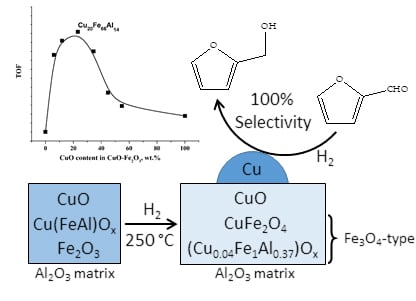
Highly Active CuFeAl-containing Catalysts for Selective Hydrogenation of Furfural to Furfuryl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
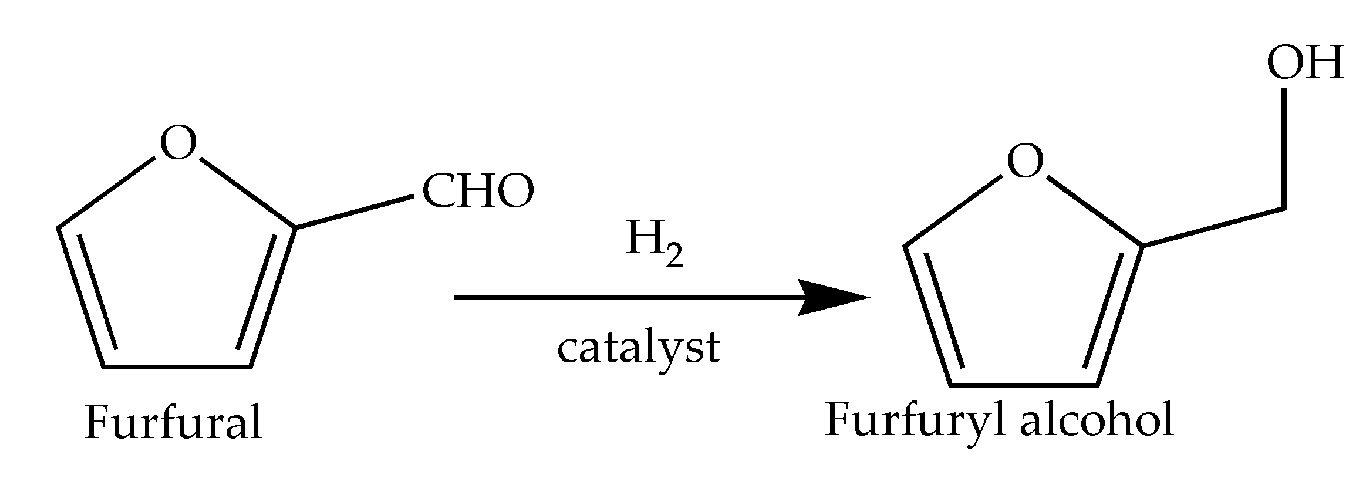
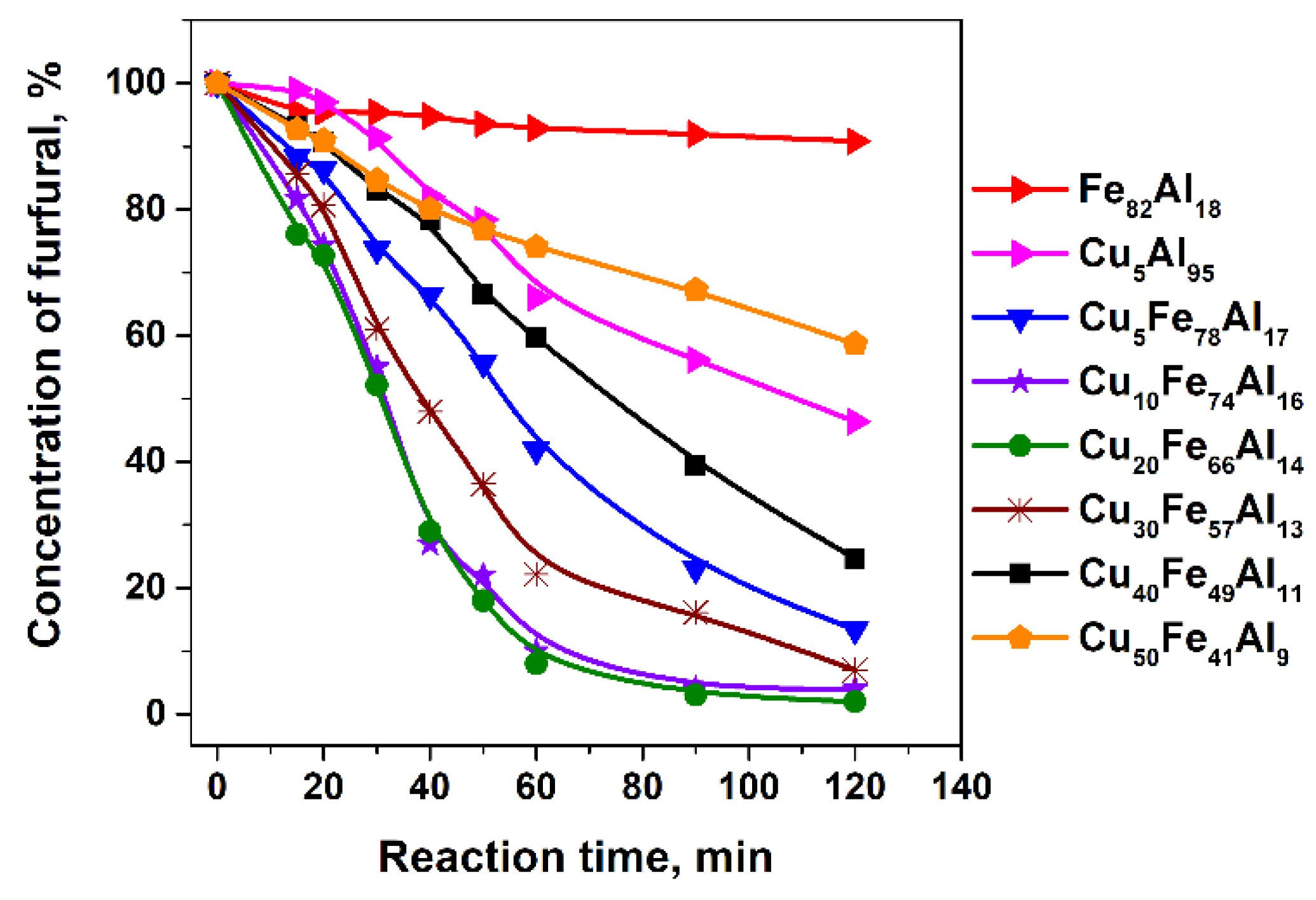
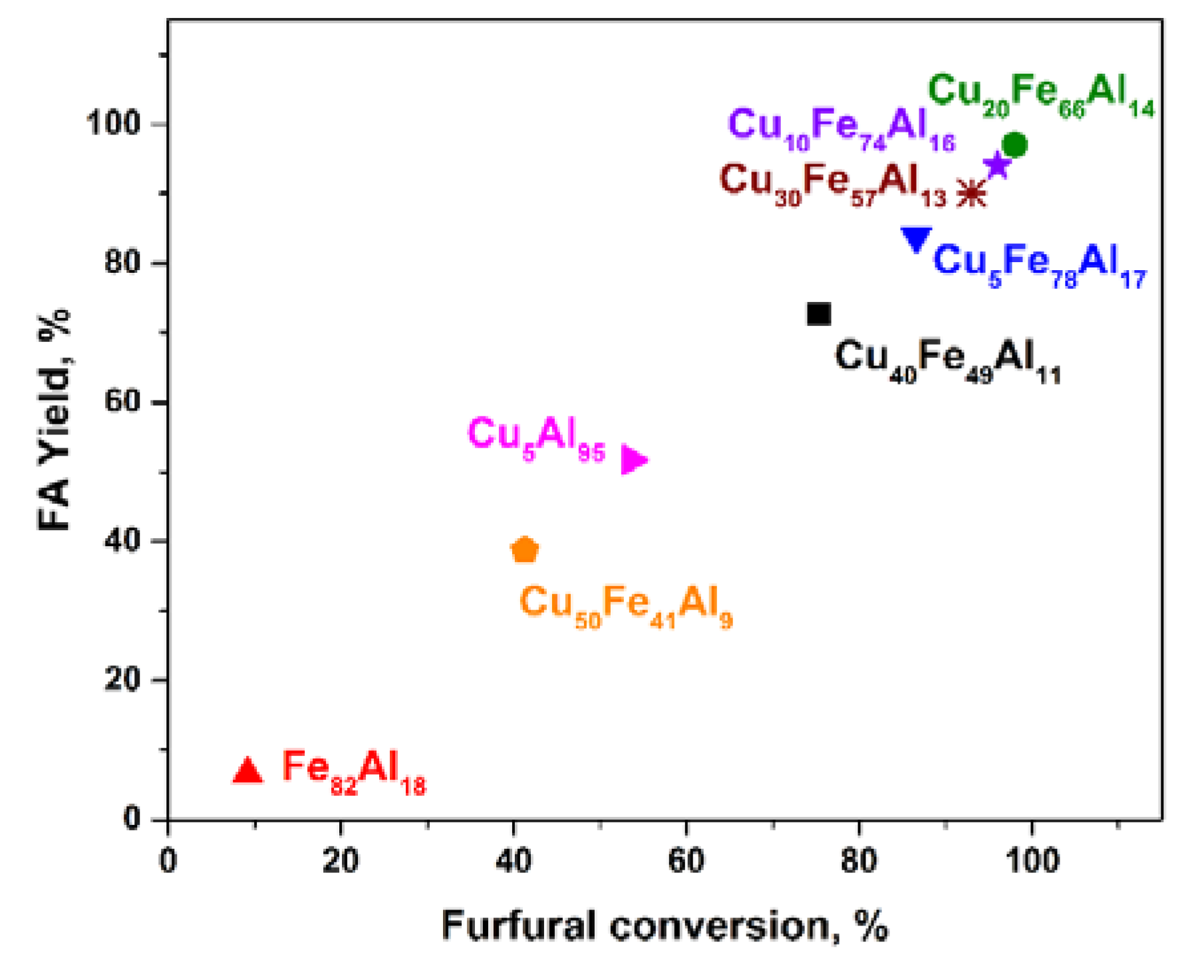
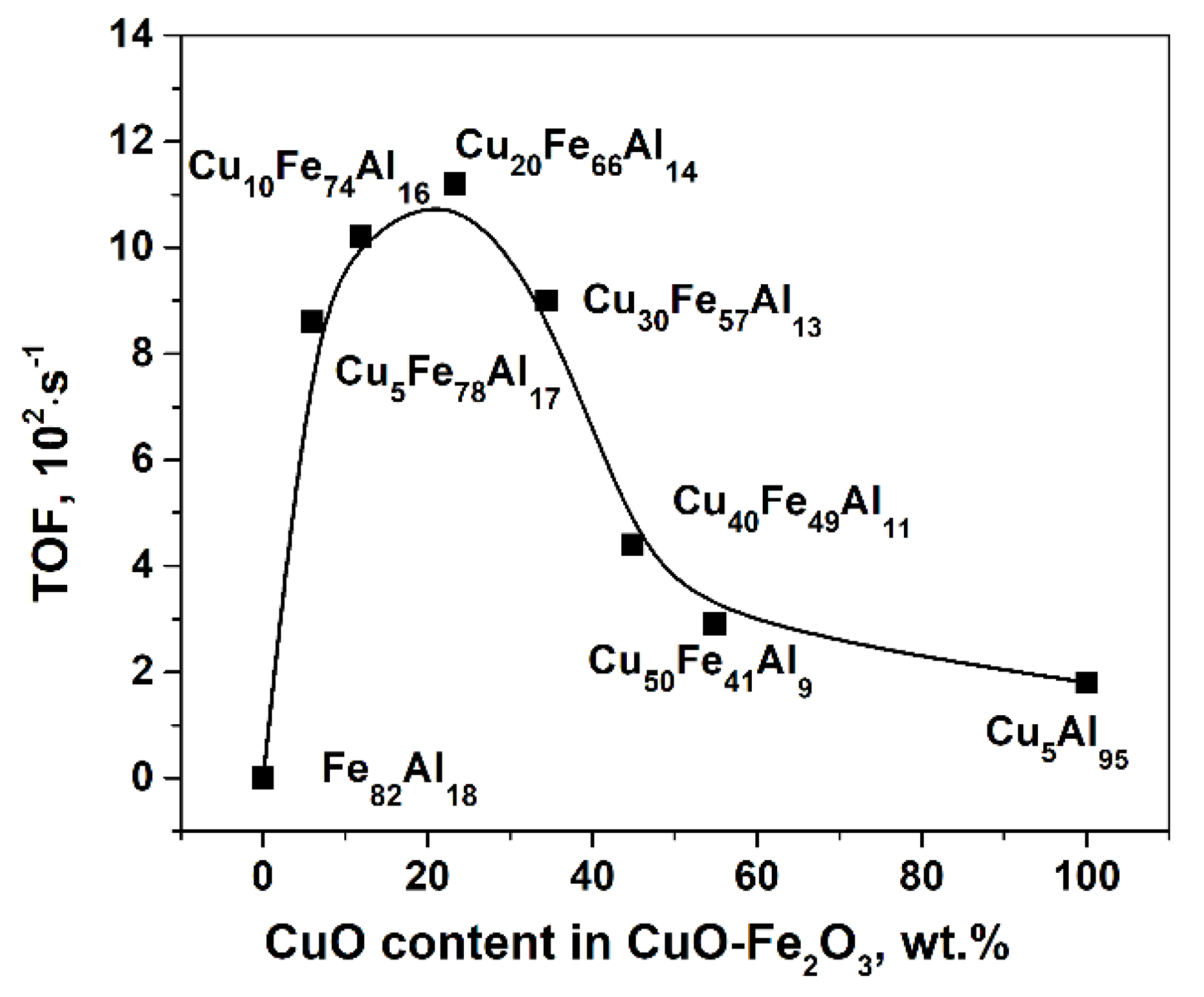
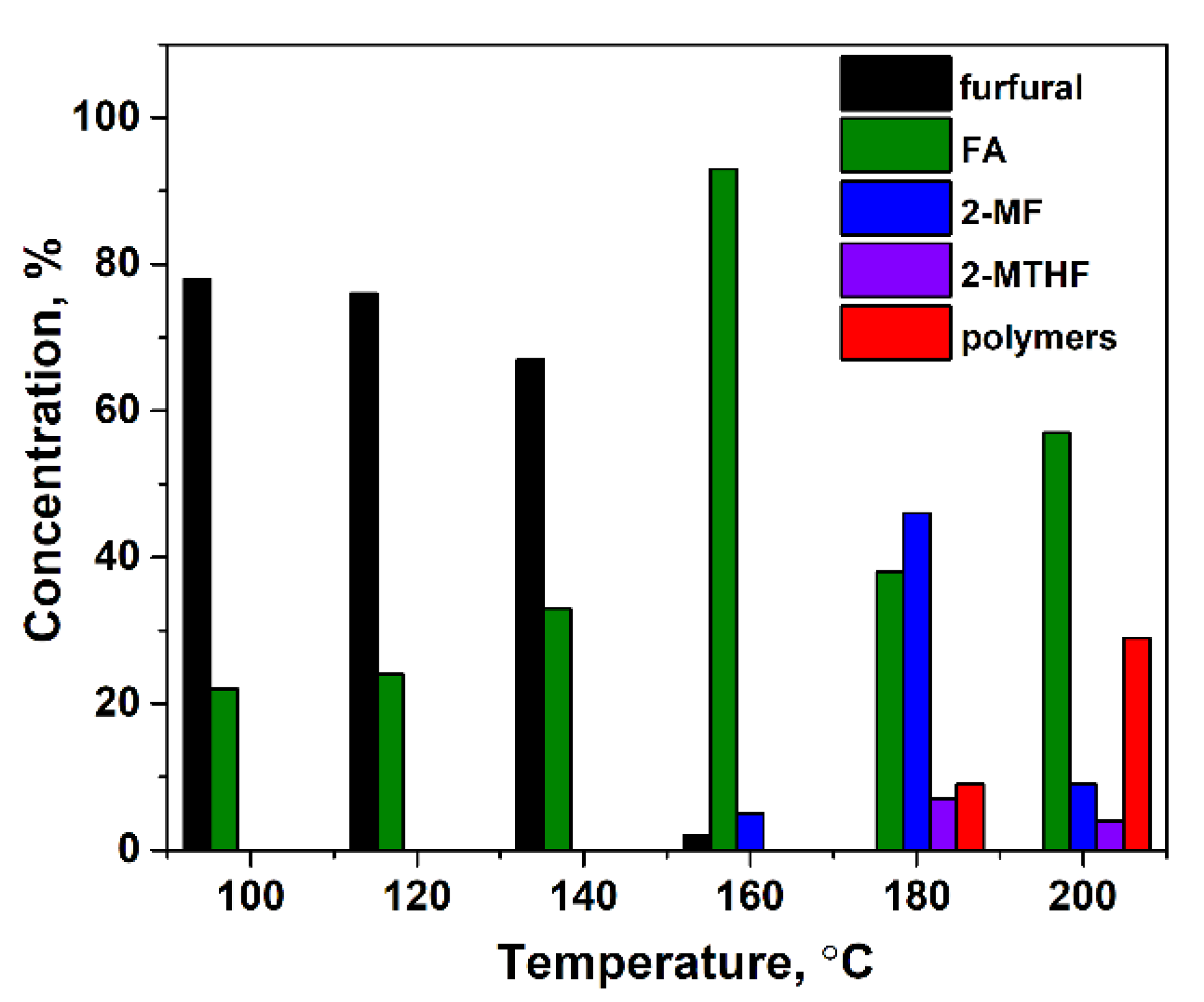
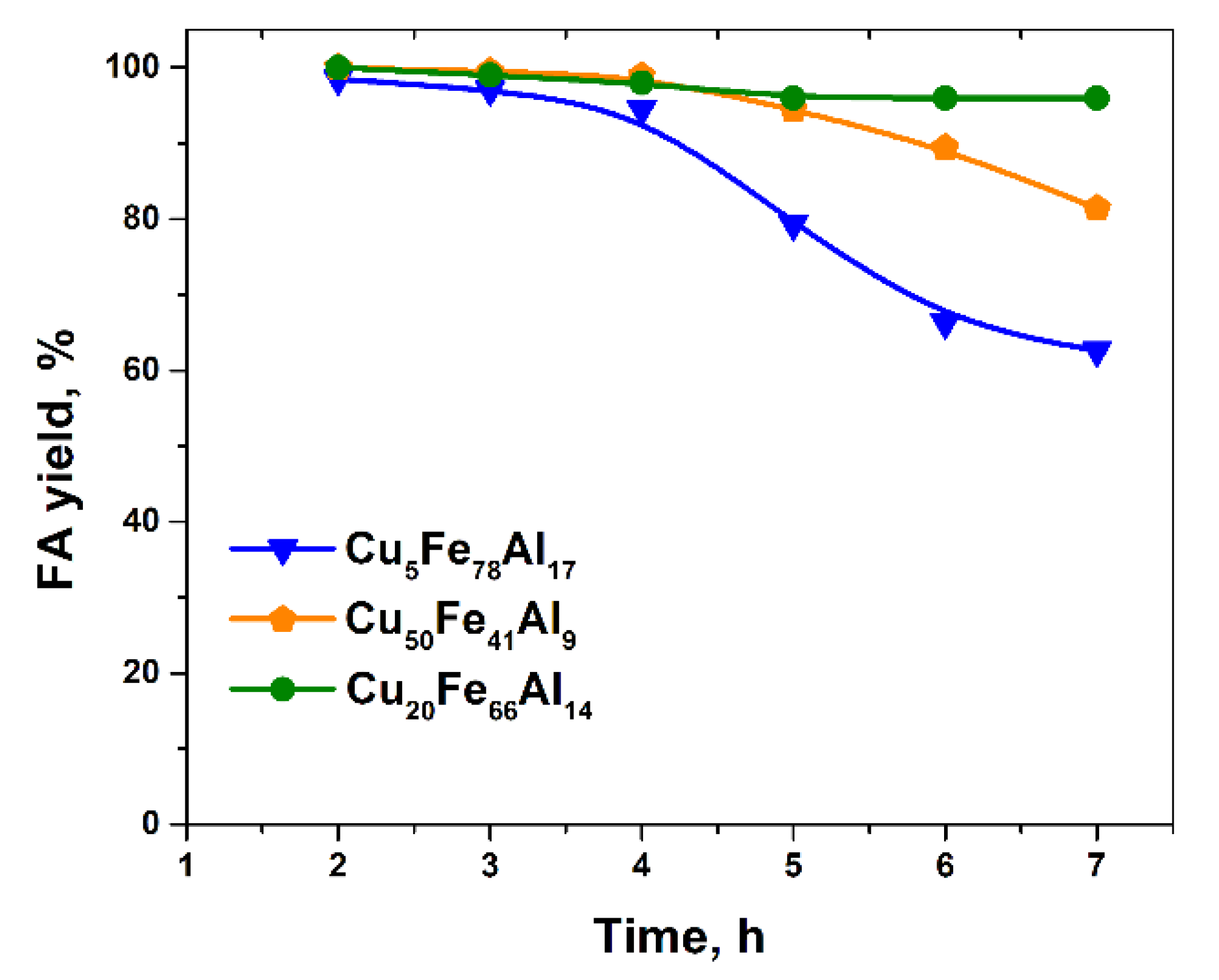
2.1. Catalytic Activity of CuFe-Containing Catalysts in a Batch Reactor
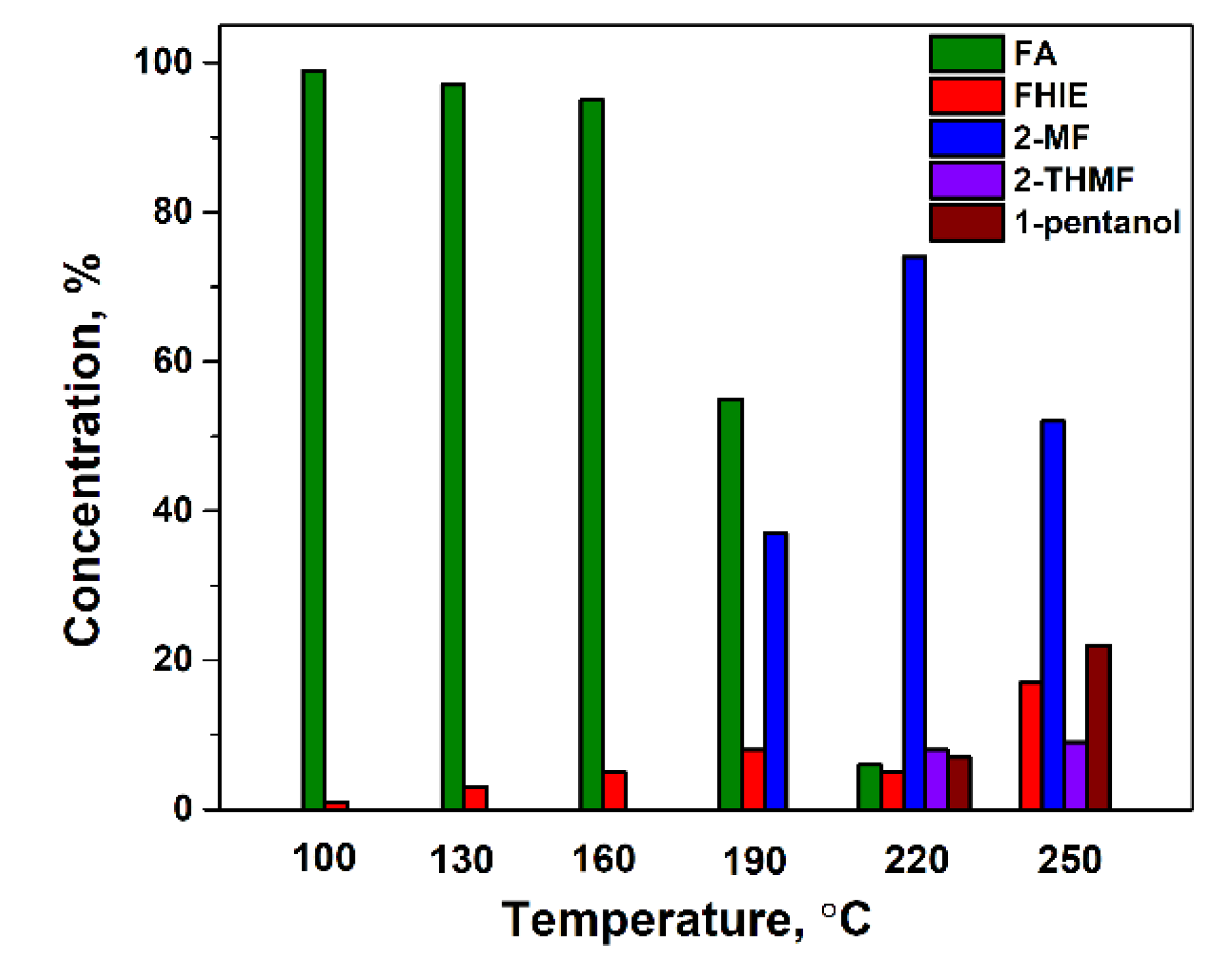
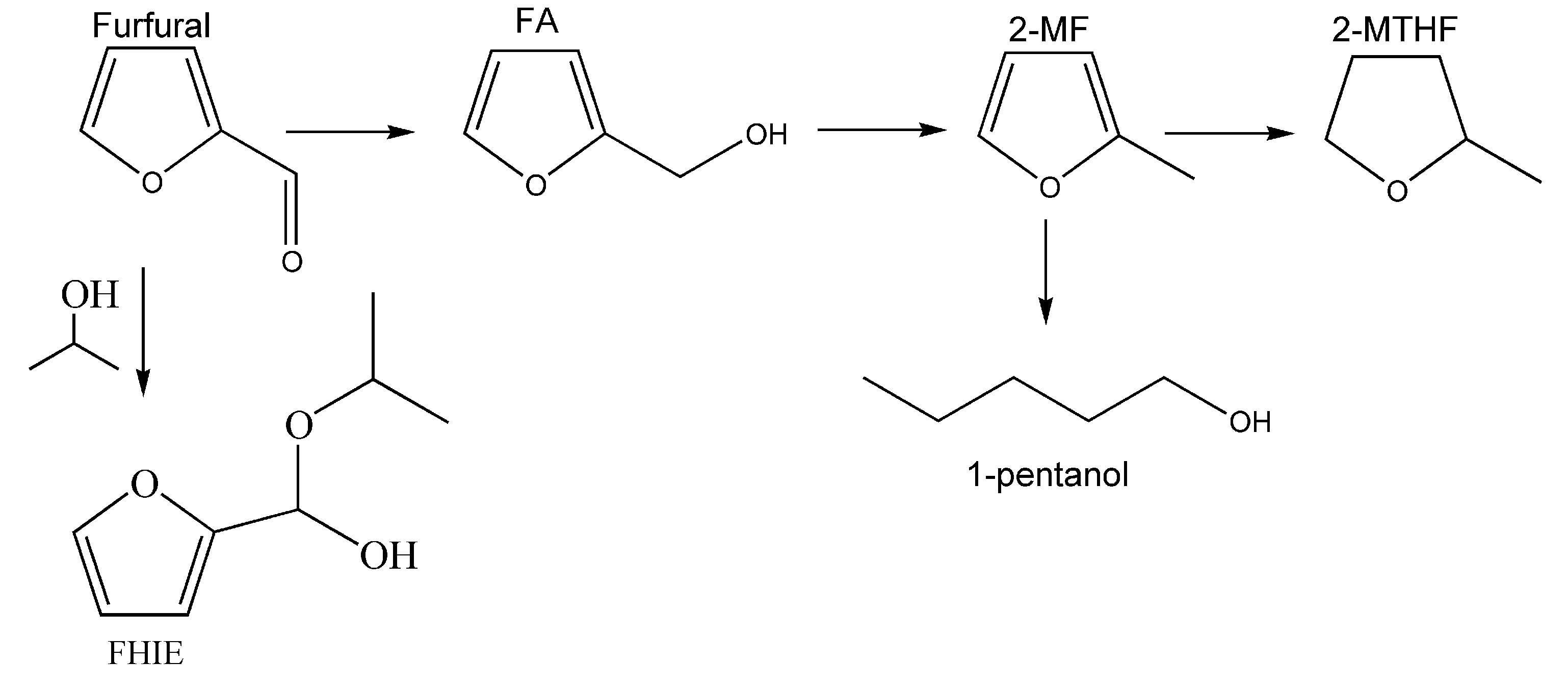
2.2. Catalytic Activity of Cu20Fe66Al14 Catalyst in a Fixed-Bed Reactor in Solvent-Free Furfural Hydrogenation
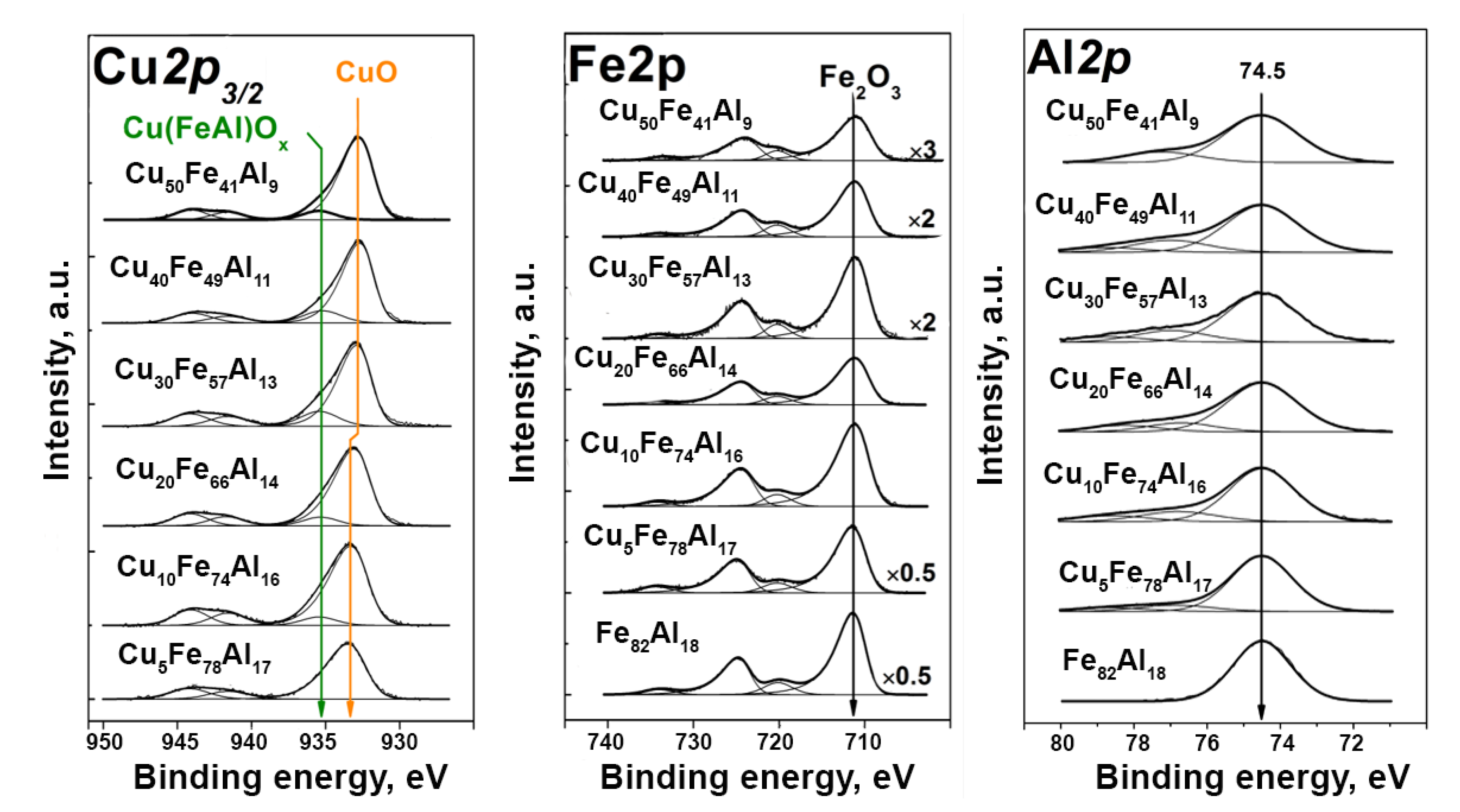
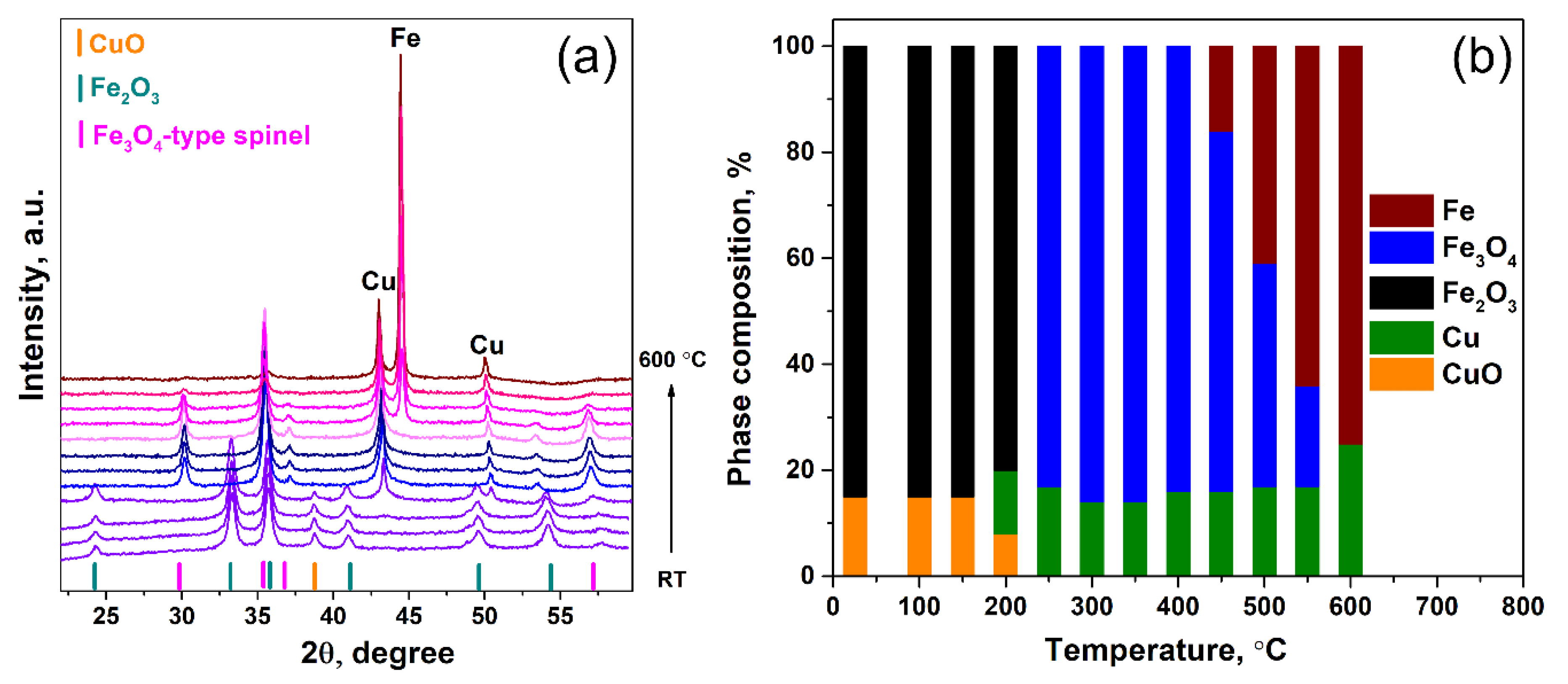
2.3. Catalysts Characterization
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Activity Tests
3.3. Products Analysis
3.4. X-Ray Diffraction
3.5. X-Ray Photoelectron Spectroscopy
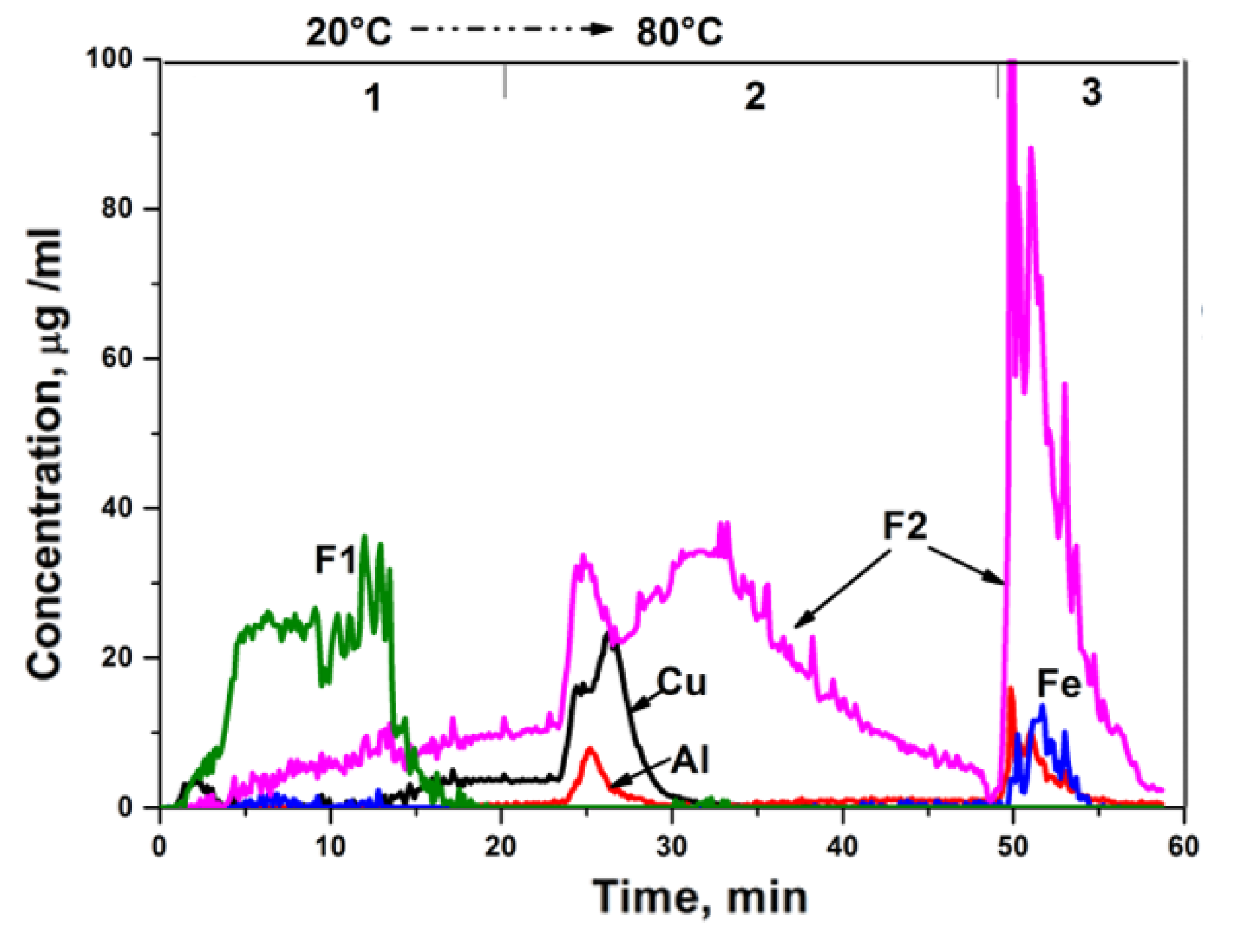
3.6. Differentiating Dissolution on an Inductively Coupled Plasma Atomic Emission Spectrometer
3.7. In Situ XANES
3.8. Elemental Analysis
3.9. CO Pulse Chemisorption Measurements
3.10. Transmission Electron Microscopy
3.11. Texture Characteristics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Espro, C.; Gumina, B.; Szumelda, T.; Paone, E.; Mauriello, F. Catalytic Transfer Hydrogenolysis as an Effective Tool for the Reductive Upgrading of Cellulose, Hemicellulose, Lignin, and Their Derived Molecules. Catalysts 2018, 8, 313. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Bonacci, S.; Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.; Oliverio, M.; Procopio, A. Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones. Catalysts 2019, 9, 301. [Google Scholar] [CrossRef]
- Smirnov, A.; Selishcheva, S.; Yakovlev, V. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595. [Google Scholar] [CrossRef]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Gomes, R.F.A.; Esteves, N.R.; Coelho, J.A.S.; Afonso, C.A.M. Copper(II) Triflate As a Reusable Catalyst for the Synthesis of trans-4,5-Diamino-cyclopent-2-enones in Water. J. Org. Chem. 2018, 83, 7509–7513. [Google Scholar] [CrossRef] [PubMed]
- Morozov, E. Furfural Production, 2nd ed.; Forest Industry: Moscow, Russia, 1988; pp. 32–56. [Google Scholar]
- Liu, D.; Zemlyanov, D.; Wu, T.; Lobo-Lapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar] [CrossRef]
- Musci, J.J.; Merlo, A.B.; Casella, M.L. Aqueous phase hydrogenation of furfural using carbon-supported Ru and RuSn catalysts. Catal. Today 2017, 296, 43–50. [Google Scholar] [CrossRef]
- Vorotnikov, V.; Mpourmpakis, G.; Vlachos, D.G. DFT study of furfural conversion to furan, furfuryl alcohol, and 2-methylfuran on Pd(111). ACS Catal. 2012, 2, 2496–2504. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Mahajani, V.V. Kinetics of Liquid-Phase Hydrogenation of Furfuraldehyde to Furfuryl Alcohol over a Pt / C Catalyst. Ind. Eng. Chem. Resour. 2003, 42, 3881–3885. [Google Scholar] [CrossRef]
- Zhao, Y. Facile synthesis of Pd nanoparticles on SiO2 for hydrogenation of biomass-derived furfural. Environ. Chem. Lett. 2014, 12, 185–190. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Gulyaeva, T.I.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Lavrenov, A.V.; Likholobov, V.A. Effect of the nature of carbon support on the formation of active sites in Pd/C and Ru/C catalysts for hydrogenation of furfural. Catal. Today 2015, 249, 145–152. [Google Scholar] [CrossRef]
- Wu, J.; Shen, Y.; Liu, C.; Wang, H.; Geng, C.; Zhang, Z. Vapor phase hydrogenation of furfural to furfuryl alcohol over environmentally friendly Cu-Ca/SiO2 catalyst. Catal. Commun. 2005, 6, 633–637. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Bertero, N.M.; Garetto, T.F.; Marchi, A.J. Selective liquid-phase hydrogenation of furfural to furfuryl alcohol over Cu-based catalysts. Catal. Today 2013, 213, 87–92. [Google Scholar] [CrossRef]
- Khromova, S.A.; Bykova, M.V.; Bulavchenko, O.A.; Ermakov, D.Y.; Saraev, A.A.; Kaichev, V.V.; Venderbosch, R.H.; Yakovlev, V.A. Furfural Hydrogenation to Furfuryl Alcohol over Bimetallic Ni–Cu Sol–Gel Catalyst: A Model Reaction for Conversion of Oxygenates in Pyrolysis Liquids. Top. Catal. 2016, 59, 1413–1423. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts. J. Catal. 2011, 277, 1–13. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Shilov, I.N.; Alekseeva, M.V.; Selishcheva, S.A.; Yakovlev, V.A. Study of the Composition Effect of Molybdenum-Modified Nickel–Copper Catalysts on Their Activity and Selectivity in the Hydrogenation of Furfural to Different Valuable Chemicals. Catal. Ind. 2018, 10, 228–236. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Garetto, T.F.; Marchi, A.J. Liquid-phase transfer hydrogenation of furfural to furfuryl alcohol on Cu–Mg–Al catalysts. Catal. Commun. 2015, 58, 6–10. [Google Scholar] [CrossRef]
- Srivastava, S.; Mohanty, P.; Parikh, J.K.; Dalai, A.K.; Amritphale, S.S.; Khare, A.K. Cr-free Co–Cu/SBA-15 catalysts for hydrogenation of biomass-derived α-, β-unsaturated aldehyde to alcohol. Chin. J. Catal. 2015, 36, 933–942. [Google Scholar] [CrossRef]
- Wei, S.; Cui, H.; Wang, J.; Zhuo, S.; Yi, W.; Wang, L.; Li, Z. Preparation and activity evaluation of NiMoB/γ-Al2O3 catalyst by liquid-phase furfural hydrogenation. Particuology 2011, 9, 69–74. [Google Scholar] [CrossRef]
- Hu, F.; Wang, Y.; Xu, S.; Zhang, Z.; Chen, Y.; Fan, J.; Yuan, H.; Gao, L.; Xiao, G. Efficient and Selective Ni/Al2O3–C Catalyst Derived from Metal–Organic Frameworks for the Hydrogenation of Furfural to Furfuryl Alcohol. Catal. Lett. 2019, 149, 2158–2168. [Google Scholar] [CrossRef]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of furfural over supported metal catalysts: A comparative study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Yan, P.; Jiang, M.; Zhao, Z.; Zhang, Z.C. Catalytic furfural hydrogenation to furfuryl alcohol over Cu/SiO2 catalysts: A comparative study of the preparation methods. Fuel Process. Technol. 2019, 193, 221–231. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaee, M.; Shirvani, S.; Ghambarian, M. Kinetic models for hydroconversion of furfural over the ecofriendly Cu-MgO catalyst: An experimental and theoretical study. Appl. Catal. A Gen. 2017, 545, 134–147. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Tsapina, A.M.; Bulavchenko, O.A.; Saraev, A.A.; Odegova, G.V.; Ermakov, D.Y.; Zubavichus, Y.V.; Yakovlev, V.A.; Kaichev, V.V. Structure and Chemistry of Cu–Fe–Al Nanocomposite Catalysts for CO Oxidation. Catal. Lett. 2018, 148, 3715–3722. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Vinokurov, Z.S.; Saraev, A.A.; Tsapina, A.M.; Trigub, A.L.; Gerasimov, E.Y.; Gladky, A.Y.; Fedorov, A.V.; Yakovlev, V.A.; Kaichev, V.V. The Influence of Cu and Al Additives on Reduction of Iron(III) Oxide: In Situ XRD and XANES Study. Inorg. Chem. 2019, 58, 4842–4850. [Google Scholar] [CrossRef]
- Selishcheva, S.A.; Smirnov, A.A.; Fedorov, A.V.; Ermakov, D.Y.; Gulyaeva, Y.K.; Yakovlev, V.A. Production of Furfuryl Alcohol in the Presence of Copper-Containing Catalysts in the Selective Hydrogenation of Furfural. Katal. V Promyshlennosti 2019, 11, 216–223. [Google Scholar] [CrossRef]
- Ferullo, R.M.; Castellani, N.J. A quantum-chemical study of CO adsorption on small Cu particles supported on reduced SiO2. J. Mol. Catal. A Chem. 2005, 234, 121–127. [Google Scholar] [CrossRef]
- Shilov, I.; Smirnov, A.; Bulavchenko, O.; Yakovlev, V. Effect of Ni–Mo Carbide Catalyst Formation on Furfural Hydrogenation. Catalysts 2018, 8, 560. [Google Scholar] [CrossRef]
- Khromova, S.A.; Smirnov, A.A.; Bulavchenko, O.A.; Saraev, A.A.; Kaichev, V.V.; Reshetnikov, S.I.; Yakovlev, V.A. Anisole hydrodeoxygenation over Ni–Cu bimetallic catalysts: The effect of Ni/Cu ratio on selectivity. Appl. Catal. A Gen. 2014, 470, 261–270. [Google Scholar] [CrossRef]
- Strohmeier, B.R.; Levden, D.E.; Field, R.S.; Hercules, D.M. Surface spectroscopic characterization of Cu/Al2O3 catalysts. J. Catal. 1985, 94, 514–530. [Google Scholar] [CrossRef]
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the determination of chemical environment and oxidation state of iron and sulfur samples: Constitution of a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution x-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–6024. [Google Scholar] [CrossRef]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural Materials Science end-station at the Kurchatov Synchrotron Radiation Source: Recent instrumentation upgrades and experimental results. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. SpectrometersDetect. Assoc. Equip. 2009, 603, 95–98. [Google Scholar] [CrossRef]


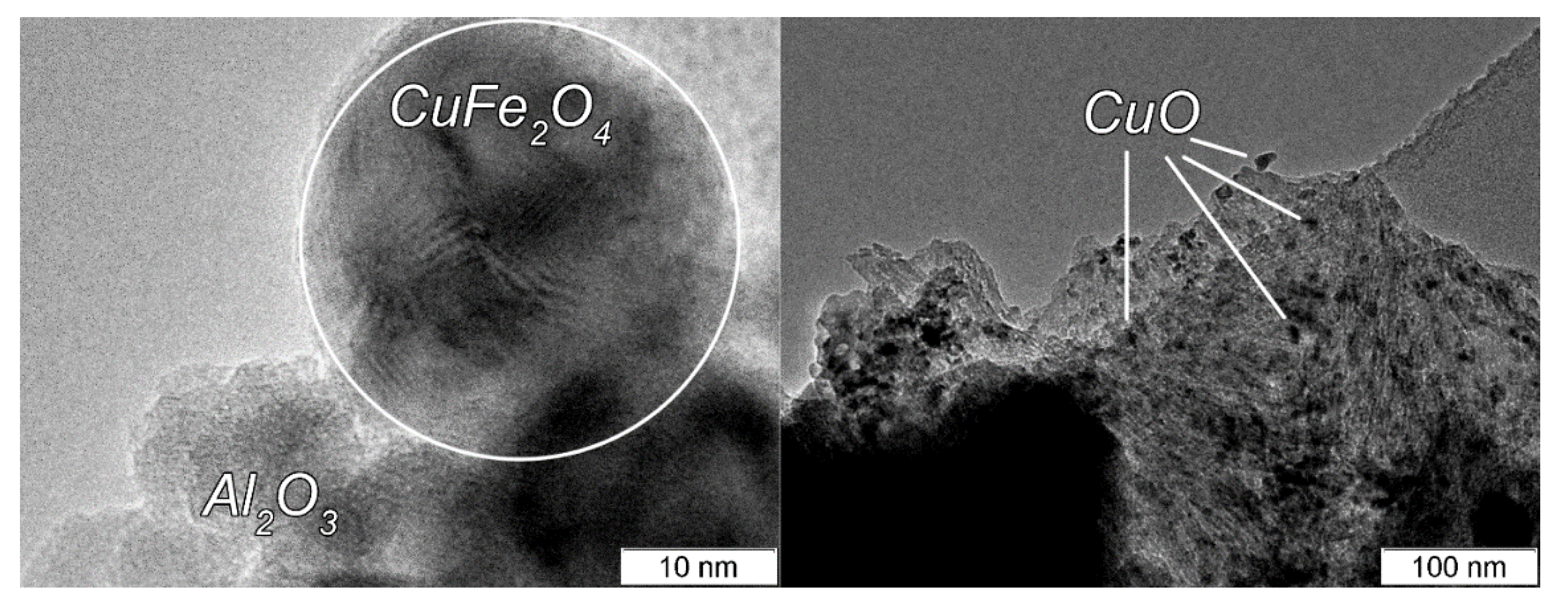
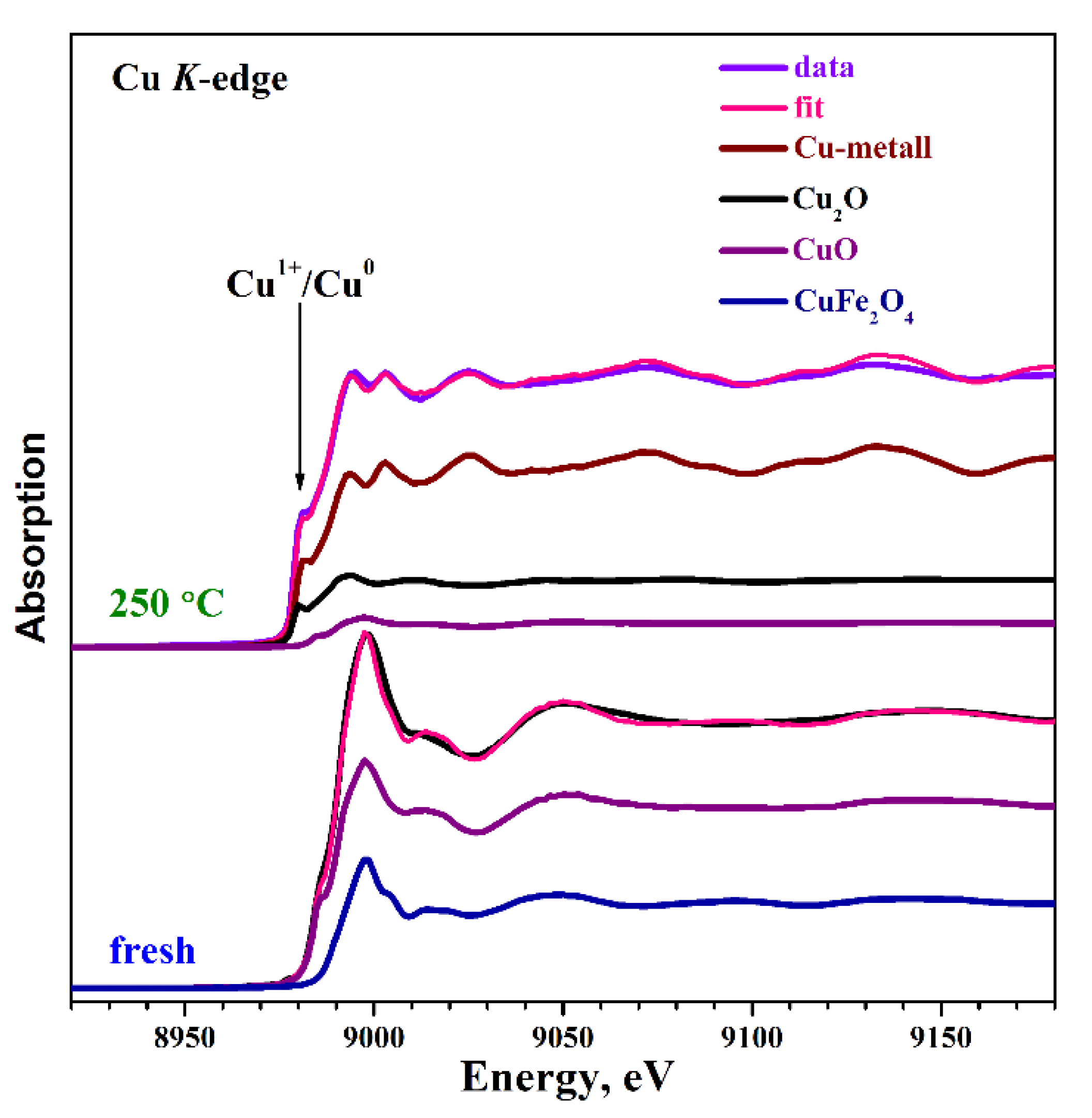
| Sample | Content, wt % | ABET, m2/g | CO Uptake, μmol/g | ||
|---|---|---|---|---|---|
| CuO | Fe2O3 | Al2O3 | |||
| Fe82Al18 | - | 82.0 | 18.0 | 83 | 0 |
| Cu5Fe78Al17 | 5.0 | 77.9 | 17.1 | 74 | 23.5 |
| Cu10Fe74Al16 | 10.0 | 73.8 | 16.2 | 58 | 30.7 |
| Cu20Fe66Al14 | 20.0 | 65.6 | 14.4 | 31 | 30.7 |
| Cu30Fe57Al13 | 30.0 | 57.4 | 12.6 | 37 | 32.7 |
| Cu40Fe49Al11 | 40.0 | 49.2 | 10.8 | 35 | 32.7 |
| Cu50Fe41Al9 | 50.0 | 41.0 | 9.0 | 22 | 26.6 |
| Cu5Al95 | 5.0 | - | 95.0 | 256 | 55.2 |
| Sample | k0·105, s−1 | TOF, 102·s−1 | Carbon Content in Spent Catalysts, wt % |
|---|---|---|---|
| Fe82Al18 | 1.28 ± 0.02 | 0 | 1.9 ± 0.1 |
| Cu5Fe78Al17 | 20 ± 1 | 8.6 ± 0.5 | 2.0 ± 0.1 |
| Cu10Fe74Al16 | 32 ± 3 | 10.2 ± 1.0 | 2.0 ± 0.1 |
| Cu20Fe66Al14 | 34 ± 2 | 11.2 ± 0.6 | 0.93 ± 0.04 |
| Cu30Fe57Al13 | 29 ± 4 | 9.0 ± 1.0 | 1.1 ± 0.1 |
| Cu40Fe49Al11 | 14 ± 1 | 4.4 ± 0.4 | 0.88 ± 0.05 |
| Cu50Fe41Al9 | 7.6 ± 0.3 | 2.9 ± 0.1 | 0.5 ± 0.1 |
| Cu5Al95 | 9.8 ± 0.9 | 1.8 ± 0.2 | 0.4 ± 0.1 |
| Sample | [Fe]/[Al] | [Cu]/[Al] | % CuO in all form of Cu |
|---|---|---|---|
| Cu5Fe78Al17 | 0.70 | 0.21 | 100 |
| Cu10Fe74Al16 | 0.42 | 0.31 | 93 |
| Cu20Fe66Al14 | 0.25 | 0.27 | 92 |
| Cu30Fe57Al13 | 0.20 | 0.29 | 87 |
| Cu40Fe49Al11 | 0.15 | 0.26 | 87 |
| Cu50Fe41Al9 | 0.08 | 0.26 | 92 |
| Cu20Fe66Al14 (fixed-bed reactor) | 0.19 | 0.20 | 81 |
| Sample | Phase Composition, % | CSD, Å |
|---|---|---|
| Fe82Al18 | Fe2O3 | 200 |
| Cu5Fe78Al17 | Fe3O4 | 200 |
| Cu10Fe74Al16 | Fe3O4 | 250 |
| Cu20Fe66Al14 | 93% Fe3O4 7% Cu | 250 450 |
| Cu30Fe57Al13 | 77% Fe3O4 23% Cu | 250 1000 |
| Cu40Fe49Al11 | 55% Fe3O4, 45% Cu | 250 1500 |
| Cu50Fe41Al9 | 45% Fe3O4 55% Cu | 300 >1500 |
| Cu5Al95 | AlOOH | 60 |
| Cu20Fe66Al14 (fixed-bed reactor) | 92% Fe3O4 8% Cu | 200 450 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selishcheva, S.A.; Smirnov, A.A.; Fedorov, A.V.; Bulavchenko, O.A.; Saraev, A.A.; Lebedev, M.Y.; Yakovlev, V.A. Highly Active CuFeAl-containing Catalysts for Selective Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts 2019, 9, 816. https://doi.org/10.3390/catal9100816
Selishcheva SA, Smirnov AA, Fedorov AV, Bulavchenko OA, Saraev AA, Lebedev MY, Yakovlev VA. Highly Active CuFeAl-containing Catalysts for Selective Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts. 2019; 9(10):816. https://doi.org/10.3390/catal9100816
Chicago/Turabian StyleSelishcheva, Svetlana A., Andrey A. Smirnov, Alexander V. Fedorov, Olga A. Bulavchenko, Andrey A. Saraev, Maksim Yu. Lebedev, and Vadim A. Yakovlev. 2019. "Highly Active CuFeAl-containing Catalysts for Selective Hydrogenation of Furfural to Furfuryl Alcohol" Catalysts 9, no. 10: 816. https://doi.org/10.3390/catal9100816
APA StyleSelishcheva, S. A., Smirnov, A. A., Fedorov, A. V., Bulavchenko, O. A., Saraev, A. A., Lebedev, M. Y., & Yakovlev, V. A. (2019). Highly Active CuFeAl-containing Catalysts for Selective Hydrogenation of Furfural to Furfuryl Alcohol. Catalysts, 9(10), 816. https://doi.org/10.3390/catal9100816