Effect of Mono-, Di-, and Triethylene Glycol on the Activity of Phosphate-Doped NiMo/Al2O3 Hydrotreating Catalysts
Abstract
1. Introduction
2. Results and Discussion
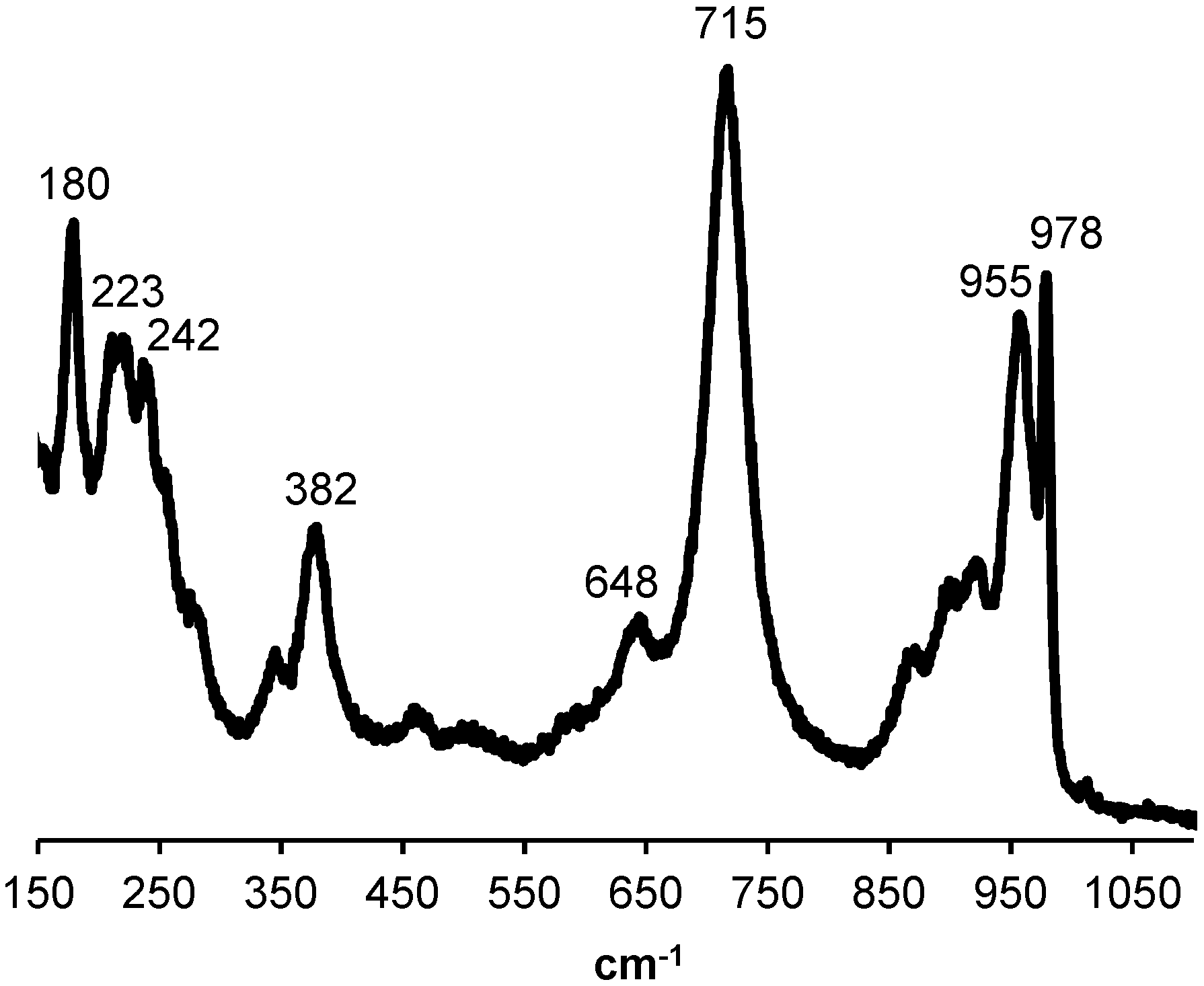
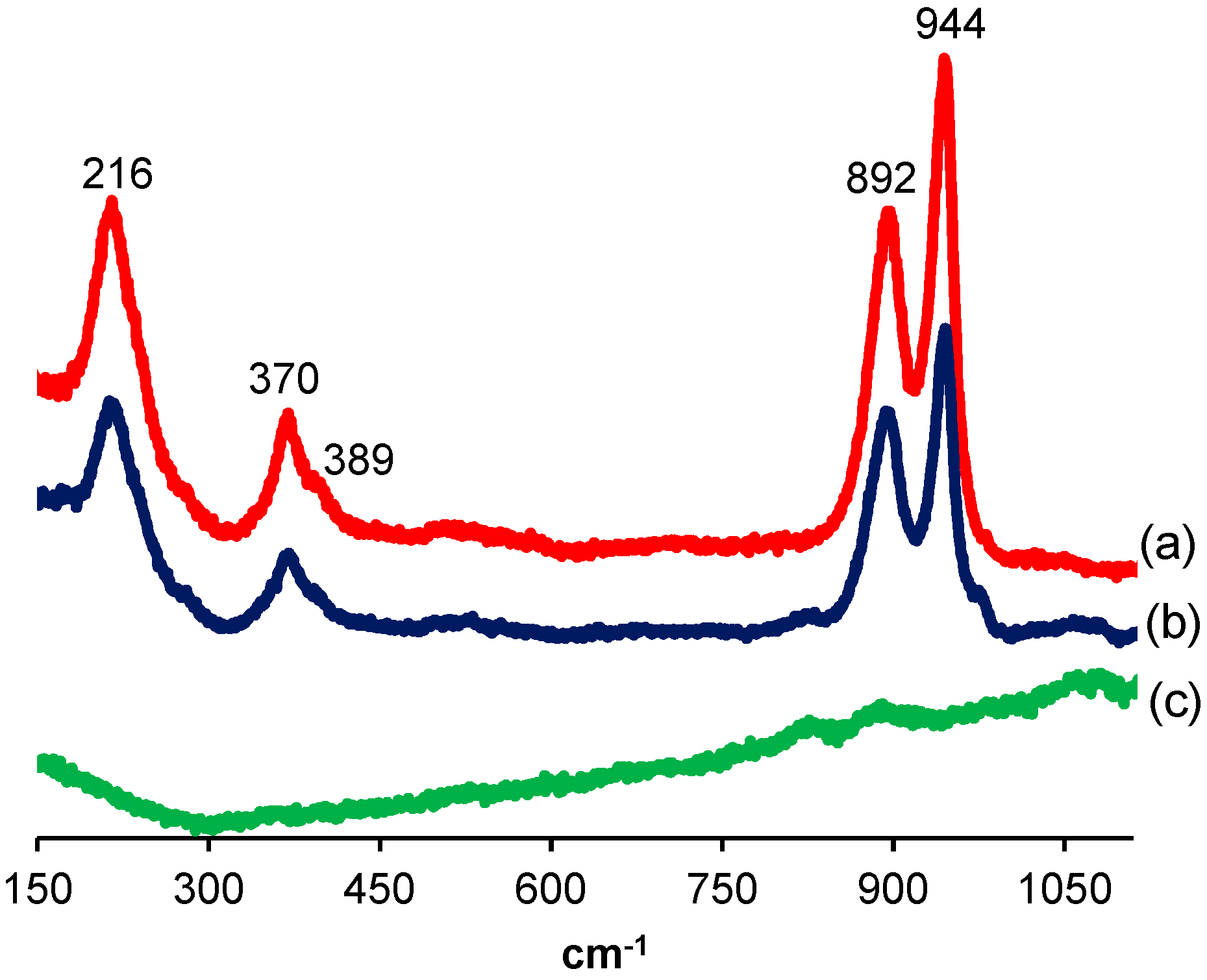
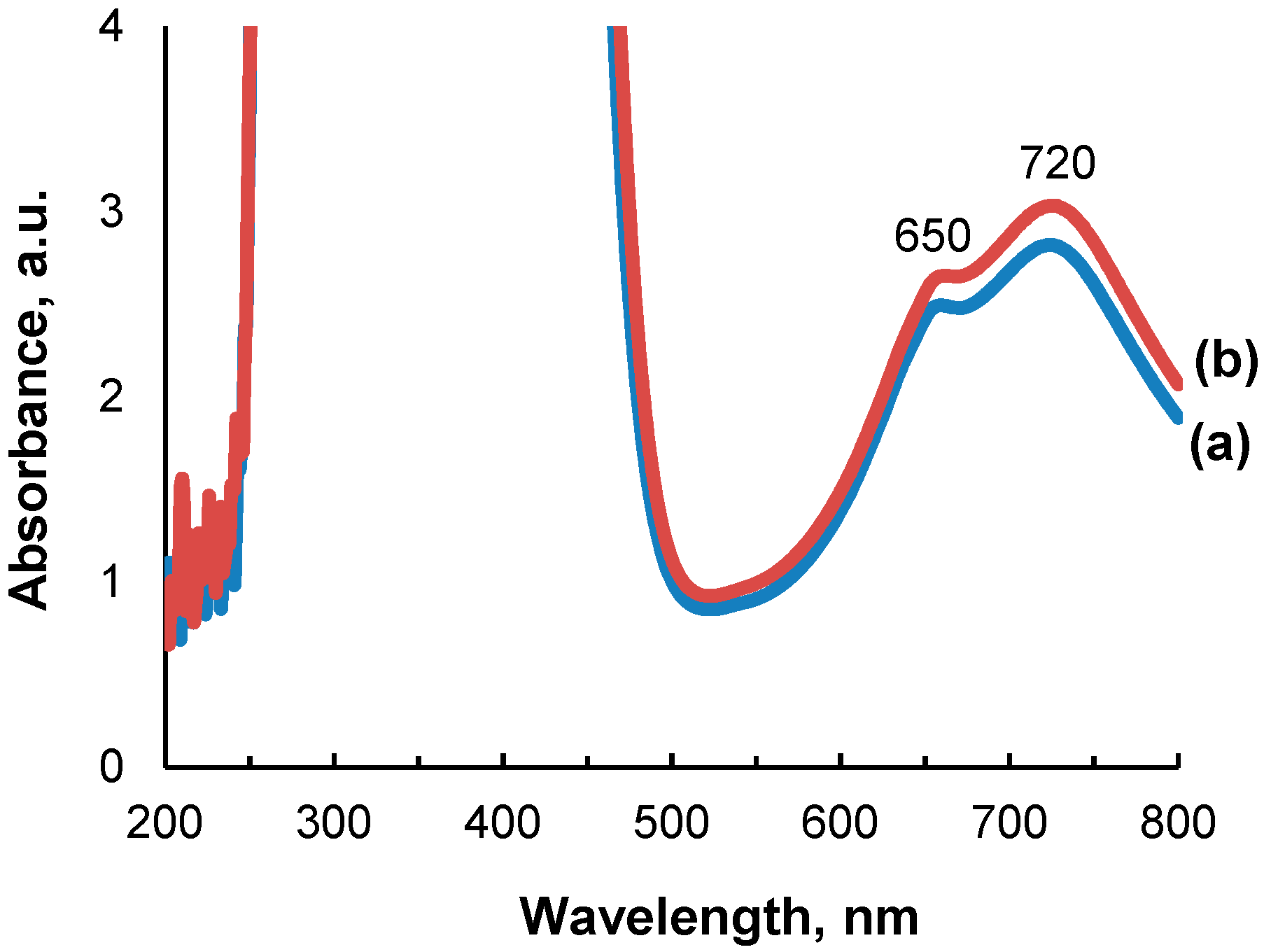
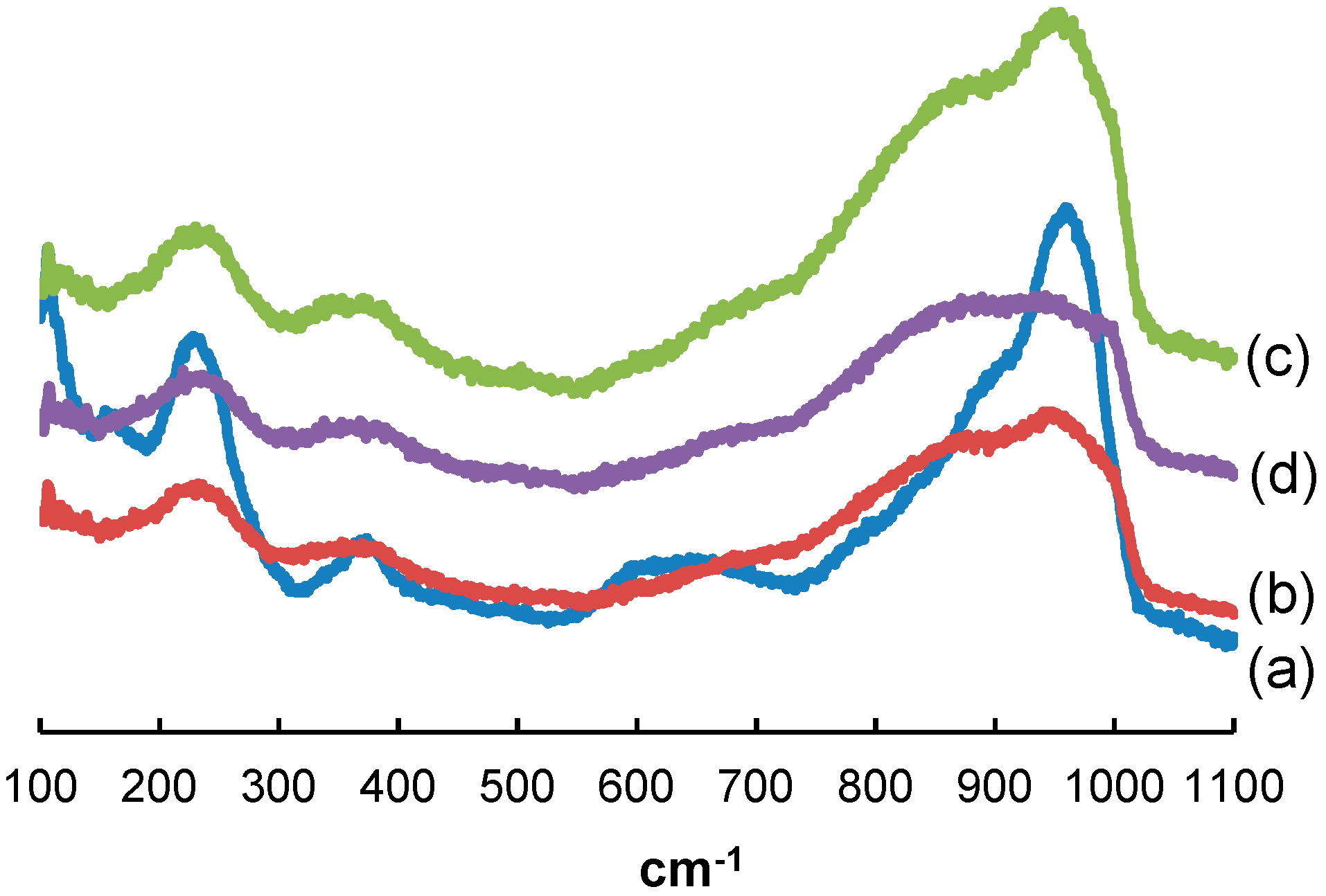
2.1. Impregnation Solutions
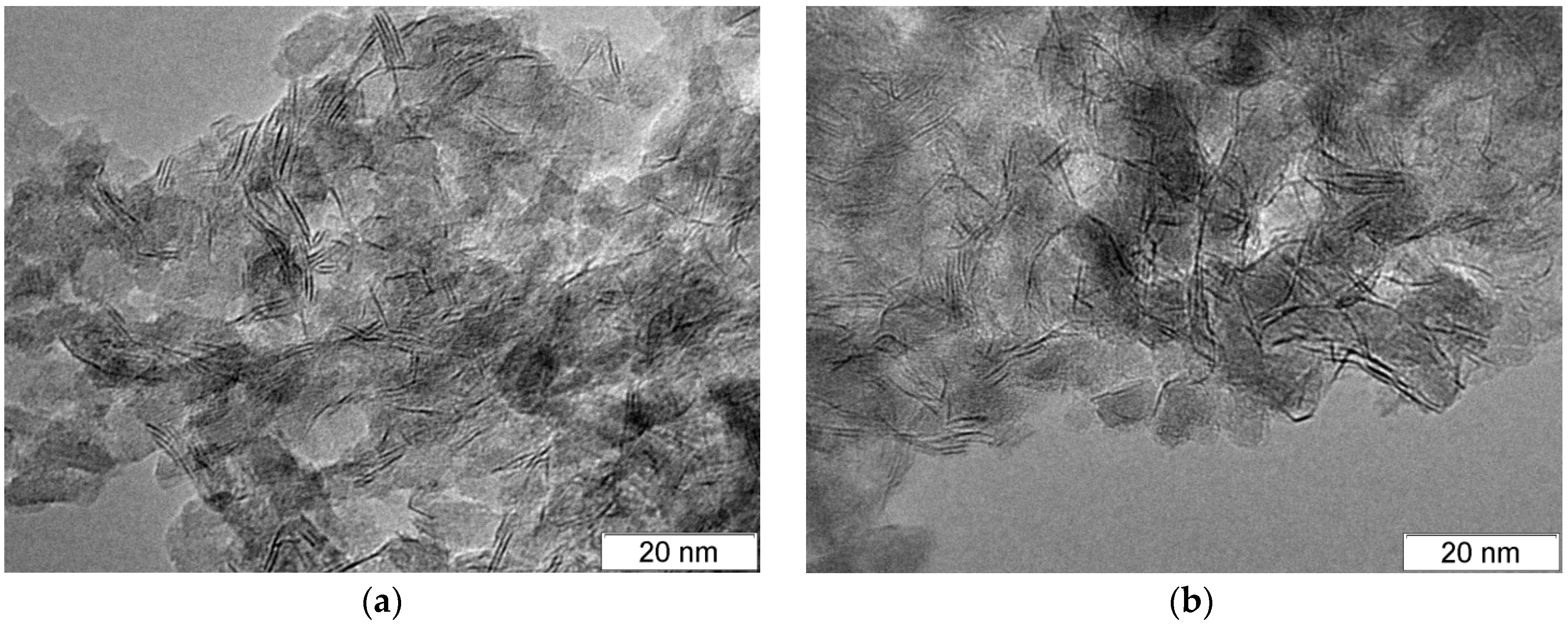
2.2. Catalyst Characterization
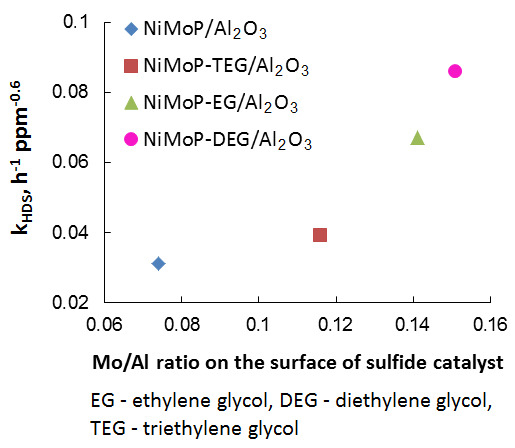
2.3. Catalytic Activity
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization Techniques
3.3. Catalytic Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Topsøe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating Catalysis Science and Technology; Springer: New York, NY, USA, 1996; ISBN 3-540-60380-8. [Google Scholar]
- Pecoraro, T.A.; Chianelli, R.R. Hydrodesulfurization catalysis by transition metal sulfides. J. Catal. 1981, 67, 430–445. [Google Scholar] [CrossRef]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra-low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Ito, E.; van Veen, J.A.R. On novel processes for removing sulphur from refinery streams. Catal. Today 2006, 116, 446–460. [Google Scholar] [CrossRef]
- Griboval, A.; Blanchard, P.; Payen, E.; Fournier, M.; Dubois, J.L. Alumina supported HDS catalysts prepared by impregnation with new heteropolycompounds. Comparison with catalysts prepared by conventional Co-Mo-P coimpregnation. Catal. Today 1998, 45, 277–283. [Google Scholar] [CrossRef]
- Nicosia, D.; Prins, R. The effect of glycol on phosphate-doped CoMo/Al2O3 hydrotreating catalysts. J. Catal. 2005, 229, 424–438. [Google Scholar] [CrossRef]
- Nicosia, D.; Prins, R. The effect of phosphate and glycol on the sulfidation mechanism of CoMo/Al2O3 hydrotreating catalysts: An in situ QEXAFS study. J. Catal. 2005, 231, 259–268. [Google Scholar] [CrossRef]
- Van Haandel, L.; Bremmer, G.M.; Hensen, E.J.M.; Weber, T. The effect of organic additives and phosphoric acid on sulfidation and activity of (Co)Mo/Al2O3 hydrodesulfurization catalysts. J. Catal. 2017, 351, 95–106. [Google Scholar] [CrossRef]
- Pimerzin, A.; Mozhaev, A.; Varakin, A.; Maslakov, K.; Nikulshin, P. Comparison of citric acid and glycol effects on the state of active phase species and catalytic properties of CoPMo/Al2O3 hydrotreating catalysts. Appl. Catal. B Environ. 2017, 205, 93–103. [Google Scholar] [CrossRef]
- Escobar, J.; Barrera, M.C.; Toledo, J.A.; Cortes-Jacome, M.A.; Angeles-Chavez, C.; Nunez, S.; Santes, V.; Gomez, E.; Dıaz, L.; Romero, E.; et al. Effect of ethyleneglycol addition on the properties of P-doped NiMo/Al2O3 HDS catalysts: Part I. Materials preparation and characterization. Appl. Catal. B Environ. 2009, 88, 564–575. [Google Scholar] [CrossRef]
- Gutiérrez-Alejandre, A.; Laurrabaquio-Rosas, G.; Ramírez, J.; Busca, G. On the role of triethyleneglycol in the preparation of highly active Ni-Mo/Al2O3 hydrodesulfurization catalysts: A spectroscopic study. Appl. Catal. B Environ. 2015, 166–167, 560–567. [Google Scholar] [CrossRef]
- Iwamoto, R.; Kagami, N.; Sakoda, Y.; Iino, A. Effect of polyethylene glycol addition on NiO-MoO3/Al2O3 and NiO-MoO3-P2O5/Al2O3 hydrodesulfurization catalyst. J. Jpn. Petrol. Inst. 2005, 48, 351–357. [Google Scholar] [CrossRef]
- Rob van Veen, J.A. What’s new? On the development of sulphidic HT catalysts before the molecular aspects. Catal. Today 2017, 292, 2–25. [Google Scholar] [CrossRef]
- Costa, V.; Marchand, K.; Digne, M.; Geantet, C. New insights into the role of glycol-based additives in the improvement of hydrotreatment catalyst performances. Catal. Today 2008, 130, 69–74. [Google Scholar] [CrossRef]
- Costa, V.; Guichard, B.; Digne, M.; Legens, C.; Lecour, P.; Marchand, K.; Raybaud, P.; Krebs, E.; Geantet, C. A rational interpretation of improved catalytic performances of additive-impregnated dried CoMo hydrotreating catalysts: A combined theoretical and experimental study. Catal. Sci. Technol. 2013, 3, 140–151. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Loridant, S.; Chantal, L.; Cholley, T.; Geantet, C. Effect of glycol on the formation of active species and sulfidation mechanism of CoMoP/Al2O3 hydrotreating catalysts. Appl. Catal. B Environ. 2011, 107, 59–67. [Google Scholar] [CrossRef]
- Iwamoto, R.; Kagami, N.; Iino, A. Effect of polyethylene glycol addition on hydrodesulfurization activity over CoO-MoO3/Al2O3 catalyst. J. Jpn. Petrol. Inst. 2005, 48, 237–242. [Google Scholar] [CrossRef]
- Blanchard, P.; Lamonier, C.; Griboval, A.; Payen, E. New insight in the preparation of alumina supported hydrotreatment oxidic precursors: A molecular approach. Appl. Catal. A Gen. 2007, 322, 33–45. [Google Scholar] [CrossRef]
- Briand, L.E.; Valle, G.M.; Thomas, H.J. Stability of the phospho-molybdic Dawson-type ion P2Mo18O626− in aqueous media. J. Mater. Chem. 2002, 12, 299–304. [Google Scholar] [CrossRef]
- Klimova, T.E.; Valencia, D.; Mendoza-Nieto, J.A.; Hernandez-Hipolito, P. Behavior of NiMo/SBA-15 catalysts prepared with citric acid in simultaneous hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene. J. Catal. 2013, 304, 29–46. [Google Scholar] [CrossRef]
- Payen, E.; Grimblot, J.; Kasztelan, S. Study of oxidic and reduced alumina-supported molybdate and heptamolybdate species by in situ laser Raman spectroscopy. J. Phys. Chem. 1987, 91, 6642–6648. [Google Scholar] [CrossRef]
- Bergwerff, J.A.; Visser, T.; Leliveld, B.R.G.; Rossenaar, B.D.; de Jong, K.P.; Weckhuysen, B.M. Envisaging the physicochemical processes during the preparation of supported catalysts: Raman microscopy on the impregnation of Mo onto Al2O3 extrudates. J. Am. Chem. Soc. 2004, 126, 14548–14556. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, J.A.R.; Hendriks, P.A.J.M.; Andrea, R.R.; Romers, E.J.G.M.; Wilson, A.E. Chemistry of phosphomolybdate adsorption on alumina surfaces. 2. The molybdate/phosphated alumina and phosphomolybdate/alumina systems. J. Phys. Chem. 1990, 94, 5282–5285. [Google Scholar] [CrossRef]
- Ahn, K.-S.; Nah, Y.-C.; Sung, Y.-E. Surface morphological, microstructural, and electrochromic properties of short-range ordered and crystalline nickel oxide thin films. Appl. Surf. Sci. 2002, 199, 259–269. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Paine, B.P.; Lau, A.; Gerson, L.W.M.; Smart, R.St.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Klimov, O.V.; Leonova, K.A.; Koryakina, G.I.; Gerasimov, E.Yu.; Prosvirin, I.P.; Cherepanova, S.V.; Budukva, S.V.; Pereyma, V.Yu.; Dik, P.P.; Parakhin, O.A.; et al. Supported on alumina Co-Mo hydrotreating catalysts: Dependence of catalytic and strength characteristics on the initial AlOOH particle morphology. Catal. Today 2014, 220–222, 66–77. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer: Eden Prairie, MN, USA, 1992; p. 261. ISBN 0962702625. [Google Scholar]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Vlasova, E.N.; Deliy, I.V.; Nuzhdin, A.L.; Aleksandrov, P.V.; Gerasimov, E.Yu.; Aleshina, G.I.; Bukhtiyarova, G.A. Catalytic properties of CoMo/Al2O3 sulfide catalysts in the hydrorefining of straight-run diesel fraction mixed with rapeseed oil. Kinet. Catal. 2014, 55, 481–491. [Google Scholar] [CrossRef]

| Catalyst | Mo, wt% | Ni, wt% | P, wt% |
|---|---|---|---|
| NiMoP/Al2O3 | 13.4 | 3.4 | 2.0 |
| NiMoP-EG/Al2O3 | 13.1 | 3.4 | 1.9 |
| NiMoP-DEG/Al2O3 | 13.2 | 3.5 | 1.6 |
| NiMoP-TEG/Al2O3 | 13.3 | 3.3 | 1.8 |
| Catalyst | Oxide Form | Sulfide Form | ||
|---|---|---|---|---|
| Ni/Al | Mo/Al | Ni/Al | Mo/Al | |
| NiMoP/Al2O3 | 0.044 | 0.113 | 0.051 | 0.074 |
| NiMoP-EG/Al2O3 | 0.051 | 0.112 | 0.041 | 0.141 |
| NiMoP-DEG/Al2O3 | 0.065 | 0.123 | 0.056 | 0.151 |
| NiMoP-TEG/Al2O3 | 0.063 | 0.120 | 0.040 | 0.116 |
| Catalyst | DXRD, nm | Average Slab Length, nm | Stacking Number | SBET, m2 g−1 | Vpore, cm3 g−1 | Dpore, nm |
|---|---|---|---|---|---|---|
| NiMoP/Al2O3 | 4.5 | 4.9 | 1.9 | 114 | 0.34 | 11.8 |
| NiMoP-EG/Al2O3 | 4.0 | 4.7 | 1.4 | 120 | 0.36 | 11.9 |
| NiMoP-DEG/Al2O3 | 4.4 | 5.2 | 1.6 | 129 | 0.34 | 10.7 |
| NiMoP-TEG/Al2O3 | 4.1 | 4.5 | 1.5 | 127 | 0.35 | 10.9 |
| Property | Method | |
|---|---|---|
| IBP 1:0.5%V, °С | 214.3 | ASTM D2887 |
| 5%V, °С | 243.5 | |
| 10%V, °С | 250.7 | |
| 20%V, °С | 259.3 | |
| 30%V, °С | 266.8 | |
| 50%V, °С | 282.9 | |
| 70%V, °С | 303.4 | |
| 80%V, °С | 315.5 | |
| 90%V, °С | 330.9 | |
| 95%V, °С | 343.2 | |
| FBP 2: 99.5%V, °С | 349.4 | |
| Sulfur content, ppm | 8870 | ASTM D 4294 |
| Nitrogen content, ppm | 110 | ASTM D 4629 |
| Total aromatics, wt% | 28.0 | IP 391 |
| Monoaromatics, wt% | 19.2 | |
| Diaromatics, wt% | 7.1 | |
| Polyaromatics, wt% | 1.7 | |
| Density, g cm−3 | 0.847 | ASTM D 4052 |
| Entry | Catalyst | T, °C | S, ppm | N, ppm | k, h−1 ppm−0.6 |
|---|---|---|---|---|---|
| 1 | NiMoP/Al2O3 | 330 | 340 | 19 | 0.031 |
| 2 | NiMoP/Al2O3 | 340 | 166 | 15 | – |
| 3 | NiMoP-EG/Al2O3 | 330 | 109 | 11 | 0.067 |
| 4 | NiMoP-EG/Al2O3 | 340 | 60 | 6 | – |
| 5 | NiMoP-DEG/Al2O3 | 330 | 74 | 4 | 0.086 |
| 6 | NiMoP-DEG/Al2O3 | 340 | 34 | 3 | – |
| 7 | NiMoP-TEG/Al2O3 | 330 | 246 | 16 | 0.039 |
| 8 | NiMoP-TEG/Al2O3 | 340 | 142 | 12 | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzhdin, A.L.; Bukhtiyarova, G.A.; Porsin, A.A.; Prosvirin, I.P.; Deliy, I.V.; Volodin, V.A.; Gerasimov, E.Y.; Vlasova, E.N.; Bukhtiyarov, V.I. Effect of Mono-, Di-, and Triethylene Glycol on the Activity of Phosphate-Doped NiMo/Al2O3 Hydrotreating Catalysts. Catalysts 2019, 9, 96. https://doi.org/10.3390/catal9010096
Nuzhdin AL, Bukhtiyarova GA, Porsin AA, Prosvirin IP, Deliy IV, Volodin VA, Gerasimov EY, Vlasova EN, Bukhtiyarov VI. Effect of Mono-, Di-, and Triethylene Glycol on the Activity of Phosphate-Doped NiMo/Al2O3 Hydrotreating Catalysts. Catalysts. 2019; 9(1):96. https://doi.org/10.3390/catal9010096
Chicago/Turabian StyleNuzhdin, Alexey L., Galina A. Bukhtiyarova, Aleksander A. Porsin, Igor P. Prosvirin, Irina V. Deliy, Vladimir A. Volodin, Evgeny Yu. Gerasimov, Evgeniya N. Vlasova, and Valerii I. Bukhtiyarov. 2019. "Effect of Mono-, Di-, and Triethylene Glycol on the Activity of Phosphate-Doped NiMo/Al2O3 Hydrotreating Catalysts" Catalysts 9, no. 1: 96. https://doi.org/10.3390/catal9010096
APA StyleNuzhdin, A. L., Bukhtiyarova, G. A., Porsin, A. A., Prosvirin, I. P., Deliy, I. V., Volodin, V. A., Gerasimov, E. Y., Vlasova, E. N., & Bukhtiyarov, V. I. (2019). Effect of Mono-, Di-, and Triethylene Glycol on the Activity of Phosphate-Doped NiMo/Al2O3 Hydrotreating Catalysts. Catalysts, 9(1), 96. https://doi.org/10.3390/catal9010096