimethyl carbonate on chloride-free Cu-precipitated zeolite Y at normal pressure. J. Catal. 2007, 245, 11–24. [Google Scholar] [CrossRef]
- Yamashita, H.; Matsuoka, M.; Tsuji, K.; Shioya, Y.; Anpo, M.; Che, M. In-situ XAFS, photoluminescence, and IR investigations of copper ions included within various kinds of zeolites. Structure of Cu(I) ions and their interaction with CO molecules. J. Phys. Chem. 1996, 100, 397–402. [Google Scholar] [CrossRef]
- Korhonen, S.T.; Fickel, D.W.; Lobo, R.F.; Weckhuysen, B.M.; Beale, A.M. Isolated Cu2+ ions: Active sites for selective catalytic reduction of NO. Chem. Commun. 2011, 47, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.; Shrivastava, B.D.; Joshi, S.K. Copper K-edge XANES of Cu(I) and Cu(II) oxide mixtures. J. Phys. Conf. Ser. 2009, 190, 012084. [Google Scholar] [CrossRef]
- Peng, Z.L. The Optimization on in Situ Synthesis Conditions of Cu-SSZ-13 Denitration Catalyst; Taiyuan University of Technology: Taiyuan, China, 2015. [Google Scholar]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef]



| Sample | Element Content from ICP (wt.%) | ||||
|---|---|---|---|---|---|
| Cu | Si | Al | Si/Al | P | |
| Cu4.3-SAPO-44 | 4.3 | 5.7 | 17.2 | 0.32 | 16.5 |
| Cu5.6-SAPO-44 | 5.6 | 6.4 | 16.7 | 0.37 | 13.6 |
| Cu7.6-SAPO-44 | 7.6 | 5.1 | 17.6 | 0.28 | 15.1 |
| Cu1.0-SAPO-44-IE | 1.0 | 6.1 | 18.8 | 0.31 | 18.2 |
| Cu1.6-SAPO-44-IE | 1.6 | 7.1 | 18.7 | 0.37 | 14.0 |
| Cu2.2-SAPO-44-IE | 2.2 | 4.8 | 20.2 | 0.23 | 18.4 |
| Sample | BET Surface Areas (m2/g) | Pore Volume (cm3/g) | Cu2+/CuO Data from LCF of XANES Spectra (wt.%/wt.%) |
|---|---|---|---|
| Cu5.6-SAPO-44 | 338.8 | 0.15 | 22.4/77.6 |
| Cu1.6-SAPO-44-IE | 363.7 | 0.17 | 69.7/30.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Xin, Y.; Li, Q.; Ma, X.; Qi, Y.; Zheng, L.; Zhang, Z. Ion Exchange of One-Pot Synthesized Cu-SAPO-44 with NH4NO3 to Promote Cu Dispersion and Activity for Selective Catalytic Reduction of NOx with NH3. Catalysts 2019, 9, 882. https://doi.org/10.3390/catal9110882
Zhang N, Xin Y, Li Q, Ma X, Qi Y, Zheng L, Zhang Z. Ion Exchange of One-Pot Synthesized Cu-SAPO-44 with NH4NO3 to Promote Cu Dispersion and Activity for Selective Catalytic Reduction of NOx with NH3. Catalysts. 2019; 9(11):882. https://doi.org/10.3390/catal9110882
Chicago/Turabian StyleZhang, Nana, Ying Xin, Qian Li, Xicheng Ma, Yongxin Qi, Lirong Zheng, and Zhaoliang Zhang. 2019. "Ion Exchange of One-Pot Synthesized Cu-SAPO-44 with NH4NO3 to Promote Cu Dispersion and Activity for Selective Catalytic Reduction of NOx with NH3" Catalysts 9, no. 11: 882. https://doi.org/10.3390/catal9110882
APA StyleZhang, N., Xin, Y., Li, Q., Ma, X., Qi, Y., Zheng, L., & Zhang, Z. (2019). Ion Exchange of One-Pot Synthesized Cu-SAPO-44 with NH4NO3 to Promote Cu Dispersion and Activity for Selective Catalytic Reduction of NOx with NH3. Catalysts, 9(11), 882. https://doi.org/10.3390/catal9110882






