Titanium Dioxide (TiO2) Mesocrystals: Synthesis, Growth Mechanisms and Photocatalytic Properties
Abstract
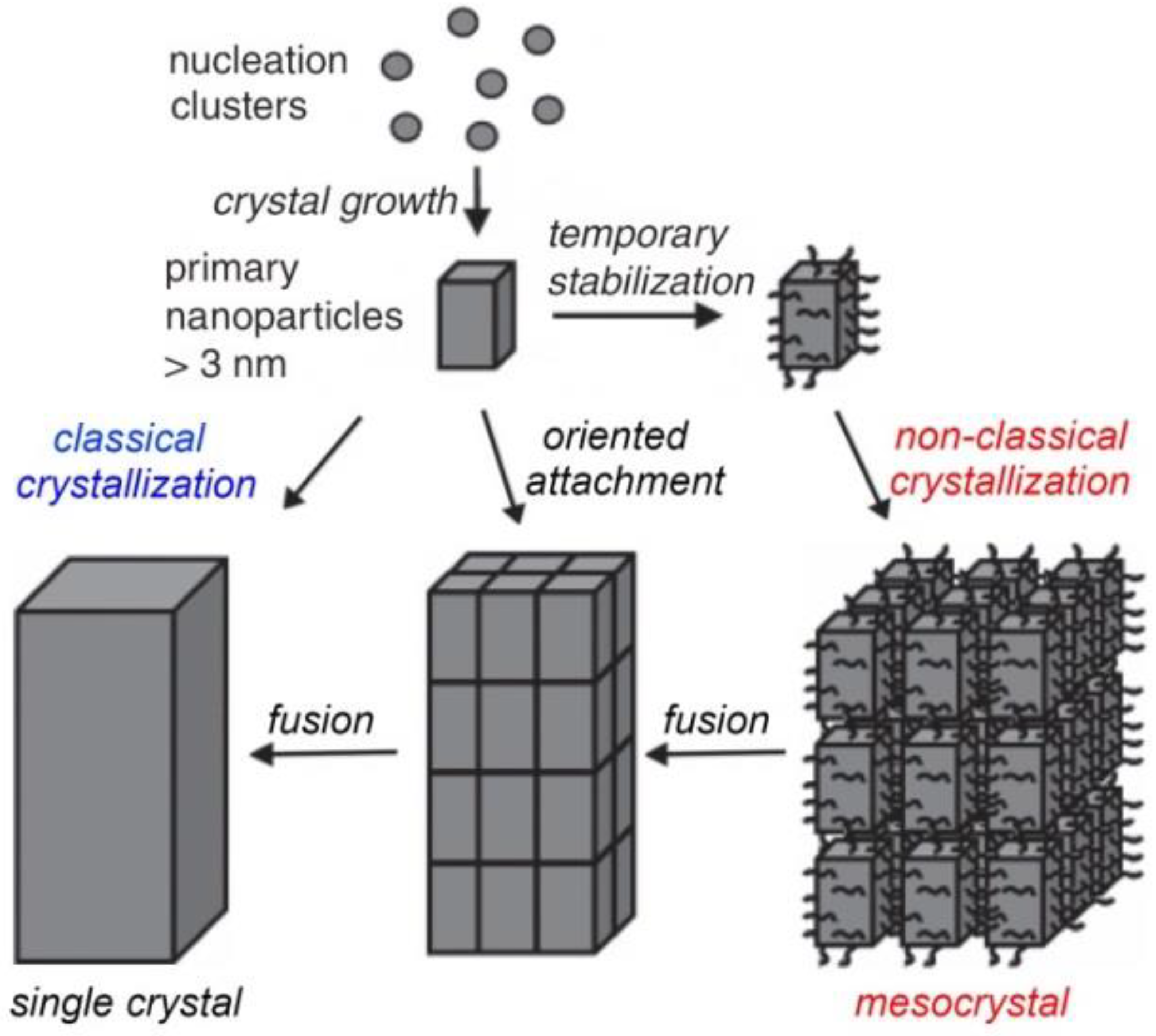
1. Introduction
2. Synthesis TiO2 Mesocrystals
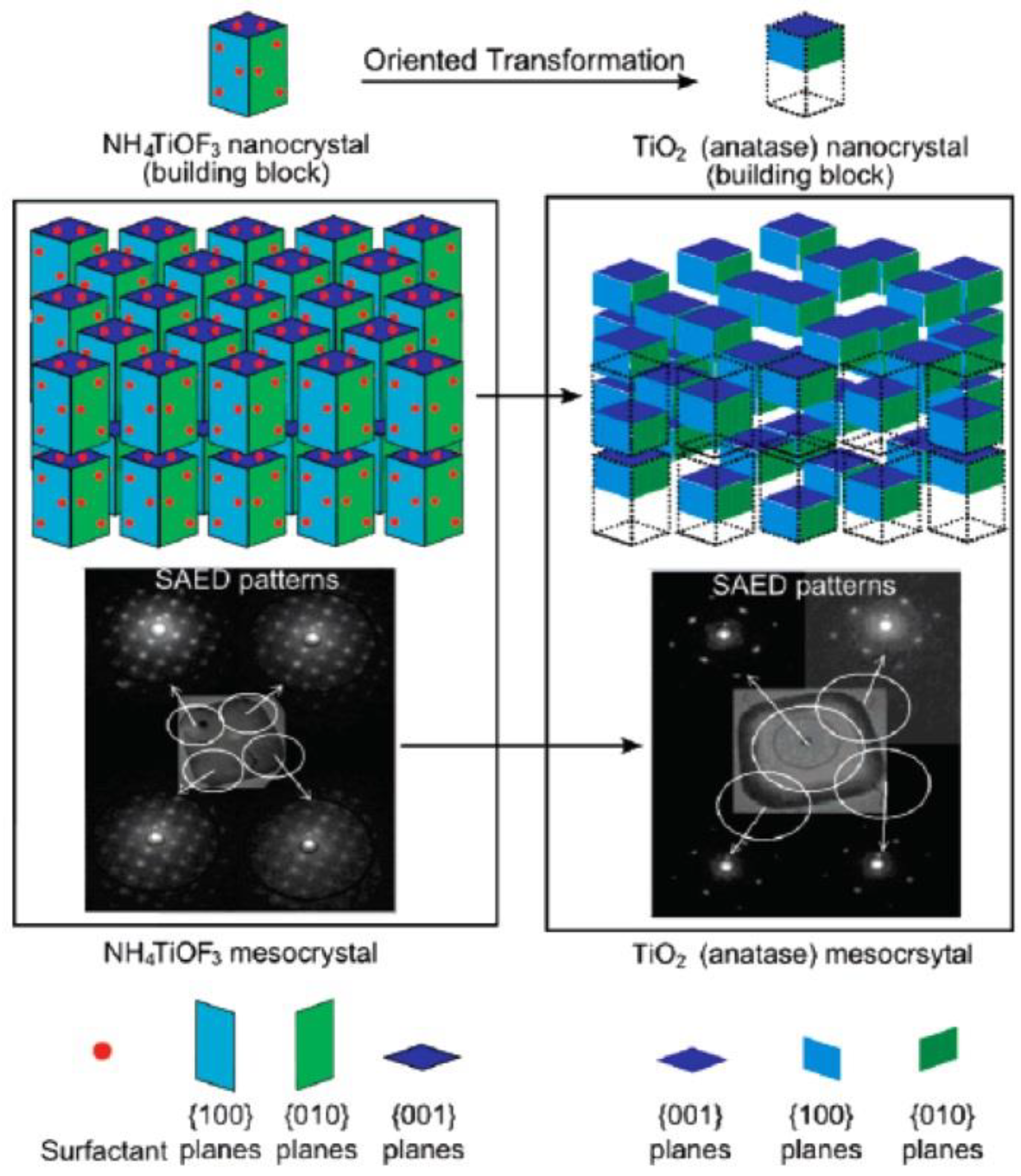
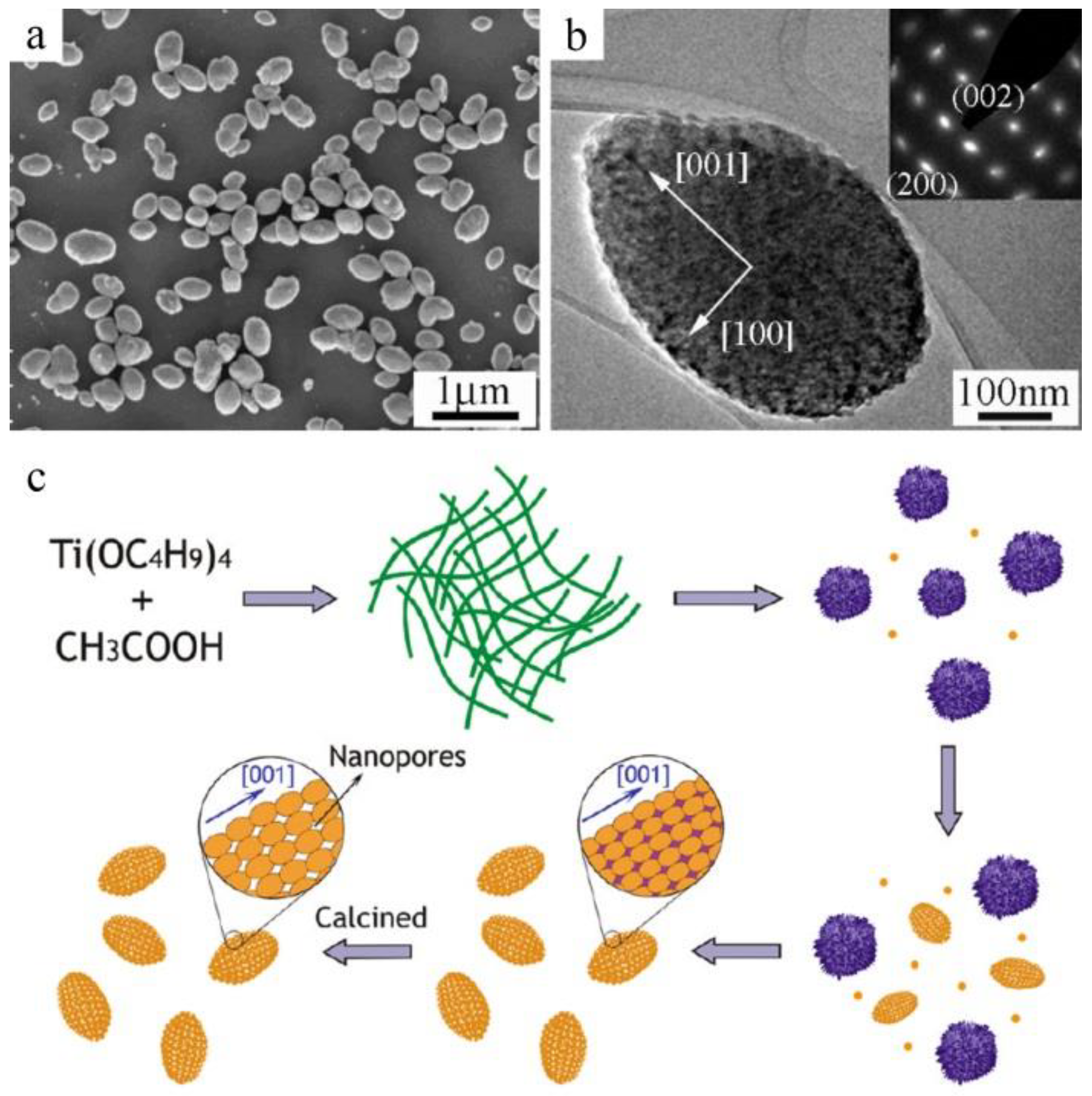
2.1. Oriented Topotactic Transformation
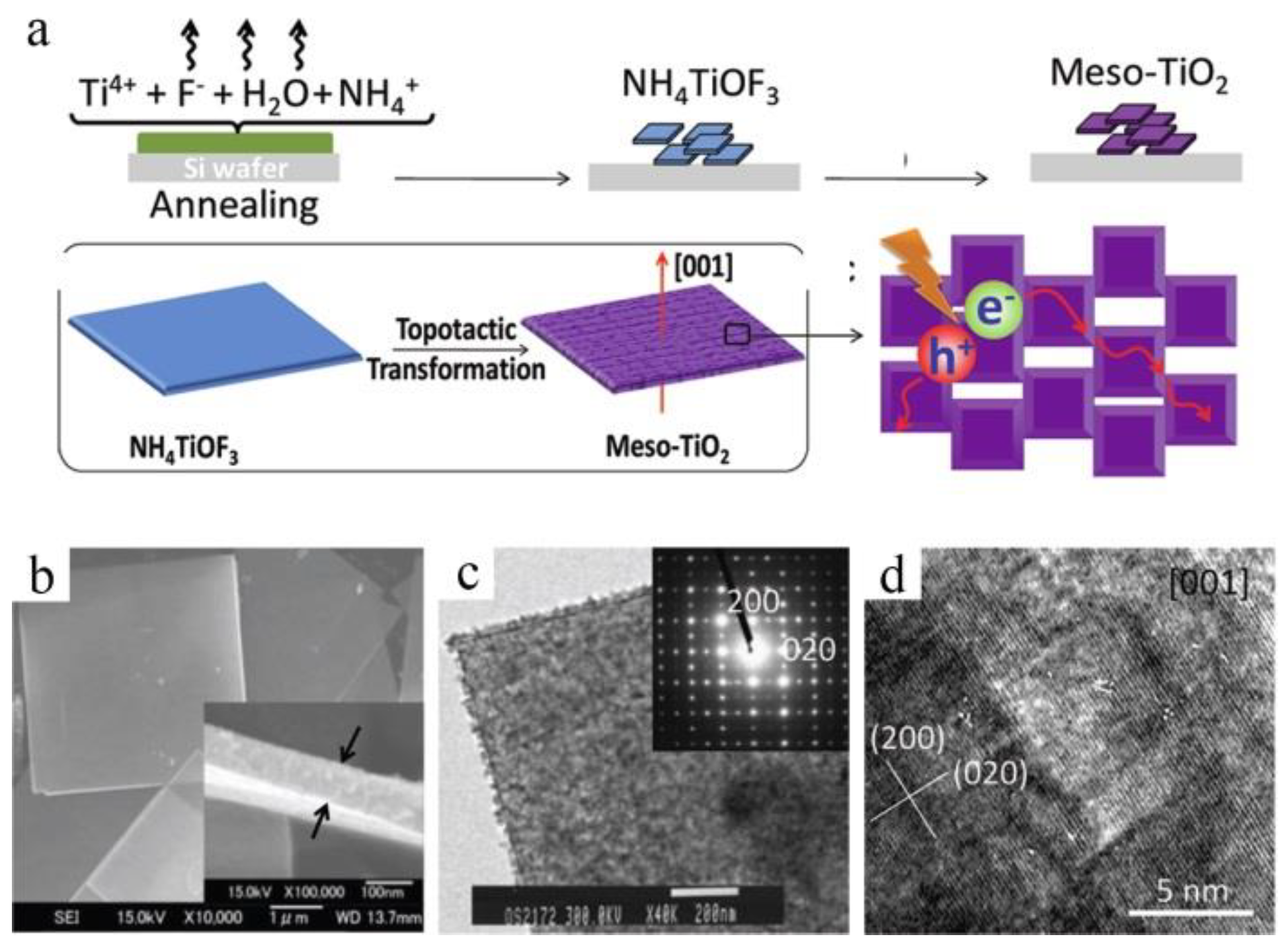
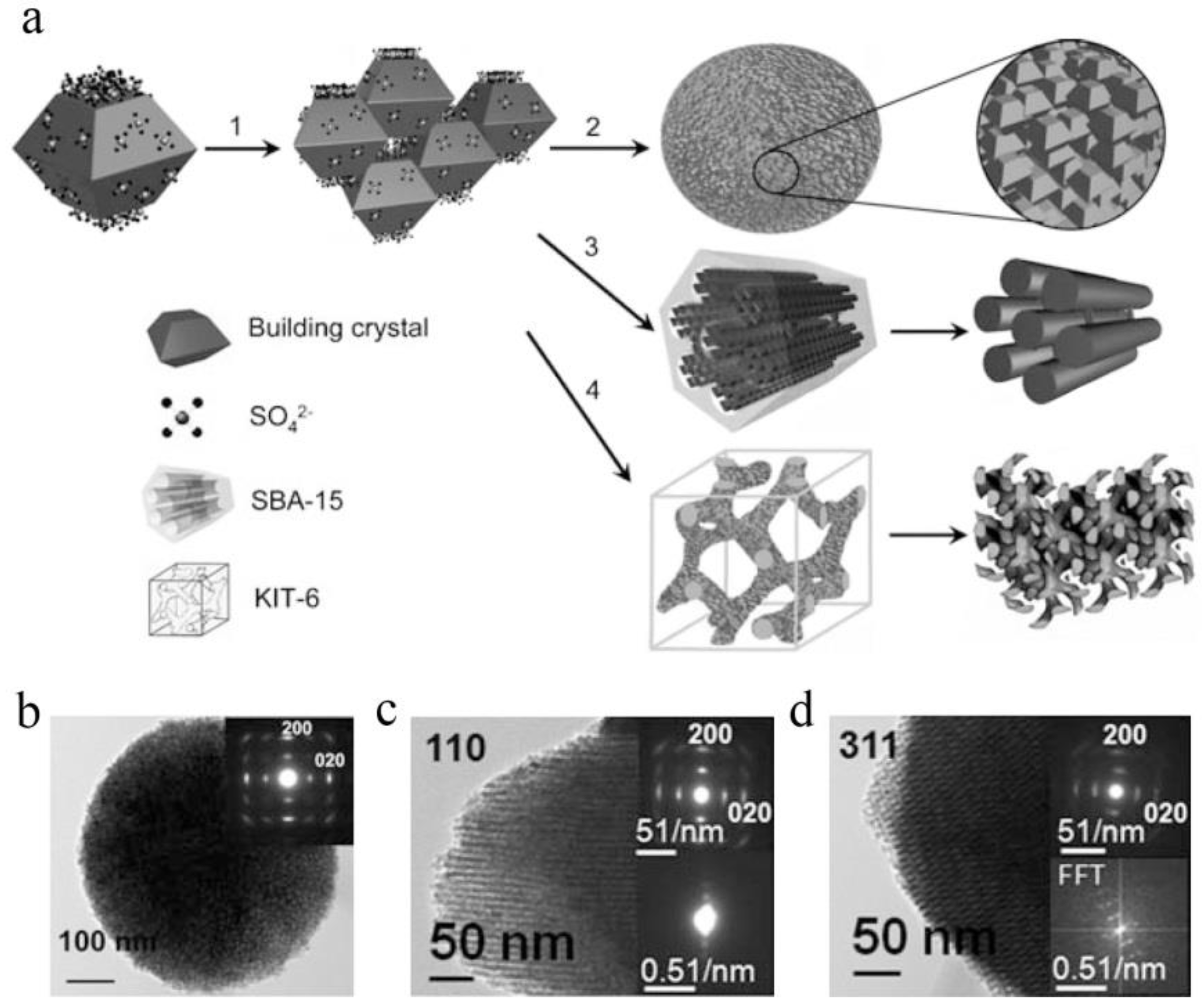
2.2. Growth on Substrates
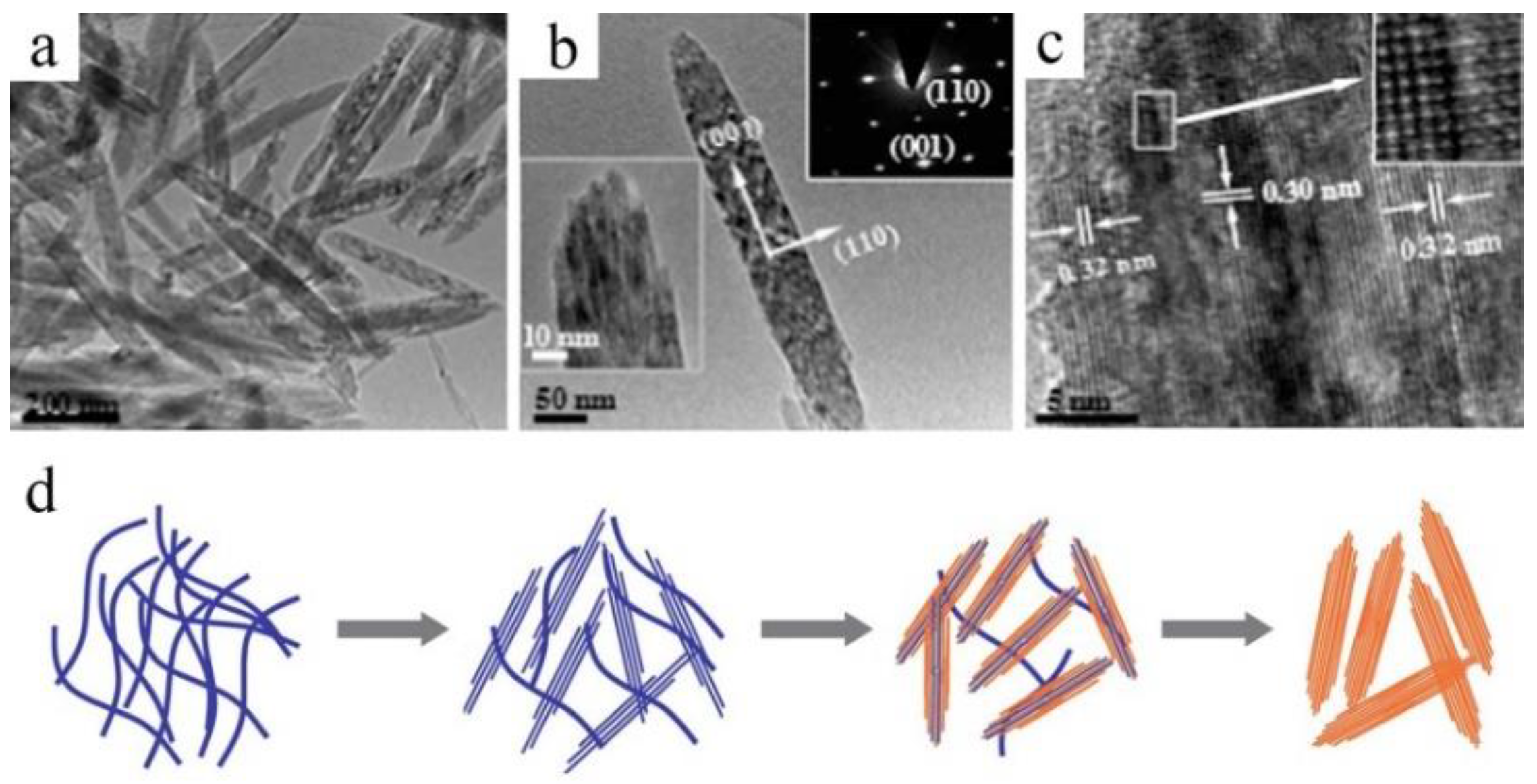
2.3. Organic-Additive-Assisted Growth in Solution
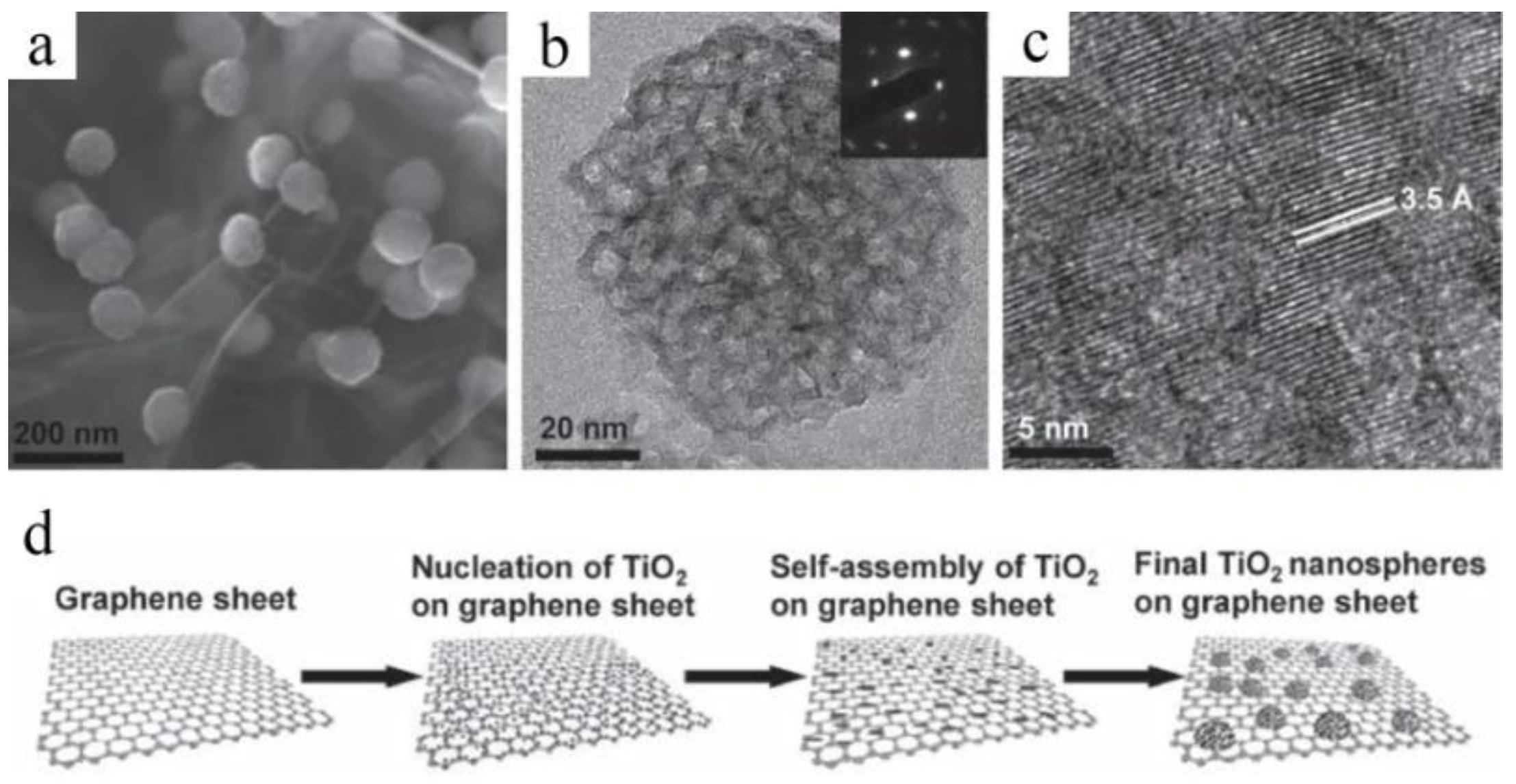
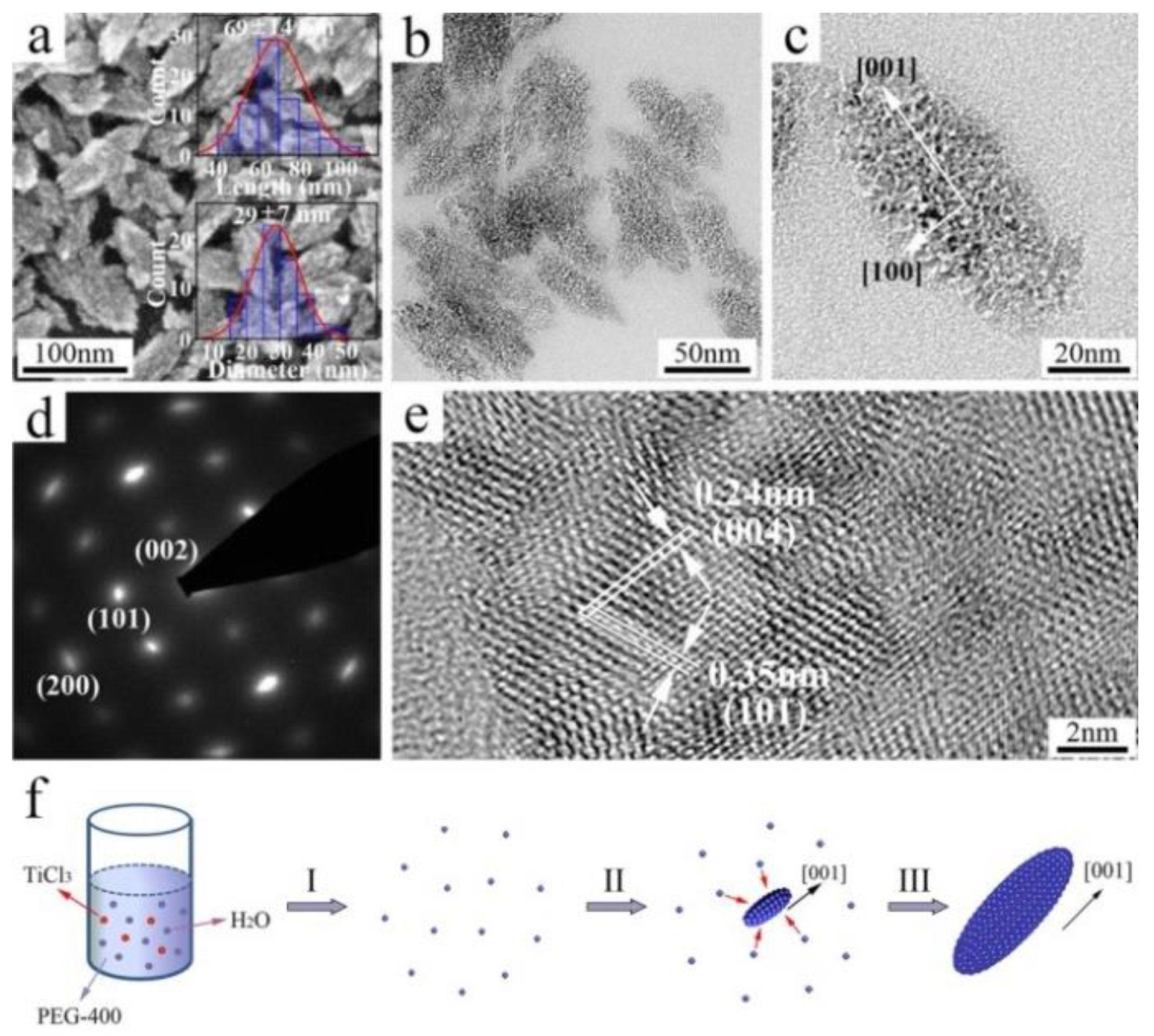
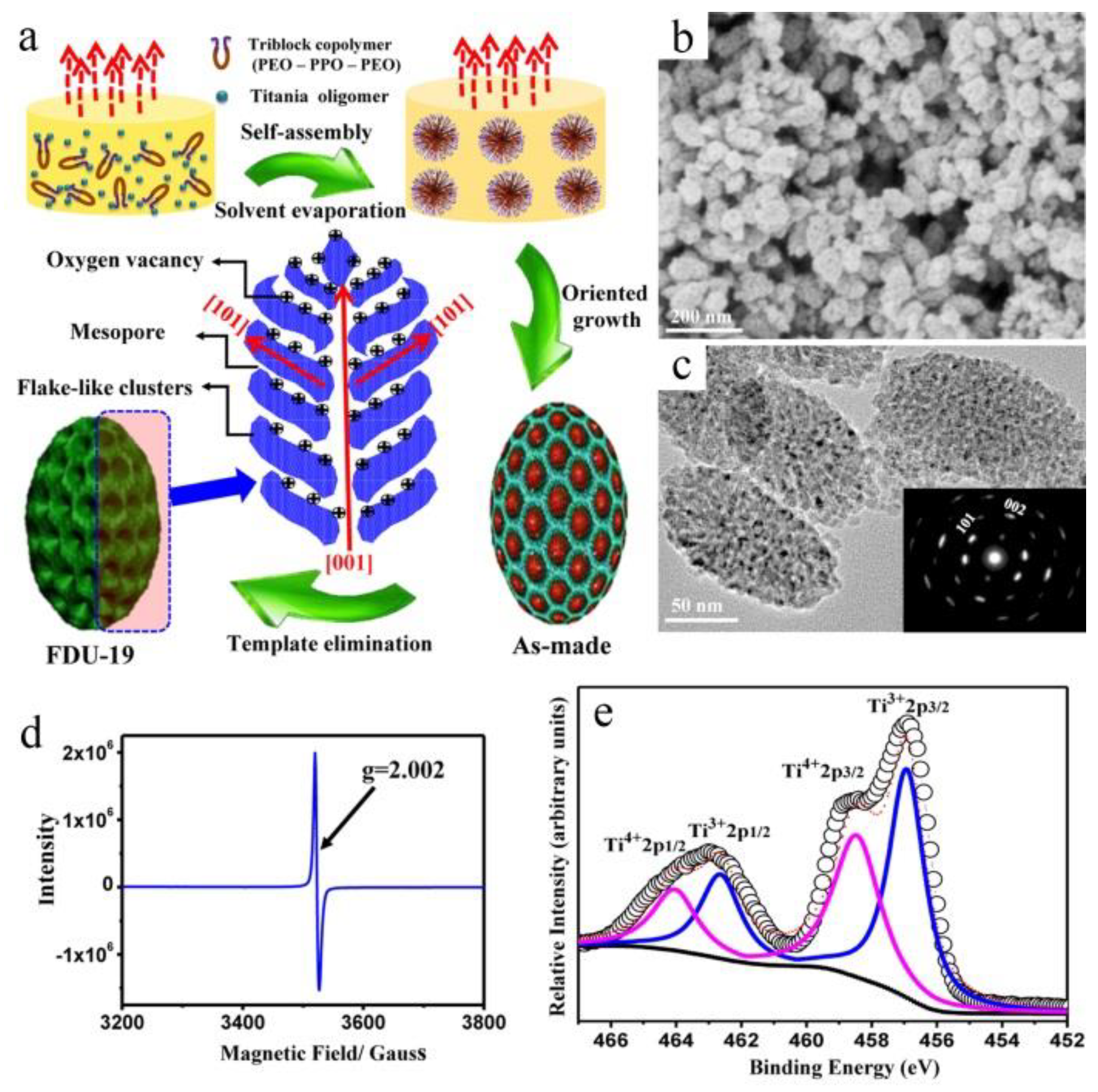
2.4. Direct Additive-Free Growth in Solution
3. Modification of TiO2 Mesocrystals
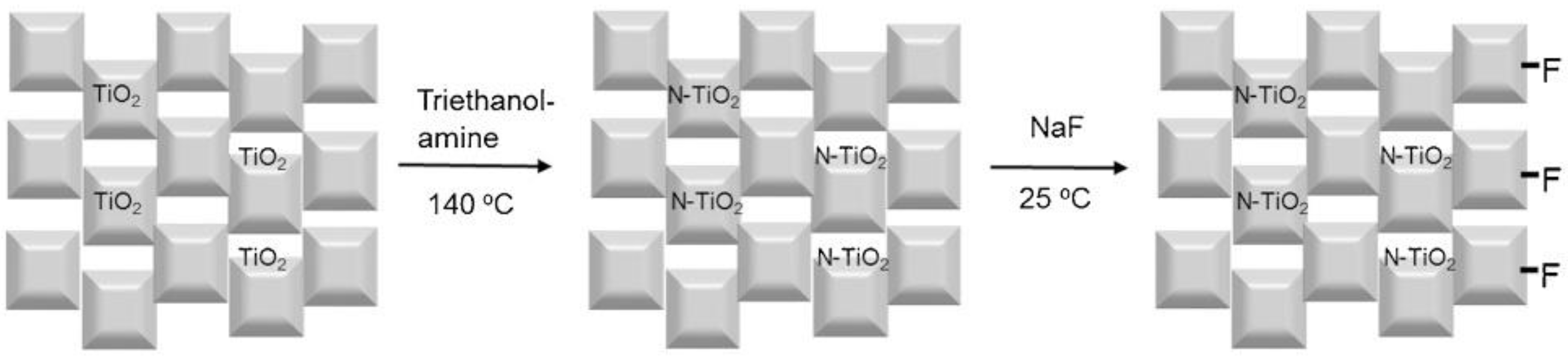
3.1. Fabrication of Doped TiO2 Mesocrystals
3.2. Construction of TiO2 Mesocrystal-Based Heterostructures
4. TiO2 Mesocrystals for Photocatalytic Applications
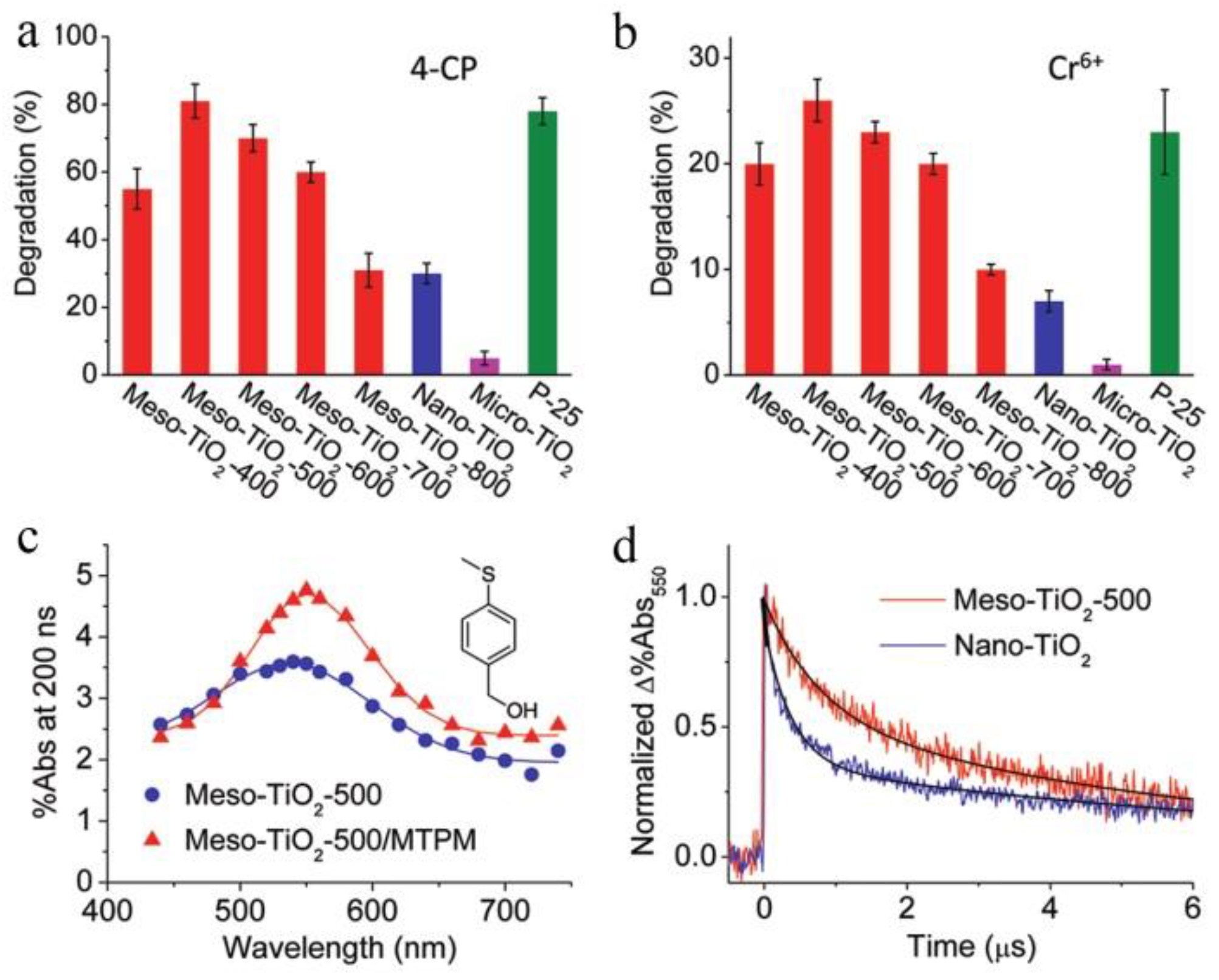
4.1. Bare TiO2 Mesocrystals for Photocatalytic Applications
4.2. Doped TiO2 Mesocrystals for Photocatalytic Applications
4.3. Composited TiO2 Mesocrystals for Photocatalytic Applications
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Keane, D.A.; McGuigan, K.G.; Ibáñez, P.F.; Polo-López, M.I.; Byrne, J.A.; Dunlop, P.S.M.; O’Shea, K.; Dionysiou, D.D.; Pillai, S.C. Solar photocatalysis for water disinfection: Materials and reactor design. Catal. Sci. Technol. 2014, 4, 1211–1226. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibanez, P.; Somma, I.D. Solar photocatalysis: Materials, reactors, some commercial and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Lee, A.F. Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J. Mater. Chem. A 2015, 3, 14487–14516. [Google Scholar] [CrossRef]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-based photocatalytic CO2 conversion. Mater. Horiz. 2015, 2, 261–278. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic principles, diverse forms of implementations and emerging scientific opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic hydrogen production: A rift into the future energy supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Kapilashrami, M.; Zhang, Y.; Liu, Y.-S.; Hagfeldt, A.; Guo, J. Probing the optical property and electronic structure of TiO2 nanomaterials for renewable energy applications. Chem. Rev. 2014, 114, 9662–9707. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Shi, J.; Yu, Y. One-dimensional titanium dioxide nanomaterials: Nanowires, nanorods, and nanobelts. Chem. Rev. 2014, 114, 9346–9384. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Wang, J.; Elzatahry, A.A.; Zhao, D. A perspective on mesoporous TiO2 materials. Chem. Mater. 2014, 26, 287–298. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Zhang, G.; Kim, G.; Choi, W. Visible light driven photocatalysis mediated via ligand-to-metal charge transfer (LMCT): An alternative approach to solar activation of titania. Energy Environ. Sci. 2014, 7, 954–966. [Google Scholar] [CrossRef]
- Etacheri, V.; Valentin, C.D.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Ong, W.; Wang, J.; Ho, G.W. Structural design of TiO2-based photocatalyst for H2 production and degradation applications. Catal. Sci. Technol. 2015, 5, 4703–4726. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Varma, R.S.; Lisowski, P. Sustainable hybrid photocatalysts: Titania immobilized on carbon materials derived from renewable and biodegradable resources. Green Chem. 2016, 18, 5736–5750. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H. Mesoporous TiO2: Preparation, doping, and as a composite for photocatalysis. ChemCatChem 2013, 5, 885–894. [Google Scholar] [CrossRef]
- Wang, M.; Ioccozia, J.; Sun, L.; Lin, C.; Li, Z. Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, B.; Sang, Y.; Liu, H. Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B Environ. 2017, 202, 620–641. [Google Scholar] [CrossRef]
- Mann, S. Self-assembly and transformation of hybrid nano-objects and nanostructures under equilibrium and non-equilibrium conditions. Nat. Mater. 2009, 8, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Petukhova, A.; Kumacheva, E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotechnol. 2010, 5, 15–25. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Liu, J.-W.; Liang, H.-W.; Yu, S.-H. Macroscopic-scale assembled nanowire thin films and their functionalities. Chem. Rev. 2012, 112, 4770–4799. [Google Scholar] [CrossRef]
- Klinkova, A.; Choueiri, R.M.; Kumacheva, E. Self-assembled plasmonic nanostructures. Chem. Soc. Rev. 2014, 43, 3976–3991. [Google Scholar] [CrossRef]
- Cargnello, M.; Johnston-Peck, A.C.; Diroll, B.T.; Wong, E.; Datta, B.; Damodhar, D.; Doan-Nguyen, V.V.T.; Herzing, A.A.; Kagan, C.R.; Murray, C.B. Substitutional doping in nanocrystal superlattices. Nature 2015, 524, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H.; Antonietti, M. Mesocrystals and Nonclassical Crystallization; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Zhou, L.; O’Brien, P. Mesocrystals: A new class of solid materials. Small 2008, 4, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Song, R.-Q.; Cölfen, H. Mesocrystals-ordered nanoparticle superstructures. Adv. Mater. 2010, 22, 1301–1330. [Google Scholar] [CrossRef]
- Fang, J.; Ding, B.; Gleiter, H. Mesocrystals: Syntheses in metals and applications. Chem. Soc. Rev. 2011, 40, 5347–5360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; O’Brien, P. Mesocrystals-properties and applications. J. Phys. Chem. Lett. 2012, 3, 620–628. [Google Scholar] [CrossRef]
- Uchaker, E.; Cao, G. Mesocrystals as electrode materials for lithium-ion batteries. Nano Today 2014, 9, 499–524. [Google Scholar] [CrossRef]
- Tachikawa, T.; Majima, T. Metal oxide mesocrystals with tailored structures and properties for energy conversion and storage applications. NPG Asia Mater. 2014, 6, e100. [Google Scholar] [CrossRef]
- Bergström, L.; Sturm (née Rosseeva), E.V.; Salazar-Alvarez, G.; Cölfen, H. Mesocrystals in biominerals and colloidal arrays. Acc. Chem. Res. 2015, 48, 1391–1402. [Google Scholar] [CrossRef]
- Zhou, L.; Boyle, D.S.; O’Brien, P. Uniform NH4TiOF3 mesocrystals prepared by an ambient temperature self-assembly process and their topotaxial conversion to anatase. Chem. Commun. 2007, 144–146. [Google Scholar] [CrossRef]
- Zhou, L.; Smyth-Boyle, D.; O’Brien, P. A facile synthesis of uniform NH4TiOF3 mesocrystals and their conversion to TiO2 mesocrystals. J. Am. Chem. Soc. 2008, 130, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yin, M.; Wang, Z.; Yan, S.; Wan, L.; Li, Z.; Zou, Z. Facile synthesis of anatase TiO2 mesocrystal sheets with dominant {001} facets based on topochemical conversion. CrystEngComm 2010, 12, 3425–3429. [Google Scholar] [CrossRef]
- Inoguchi, M.; Afzaal, M.; Tanaka, N.; O’Brien, P. The poly(ethylene glycol) assisted preparation of NH4TiOF3 mesocrystals and their topotactic conversion to TiO2. J. Mater. Chem. 2012, 22, 25123–25129. [Google Scholar] [CrossRef]
- Liu, S.-J.; Gong, J.-Y.; Hu, B.; Yu, S.-H. Mesocrystals of rutile TiO2: Mesoscale transformation, crystallization, and growth by a biologic molecules-assisted hydrothermal process. Cryst. Growth Des. 2009, 9, 203–209. [Google Scholar] [CrossRef]
- Ye, J.; Liu, W.; Cai, J.; Chen, S.; Zhao, X.; Zhou, H.; Qi, L. Nanoporous anatase TiO2 mesocrystals: Additive-free synthesis, remarkable crystalline-phase stability, and improved lithium insertion behavior. J. Am. Chem. Soc. 2011, 133, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Liu, Z.; Xu, H.; Deng, Y.; Shen, H. Hierarchical rutile TiO2 mesocrystals assembled by nanocrystals-oriented attachment mechanism. CrystEngComm 2012, 14, 2278–2282. [Google Scholar] [CrossRef]
- Zhen, M.; Guo, X.; Gao, G.; Zhou, Z.; Liu, L. Rutile TiO2 nanobundles on reduced graphene oxides as anode materials for Li ion batteries. Chem. Commun. 2014, 50, 11915–11918. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Wang, H.; Xin, L.; Wang, Q.; Liu, Y.; Wang, L. Rutile TiO2 mesocrystallines with aggregated nanorod clusters: Extremely rapid self-reaction of the single source and enhanced dye-sensitized solar cell performance. RSC Adv. 2014, 4, 58615–58623. [Google Scholar] [CrossRef]
- Fu, X.; Wang, B.; Chen, C.; Ren, Z.; Fan, C.; Wang, Z. Controllable synthesis of spherical anatase mesocrystals for lithium ion batteries. New J. Chem. 2014, 38, 4754–4759. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Wang, H.; Song, Y.; Fang, L.; Ye, N.; Wang, L. Enhanced dye-sensitized solar cells performance using anatase TiO2 mesocrystals with the Wulff construction of nearly 100% exposed {101} facets as effective light scattering layer. Dalton Trans. 2014, 43, 4711–4719. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zhou, K.; Zhang, J.; Huang, Z.; Wei, M. Facile synthesis of rutile TiO2 mesocrystals with enhanced sodium storage properties. J. Mater. Chem. A 2015, 3, 17412–17416. [Google Scholar] [CrossRef]
- Amarilla, J.M.; Morales, E.; Sanz, J.; Sobrados, I.; Tartaj, P. Electrochemical response in aprotic ionic liquid electrolytes of TiO2 anatase anodes based on mesoporous mesocrystals with uniform colloidal size. J. Power Sources 2015, 273, 368–374. [Google Scholar] [CrossRef]
- Hong, Z.; Zhou, K.; Huang, Z.; Wei, M. Iso-oriented anatase TiO2 mesocages as a high performance anode material for sodium-ion storage. Sci. Rep. 2015, 5, 11960. [Google Scholar] [CrossRef]
- Wu, D.; Cao, K.; Wang, H.; Wang, F.; Gao, Z.; Xu, F.; Guo, Y.; Jiang, K. Tunable synthesis of single-crystalline-like TiO2 mesocrystals and their application as effective scattering layer in dye-sensitized solar cells. J. Colloid Interface Sci. 2015, 456, 125–131. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, X.; Zhou, W.; Gao, Q.; Lu, F.; Zhuang, J.; Xu, X.; Wu, M.; Fan, H.J. “Isofacet” anatase TiO2 microcages: Topotactic synthesis and ultrastable Li-ion storage. Adv. Mater. Interfaces 2015, 2, 1500210. [Google Scholar] [CrossRef]
- Hong, Z.; Hong, J.; Xie, C.; Huang, Z.; Wei, M. Hierarchical rutile TiO2 with mesocrystalline structure for Li-ion and Na-ion storage. Electrochim. Acta 2016, 202, 203–208. [Google Scholar] [CrossRef]
- Le, Z.; Liu, F.; Nie, P.; Li, X.; Liu, X.; Bian, Z.; Chen, G.; Wu, H.B.; Lu, Y. Pseudocapacitive sodium storage in mesoporous single-crystal-like TiO2-graphene nanocomposite enables high-performance sodium-ion capacitors. ACS Nano 2017, 11, 2952–2960. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Le, Z.; Wen, M.; Zhang, D.; Chen, Z.; Wu, H.B.; Li, H.; Lu, Y. Mesoporous single-crystal-like TiO2 mesocages threaded with carbon nanotubes for high-performance electrochemical energy storage. Nano Energy 2017, 35, 44–51. [Google Scholar] [CrossRef]
- Hong, Z.; Wei, M.; Lan, T.; Jiang, L.; Cao, G. Additive-free synthesis of unique TiO2 mesocrystals with enhanced lithium-ion intercalation properties. Energy Environ. Sci. 2012, 5, 5408–5413. [Google Scholar] [CrossRef]
- Hong, Z.; Wei, M.; Lan, T.; Cao, G. Self-assembled nanoporous rutile TiO2 mesocrystals with tunable morphologies for high rate lithium-ion batteries. Nano Energy 2012, 1, 466–471. [Google Scholar] [CrossRef]
- Hong, Z.; Xu, Y.; Liu, Y.; Wei, M. Unique ordered TiO2 superstructures with tunable morphology and crystalline phase for improved lithium storage properties. Chem. Eur. J. 2012, 18, 10753–10760. [Google Scholar] [CrossRef]
- Liu, Y.; Che, R.; Chen, G.; Fan, J.; Sun, Z.; Wu, Z.; Wang, M.; Li, B.; Wei, J.; Wei, Y.; et al. Radially oriented mesoporous TiO2 microspheres with single-crystal–like anatase walls for high-efficiency optoelectronic devices. Sci. Adv. 2015, 1, e1500166. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Elzatahry, A.A.; Luo, W.; Che, R.; Fan, J.; Lan, K.; Al-Enizi, A.M.; Sun, Z.; Li, B.; et al. Mesoporous TiO2 mesocrystals: Remarkable defects-induced crystallite-interface reactivity and their in situ conversion to single crystals. ACS Cent. Sci. 2015, 1, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ye, J.; Chen, S.; Zhao, X.; Zhang, D.; Chen, S.; Ma, Y.; Jin, S.; Qi, L. Self-cleaning, broadband and quasi-omnidirectional antireflective structures based on mesocrystalline rutile TiO2 nanorod arrays. Energy Environ. Sci. 2012, 5, 7575–7581. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, S.; Hong, Z.; Li, X.; Xu, G.; Lin, Y.; Chen, G. Enhanced photoelectrochemical activity of a hierarchical-ordered TiO2 mesocrystal and its sensing application on a carbon nanohorn support scaffold. Anal. Chem. 2014, 86, 6418–6424. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, S.; Gong, L.; Li, Y.; Xu, G.; Lin, Y.; Hong, Z. The photoelectrochemical exploration of multifunctional TiO2 mesocrystals and its enzyme-assisted biosensing application. Biosens. Bioelectron. 2015, 72, 18–24. [Google Scholar] [CrossRef]
- Li, Z.; Gessner, A.; Richters, J.-P.; Kalden, J.; Voss, T.; Kuebel, C.; Taubert, A. Hollow zinc oxide mesocrystals from an ionic liquid precursor (ILP). Adv. Mater. 2008, 20, 1279–1285. [Google Scholar] [CrossRef]
- Liu, Z.; Wen, X.D.; Wu, X.L.; Gao, Y.J.; Chen, H.T.; Zhu, J.; Chu, P.K. Intrinsic dipole-field-driven mesoscale crystallization of core-shell ZnO mesocrystal microspheres. J. Am. Chem. Soc. 2009, 131, 9405–9412. [Google Scholar] [CrossRef]
- Wu, X.L.; Xiong, S.J.; Liu, Z.; Chen, J.; Shen, J.C.; Li, T.H.; Wu, P.H.; Chu, P.K. Green light stimulates terahertz emission from mesocrystal microspheres. Nat. Nanotechnol. 2011, 6, 103–106. [Google Scholar] [CrossRef]
- Distaso, M.; Klupp Taylor, R.N.; Taccardi, N.; Wasserscheid, P.; Peukert, W. Influence of the counterion on the synthesis of ZnO mesocrystals under solvothermal conditions. Chem. Eur. J. 2011, 17, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Distaso, M.; Segets, D.; Wernet, R.; Taylor, R.K.; Peukert, W. Tuning the size and the optical properties of ZnO mesocrystals synthesized under solvothermal conditions. Nanoscale 2012, 4, 864–873. [Google Scholar] [CrossRef]
- Hosono, E.; Tokunaga, T.; Ueno, S.; Oaki, Y.; Imai, H.; Zhou, H.; Fujihara, S. Crystal growth process of single-crystal-like mesoporous ZnO through a competitive reaction in solution. Cryst. Growth Des. 2012, 12, 2923–2931. [Google Scholar] [CrossRef]
- Liu, M.-H.; Tseng, Y.-H.; Greer, H.F.; Zhou, W.; Mou, C.-Y. Dipole field guided orientated attachment of nanocrystals to twin-brush ZnO mesocrystals. Chem. Eur. J. 2012, 18, 16104–16113. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, X.; Zhang, J.; Song, X.; Yang, Z. Unusual designated-tailoring on zone-axis preferential growth of surfactant-free ZnO mesocrystals. Cryst. Growth Des. 2012, 12, 2411–2418. [Google Scholar] [CrossRef]
- Waltz, F.; Wissmann, G.; Lippke, J.; Schneider, A.M.; Schwarz, H.-C.; Feldhoff, A.; Eiden, S.; Behrens, P. Evolution of the morphologies of zinc oxide mesocrystals under the influence of natural polysaccharides. Cryst. Growth Des. 2012, 12, 3066–3075. [Google Scholar] [CrossRef]
- Wang, H.; Xin, L.; Wang, H.; Yu, X.; Liu, Y.; Zhou, X.; Li, B. Aggregation-induced growth of hexagonal ZnO hierarchical mesocrystals with interior space: Nonaqueous synthesis, growth mechanism, and optical properties. RSC Adv. 2013, 3, 6538–6544. [Google Scholar] [CrossRef]
- Wang, S.-S.; Xu, A.-W. Template-free facile solution synthesis and optical properties of ZnO mesocrystals. Cryst. Eng. Commun. 2013, 15, 376–381. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Chen, Q.-G.; Zhu, Q.; Xu, A.W. Stable yellow ZnO mesocrystals with efficient visible-light photocatalytic activity. CrystEngComm 2014, 16, 7906–7913. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.-Y.; Peng, Y.; Huang, H.-W.; Li, Y.; Wu, M.; Ke, X.-X.; Tendeloo, G.V.; Su, B.-L. 2D ZnO mesoporous single-crystal nanosheets with exposed {0001} polar facets for the depollution of cationic dye molecules by highly selective adsorption and photocatalytic decomposition. Appl. Catal. B Environ. 2016, 181, 138–145. [Google Scholar] [CrossRef]
- Liu, M.-H.; Chen, Y.-W.; Liu, X.; Kuo, J.-L.; Chu, M.-W.; Mou, C.-Y. Defect-mediated gold substitution doping in ZnO mesocrystals and catalysis in CO oxidation. ACS Catal. 2016, 6, 115–122. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Chen, Q.; Ren, B.; Guan, R.; Cao, X.; Yang, X.; Duan, R. Interface-defect-mediated photocatalysis of mesocrystalline ZnO assembly synthesized in-situ via a template-free hydrothermal approach. Appl. Surf. Sci. 2017, 412, 517–528. [Google Scholar] [CrossRef]
- Liu, M.-H.; Chen, Y.-W.; Lin, T.-S.; Mou, C.-Y. Defective mesocrystal ZnO-supported gold catalysts: Facilitating CO oxidation via vacancy defects in ZnO. ACS Catal. 2018, 8, 6862–6869. [Google Scholar] [CrossRef]
- Liang, S.; Gou, X.; Cui, J.; Luo, Y.; Qu, H.; Zhang, T.; Yang, Z.; Yang, Q.; Sun, S. Novel cone-like ZnO mesocrystals with coexposed (10-11) and (000-1) facets and enhanced photocatalytic activity. Inorg. Chem. Front. 2018, 5, 2257–2267. [Google Scholar] [CrossRef]
- Park, G.-S.; Shindo, D.; Waseda, Y.; Sugimoto, T. Internal structure analysis of monodispersed pseudocubic hematite particles by electron microscopy. J. Colloid Interface Sci. 1996, 177, 198–207. [Google Scholar] [CrossRef]
- Ahniyaz, A.; Sakamoto, Y.; Bergström, L. Magnetic field-induced assembly of oriented superlattices from maghemite nanocubes. Proc. Natl. Acad. Sci. USA 2007, 104, 17570–17574. [Google Scholar] [CrossRef]
- Fang, X.-L.; Chen, C.; Jin, M.-S.; Kuang, Q.; Xie, Z.-X.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Single-crystal-like hematite colloidal nanocrystal clusters: Synthesis and applications in gas sensors, photocatalysis and water treatment. J. Mater. Chem. 2009, 19, 6154–6160. [Google Scholar] [CrossRef]
- An, Z.; Zhang, J.; Pan, S.; Yu, F. Facile template-free synthesis and characterization of elliptic a-Fe2O3 superstructures. J. Phys. Chem. C 2009, 113, 8092–8096. [Google Scholar] [CrossRef]
- Chen, J.S.; Zhu, T.; Li, C.M.; Lou, X.W. Building hematite nanostructures by oriented attachment. Angew. Chem. Int. Ed. 2011, 50, 650–653. [Google Scholar] [CrossRef]
- Ma, J.; Teo, J.; Mei, L.; Zhong, Z.; Li, Q.; Wang, T.; Duan, X.; Lian, J.; Zheng, W. Porous platelike hematite mesocrystals: Synthesis, catalytic and gas-sensing applications. J. Mater. Chem. 2012, 22, 11694–11700. [Google Scholar] [CrossRef]
- Duan, X.; Mei, L.; Ma, J.; Li, Q.; Wang, T.; Zheng, W. Facet-induced formation of hematite mesocrystals with improved lithium storage properties. Chem. Commun. 2012, 48, 12204–12206. [Google Scholar] [CrossRef]
- Fei, X.; Li, W.; Shao, Z.; Seeger, S.; Zhao, D.; Chen, X. Protein biomineralized nanoporous inorganic mesocrystals with tunable hierarchical nanostructures. J. Am. Chem. Soc. 2014, 136, 15781–15786. [Google Scholar] [CrossRef]
- Cai, J.; Chen, S.; Ji, M.; Hu, J.; Ma, Y.; Qi, L. Organic additive-free synthesis of mesocrystalline hematite nanoplates via two-dimensional oriented attachment. CrystEngComm 2014, 16, 1553–1559. [Google Scholar] [CrossRef]
- Agthe, M.; Plivelic, T.S.; Labrador, A.; Bergström, L.; Salazar-Alvarez, G. Following in real time the two-step assembly of nanoparticles into mesocrystals in levitating drops. Nano Lett. 2016, 16, 6838–6843. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Mesoscale organization of CuO nanoribbons: Formation of “dandelions”. J. Am. Chem. Soc. 2004, 126, 8124–8125. [Google Scholar] [CrossRef]
- Yao, W.-T.; Yu, S.-H.; Zhou, Y.; Jiang, J.; Wu, Q.-S.; Zhang, L.; Jiang, J. Formation of uniform CuO nanorods by spontaneous aggregation: Selective synthesis of CuO, Cu2O, and Cu nanoparticles by a solid-liquid phase arc discharge process. J. Phys. Chem. B 2005, 109, 14011–14016. [Google Scholar] [CrossRef]
- Xu, M.; Wang, F.; Ding, B.; Song, X.; Fang, J. Electrochemical synthesis of leaf-like CuO mesocrystals and their lithium storage properties. RSC Adv. 2012, 2, 2240–2243. [Google Scholar] [CrossRef]
- Jia, B.; Qin, M.; Zhang, Z.; Cao, Z.; Wu, H.; Chen, P.; Zhang, L.; Lu, X.; Qu, X. The formation of CuO porous mesocrystal ellipsoids via tuning the oriented attachment mechanism. CrystEngComm 2016, 18, 1376–1383. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, Y.; Qin, Q.; Zhang, G.; Luo, W.; Zheng, W. Nanoporous CuO mesocrystals: Low-temperature synthesis and improved structure-performance relationship for energy storage system. Chem. Eng. J. 2018, 331, 326–334. [Google Scholar] [CrossRef]
- Hu, J.; Zou, C.; Su, Y.; Li, M.; Han, Y.; Kong, E.S.-W.; Yang, Z.; Zhang, Y. Ultrasensitive NO2 gas sensor based on hierarchical Cu2O/CuO mesocrystals nanoflower. J. Mater. Chem. A 2018, 6, 17120–17131. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, R.; Guo, Y.; Lu, Y.; Xu, W.; Song, W. SnO mesocrystals: Additive-free synthesis, oxidation, and top-down fabrication of quantum dots. CrystEngComm 2012, 14, 4575–4577. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Ye, J.; Cai, J.; Ma, Y.; Zhou, H.; Qi, L. Kinetics-controlled growth of aligned mesocrystalline SnO2 nanorod arrays for lithium-ion batteries with superior rate performance. Nano Research 2013, 6, 243–252. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Ge, B.; Zhou, H.; Yuan, A.; Shen, X. Concave Co3O4 octahedral mesocrystal: Polymer-mediated synthesis and sensing properties. CrystEngComm 2012, 14, 6264–6270. [Google Scholar] [CrossRef]
- Wang, F.; Lu, C.; Qin, Y.; Liang, C.; Zhao, M.; Yang, S.; Sun, Z.; Song, X. Solid state coalescence growth and electrochemical performance of plate-like Co3O4 mesocrystals as anode materials for lithium-ion batteries. J. Power Sources 2013, 235, 67–73. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Mesocrystal Co3O4 nanoplatelets as high capacity anode materials for Li-ion batteries. Nano Res. 2014, 7, 794–803. [Google Scholar] [CrossRef]
- Hassen, D.; El-Safty, S.A.; Tsuchiya, K.; Chatterjee, A.; Elmarakbi, A.; Shenashen, M.A.; Sakai, M. Longitudinal hierarchy Co3O4 mesocrystals with high-dense exposure facets and anisotropic interfaces for direct-ethanol fuel cells. Sci. Rep. 2016, 6, 24330. [Google Scholar] [CrossRef]
- Cao, W.; Wang, W.; Shi, H.; Wang, J.; Cao, M.; Liang, Y.; Zhu, M. Hierarchical three-dimensional flower-like Co3O4 architectures with a mesocrystal structure as high capacity anode materials for long-lived lithium-ion batteries. Nano Res. 2018, 11, 1437–1446. [Google Scholar] [CrossRef]
- Fang, J.; Leufke, P.M.; Kruk, R.; Wang, D.; Scherer, T.; Hahn, H. External electric field driven 3D ordering architecture of silver (I) oxide meso-superstructures. Nano Today 2010, 5, 175–182. [Google Scholar] [CrossRef]
- Belman, N.; Israelachvili, J.N.; Li, Y.; Safinya, C.R.; Ezersky, V.; Rabkin, A.; Sima, O.; Golan, Y. Hierarchical superstructure of alkylamine-coated ZnS nanoparticle assemblies. Phys. Chem. Chem. Phys. 2011, 13, 4974–4979. [Google Scholar] [CrossRef]
- Querejeta-Fernandez, A.; Hernandez-Garrido, J.C.; Yang, H.; Zhou, Y.; Varela, A.; Parras, M.; Calvino-Gamez, J.J.; Gonzalez-Calbet, J.M.; Green, P.F.; Kotov, N.A. Unknown aspects of self-assembly of PbS microscale superstructures. ACS Nano 2012, 6, 3800–3812. [Google Scholar] [CrossRef]
- Simon, P.; Rosseeva, E.; Baburin, I.A.; Liebscher, L.; Hickey, S.G.; Cardoso-Gil, R.; Eychmüller, A.; Kniep, R.; Carrillo-Cabrera, W. PbS-organic mesocrystals: The relationship between nanocrystal orientation and superlattice array. Angew. Chem. Int. Ed. 2012, 51, 10776–10781. [Google Scholar] [CrossRef]
- Simon, P.; Bahrig, L.; Baburin, I.A.; Formanek, P.; Röder, F.; Sickmann, J.; Hickey, S.G.; Eychmüller, A.; Lichte, H.; Kniep, R.; et al. Interconnection of nanoparticles within 2D superlattices of PbS/oleic acid thin films. Adv. Mater. 2014, 26, 3042–3049. [Google Scholar] [CrossRef]
- De la Rica, R.; Velders, A.H. Biomimetic crystallization of Ag2S nanoclusters in nanopore assemblies. J. Am. Chem. Soc. 2011, 133, 2875–2877. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, Y.; Chen, O.; Wang, Z.; Cao, Y.C. Structural control of nanocrystal superlattices using organic guest molecules. J. Am. Chem. Soc. 2012, 134, 2868–2871. [Google Scholar] [CrossRef] [PubMed]
- Soejima, T.; Kimizuka, N. One-pot room-temperature synthesis of single-crystalline gold nanocorolla in water. J. Am. Chem. Soc. 2009, 131, 14407–14412. [Google Scholar] [CrossRef]
- Fang, J.; Du, S.; Lebedkin, S.; Li, Z.; Kruk, R.; Kappes, M.; Hahn, H. Gold mesostructures with tailored surface topography and their self-assembly arrays for surface-enhanced raman spectroscopy. Nano Lett. 2010, 10, 5006–5013. [Google Scholar] [CrossRef]
- You, H.; Ji, Y.; Wang, L.; Yang, S.; Yang, Z.; Fang, J.; Song, X.; Ding, B. Interface synthesis of gold mesocrystals with highly roughened surfaces for surface-enhanced Raman spectroscopy. J. Mater. Chem. 2012, 22, 1998–2006. [Google Scholar] [CrossRef]
- Fang, J.; Ding, B.; Song, X. Self-assembly mechanism of platelike silver mesocrystal. Appl. Phys. Lett. 2007, 91, 083108. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, J.; Bai, L.; Hu, P.; Yang, G.; Yuan, F.; Chen, Y. Formation of cubic Cu mesocrystals by a solvothermal reaction. CrystEngComm 2010, 12, 3894–3899. [Google Scholar] [CrossRef]
- Li, T.; You, H.; Xu, M.; Song, X.; Fang, J. Electrocatalytic properties of hollow coral-like platinum mesocrystals. ACS Appl. Mater. Interfaces 2012, 4, 6942–6948. [Google Scholar] [CrossRef]
- Zhong, P.; Liu, H.; Zhang, J.; Yin, Y.; Gao, C. Controlled Synthesis of octahedral platinum-based mesocrystals by oriented aggregation. Chem. Eur. J. 2017, 23, 6803–6810. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, S.; Yang, J.; Tan, Y.; Zheng, N. Etching growth under surface confinement: An effective strategy to prepare mesocrystalline Pd nanocorolla. J. Am. Chem. Soc. 2011, 133, 15946–15949. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Qi, L. TiO2 mesocrystals: Synthesis, formation mechanisms and applications. Sci. China Chem. 2012, 55, 2318–2326. [Google Scholar] [CrossRef]
- Hong, Z.; Wei, M. Recent progress in preparation and lithium-ion storage properties of TiO2 mesocrystals. J. Chin. Chem. Soc. 2015, 62, 209–216. [Google Scholar] [CrossRef]
- Zhang, P.; Tachikawa, T.; Fujitsuka, M.; Majima, T. The development of functional mesocrystals for energy harvesting, storage, and conversion. Chem. Eur. J. 2018, 24, 6295–6307. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.-Y. Organic small molecule-assisted synthesis of high active TiO2 rod-like mesocrystals. CrystEngComm 2010, 12, 2073–2078. [Google Scholar] [CrossRef]
- Zhang, D.; Li, G.; Wang, F.; Yu, J.C. Green synthesis of a self-assembled rutile mesocrystalline photocatalyst. CrystEngComm 2010, 12, 1759–1763. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Cao, C.; Luo, H.; Wang, W. Highly crystalline spindle-shaped mesoporous anatase titania particles: Solution-phase synthesis, characterization, and photocatalytic properties. Langmuir 2010, 26, 7671–7674. [Google Scholar] [CrossRef]
- Bian, Z.; Zhu, J.; Wen, J.; Cao, F.; Huo, Y.; Qian, X.; Cao, Y.; Shen, M.; Li, H.; Lu, Y. Single-crystal-like titania mesocages. Angew. Chem. Int. Ed. 2011, 123, 1137–1140. [Google Scholar] [CrossRef]
- Da Silva, R.O.; Gonçalves, R.H.; Stroppa, D.G.; Ramirez, A.J.; Leite, E.R. Synthesis of recrystallized anatase TiO2 mesocrystals with Wulff shape assisted by oriented attachment. Nanoscale 2011, 3, 1910–1916. [Google Scholar] [CrossRef]
- Tartaj, P. Sub-100 nm TiO2 mesocrystalline assemblies with mesopores: Preparation, characterization, enzyme immobilization and photocatalytic properties. Chem. Commun. 2011, 47, 256–258. [Google Scholar] [CrossRef]
- Tartaj, P.; Amarilla, J.M. Multifunctional response of anatase nanostructures based on 25 nm mesocrystal-like porous assemblies. Adv. Mater. 2011, 23, 4904–4907. [Google Scholar] [CrossRef]
- Jiao, W.; Wang, L.; Liu, G.; Lu, G.Q.; Cheng, H.-M. Hollow anatase TiO2 single crystals and mesocrystals with dominant {101} facets for improved photocatalysis activity and tuned reaction preference. ACS Catal. 2012, 2, 1854–1859. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Majima, T. Superstructure of TiO2 crystalline nanoparticles yields effective conduction pathways for photogenerated charges. J. Phys. Chem. Lett. 2012, 3, 1422–1427. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, W.; Chen, C.; Ji, H.; Zhao, J. Anatase TiO2 mesocrystals enclosed by (001) and (101) facets: Synergistic effects between Ti3+ and facets for their photocatalytic performance. Chem. Eur. J. 2012, 18, 12584–12589. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Li, H.; Wang, J. Manipulating the formation of NH4TiOF3 mesocrystals: Effects of temperature, surfactant, and pH. Cryst. Growth Des. 2012, 12, 2625–2633. [Google Scholar] [CrossRef]
- Aoyama, Y.; Oaki, Y.; Ise, R.; Imai, H. Mesocrystal nanosheet of rutile TiO2 and its reaction selectivity as a photocatalyst. CrystEngComm 2012, 14, 1405–1411. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, J.; Ji, C.; Zhou, L.; O’Brien, P. A facile solid phase reaction to prepare TiO2 mesocrystals with exposed {001} facets and high photocatalytic activity. CrystEngComm 2013, 15, 5012–5015. [Google Scholar] [CrossRef]
- Yao, X.; Liu, X.; Liu, T.; Wang, K.; Lu, L. One-step and large-scale synthesis of anatase TiO2 mesocrystals along [001] orientation with enhanced photocatalytic performance. CrystEngComm 2013, 15, 10246–10254. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Chen, J.; Wu, X.; Zhou, L. TiO2 mesocrystals built of nanocrystals with exposed {001} facets: Facile synthesis and superior photocatalytic ability. J. Mater. Chem. A 2014, 2, 19589–19593. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Zhang, H.; Liu, P.; Zhao, H.; An, T. Anatase TiO2 mesocrystals with exposed (001) surface for enhanced photocatalytic decomposition capability toward gaseous styrene. Catal. Today 2014, 224, 216–224. [Google Scholar] [CrossRef]
- Fang, Z.; Long, L.; Hao, S.; Song, Y.; Qiang, T.; Geng, B. Mesocrystal precursor transformation strategy for synthesizing ordered hierarchical hollow TiO2 nanobricks with enhanced photocatalytic property. CrystEngComm 2014, 16, 2061–2069. [Google Scholar] [CrossRef]
- Lai, L.-L.; Huang, L.-L.; Wu, J.-M. K2TiO(C2O4)2-mediated synthesis of rutile TiO2 mesocrystals and their ability to assist photodegradation of sulfosalicylic acid in water. RSC Adv. 2014, 4, 49280–49286. [Google Scholar] [CrossRef]
- Zhang, P.; Tachikawa, T.; Bian, Z.; Majima, T. Selective photoredox activity on specific facet-dominated TiO2 mesocrystal superstructures incubated with directed nanocrystals. Appl. Catal. B 2015, 176–177, 678–686. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, W.; Tanaka, Y.; Kusunose, N.; Peng, Y.; Feng, Q. Mesocrystalline nanocomposites of TiO2 polymorphs: Topochemical mesocrystal conversion, characterization, and photocatalytic response. Cryst. Growth Des. 2015, 15, 1214–1225. [Google Scholar] [CrossRef]
- Fu, X.X.; Ren, Z.M.; Fan, C.Y.; Sun, C.X.; Shi, L.; Yu, S.Q.; Qian, G.D.; Wang, Z.Y. Designed fabrication of anatase mesocrystals constructed from crystallographically oriented nanocrystals for improved photocatalytic activity. RSC Adv. 2015, 5, 41218–41223. [Google Scholar] [CrossRef]
- Lai, L.-L.; Wu, J.-M. Hollow TiO2 microspheres assembled with rutile mesocrystals: Low-temperature one-pot synthesis and the photocatalytic performance. Ceram. Int. 2015, 41, 12317–12322. [Google Scholar] [CrossRef]
- Fu, X.; Fan, C.; Yu, S.; Shi, L.; Wang, Z. TiO2 mesocrystals with exposed {001} facets as efficient photocatalysts. J. Alloys Compd. 2016, 680, 80–86. [Google Scholar] [CrossRef]
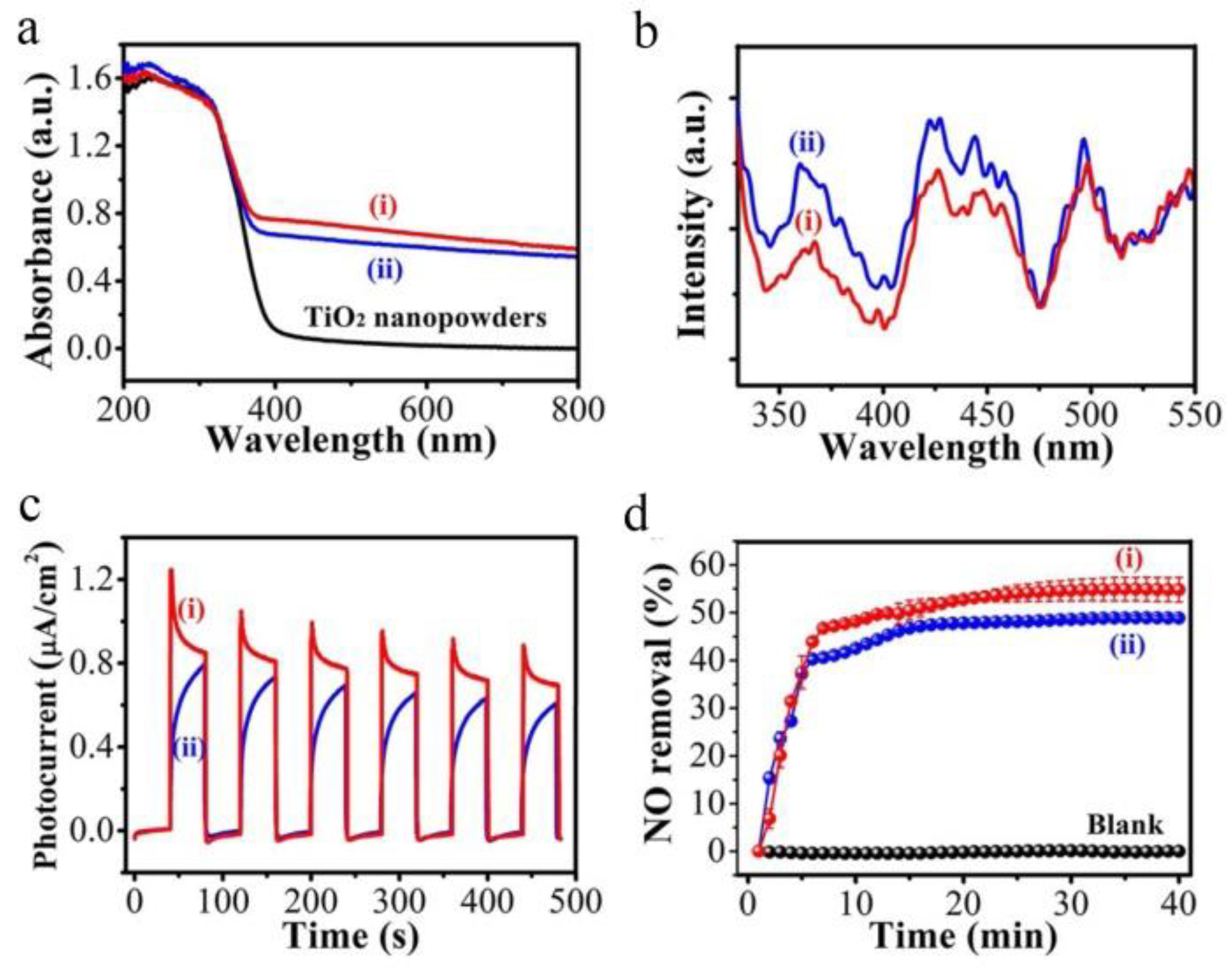
- Tan, B.; Zhang, X.; Li, Y.; Chen, H.; Ye, X.; Wang, Y.; Ye, J. Anatase TiO2 mesocrystals: Green synthesis, in situ conversion to porous single crystals, and self-doping Ti3+ for enhanced visible light driven photocatalytic removal of NO. Chem. Eur. J. 2017, 23, 5478–5487. [Google Scholar] [CrossRef]
- Tang, C.; Liu, L.; Li, Y.; Bian, Z. Aerosol spray assisted assembly of TiO2 mesocrystals into hierarchical hollow microspheres with enhanced photocatalytic performance. Appl. Catal. B 2017, 201, 41–47. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Luan, Q.; Duan, R.; Guan, R.; Cao, X.; Hu, X. Photocatalytic properties dependent on the interfacial defects of intergrains within TiO2 mesocrystals. Chem. Eur. J. 2018, 24, 17105–17116. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Dai, H.; Huang, Z.; Wei, M. Understanding the growth and photoelectrochemical properties of mesocrystals and single crystals: A case of anatase TiO2. Phys. Chem. Chem. Phys. 2014, 16, 7441–7447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, J.; Ma, Y.; Qi, L. Mesocrystalline TiO2 nanosheet arrays with exposed {001} facets: Synthesis via topotactic transformation and applications in dye-sensitized solar cells. Nano Res. 2017, 10, 2610–2625. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Carbon nanotubes supported mesoporous mesocrystals of anatase TiO2. Chem. Mater. 2008, 20, 2711–2718. [Google Scholar] [CrossRef]
- Li, N.; Liu, G.; Zhen, C.; Li, F.; Zhang, L.; Cheng, H.-M. Battery performance and photocatalytic activity of mesoporous anatase TiO2 nanospheres/graphene composites by template-free self-assembly. Adv. Funct. Mater. 2011, 21, 1717–1722. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, D.; Liu, Z.; Wu, N.-L.; Wei, M. Brookite TiO2 mesocrystals with enhanced lithium-ion intercalation properties. Chem. Commun. 2018, 54, 11491–11494. [Google Scholar] [CrossRef]
- Elbanna, O.; Zhang, P.; Fujitsuka, M.; Majima, T. Facile preparation of nitrogen and fluorine codoped TiO2 mesocrystal with visible light photocatalytic activity. Appl. Catal. B 2016, 192, 80–87. [Google Scholar] [CrossRef]
- Zhang, P.; Fujitsuka, M.; Majima, T. TiO2 mesocrystal with nitrogen and fluorine codoping during topochemical transformation: Efficient visible light induced photocatalyst with the codopants. Appl. Catal. B 2016, 185, 181–188. [Google Scholar] [CrossRef]
- Primc, D.; Niederberger, M. Synthesis and formation mechanism of multicomponent Sb-Nb:TiO2 mesocrystals. Chem. Mater. 2017, 29, 10113–10121. [Google Scholar] [CrossRef]
- Lan, T.; Zhang, W.; Wu, N.-L.; Wei, M. Nb-doped rutile TiO2 mesocrystals with enhanced lithium storage properties for lithium ion battery. Chem. Eur. J. 2017, 23, 5059–5065. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Santo, V.D. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 nanomaterials: A review of recent advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H. Defect-mediated electron-hole separation in semiconductor photocatalysis. Inorg. Chem. Front. 2018, 5, 1240–1254. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, O.; Fujitsuka, M.; Kim, S.; Majima, T. Charge carrier dynamics in TiO2 mesocrystals with oxygen vacancies for photocatalytic hydrogen generation under solar light irradiation. J. Phys. Chem. C 2018, 122, 15163–15170. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Kim, W.; Choi, W.; Majima, T. Superior electron transport and photocatalytic abilities of metal-nanoparticle-loaded TiO2 superstructures. J. Phys. Chem. C 2012, 116, 25444–25453. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Zhang, T.; Sun, D.D.; Ng, W. Hierarchical TiO2/CdS “spindle-like” composite with high photodegradation and antibacterial capability under visible light irradiation. J. Hazard. Mater. 2012, 229–230, 209–216. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Li, Y.; Zhang, R.; Tang, H. Graphene-spindle shaped TiO2 mesocrystal composites: Facile synthesis and enhanced visible light photocatalytic performance. J. Hazard. Mater. 2013, 261, 342–350. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, T.; Zhang, P.; Bian, Z.; Majima, T. Efficient charge separation and photooxidation on cobalt phosphate-loaded TiO2 mesocrystal superstructures. J. Mater. Chem. A 2014, 2, 3381–3388. [Google Scholar] [CrossRef]
- Zhang, P.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Efficient charge separation on 3D architectures of TiO2 mesocrystals packed with a chemically exfoliated MoS2 shell in synergetic hydrogen evolution. Chem. Commun. 2015, 51, 7187–7190. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Men, Y.; Bian, Z. TiO2 mesocrystal with exposed (001) facets and CdS quantum dots as an active visible photocatalyst for selective oxidation reactions. Appl. Catal. B 2016, 187, 115–121. [Google Scholar] [CrossRef]
- Han, T.; Wang, H.; Zheng, X. Gold nanoparticle incorporation into nanoporous anatase TiO2 mesocrystal using a simple deposition-precipitation method for photocatalytic applications. RSC Adv. 2016, 6, 7829–7837. [Google Scholar] [CrossRef]
- Yan, D.; Liu, Y.; Liu, C.-Y.; Zhang, Z.-Y.; Niea, S.-D. Multi-component in situ and in-step formation of visible-light response C-dots composite TiO2 mesocrystals. RSC Adv. 2016, 6, 14306–14313. [Google Scholar] [CrossRef]
- Tang, H.; Chang, S.; Jiang, L.; Tang, G.; Liang, W. Novel spindle-shaped nanoporous TiO2 coupled graphitic g-C3N4 nanosheets with enhanced visible-light photocatalytic activity. Ceram. Int. 2016, 42, 18443–18452. [Google Scholar] [CrossRef]
- Elbanna, O.; Kim, S.; Fujitsuka, M.; Majima, T. TiO2 mesocrystals composited with gold nanorods for highly efficient visible-NIR-photocatalytic hydrogen production. Nano Energy 2017, 35, 1–8. [Google Scholar] [CrossRef]
- Elbanna, O.; Fujitsuka, M.; Majima, T. g-C3N4/TiO2 mesocrystals composite for H2 evolution under visible-light irradiation and its charge carrier dynamics. ACS Appl. Mater. Interfaces 2017, 9, 34844–34854. [Google Scholar] [CrossRef]
- Yu, X.; Fan, X.; An, L.; Liu, G.; Li, Z.; Liu, J.; Hu, P.A. Mesocrystalline Ti3+-TiO2 hybridized g-C3N4 for efficient visible-light photocatalysis. Carbon 2018, 128, 21–30. [Google Scholar] [CrossRef]
- Xue, J.; Elbanna, O.; Kim, S.; Fujitsuka, M.; Majima, T. Defect state-induced efficient hot electron transfer in Au nanoparticles/reduced TiO2 mesocrystal photocatalysts. Chem. Commun. 2018, 54, 6052–6055. [Google Scholar] [CrossRef]
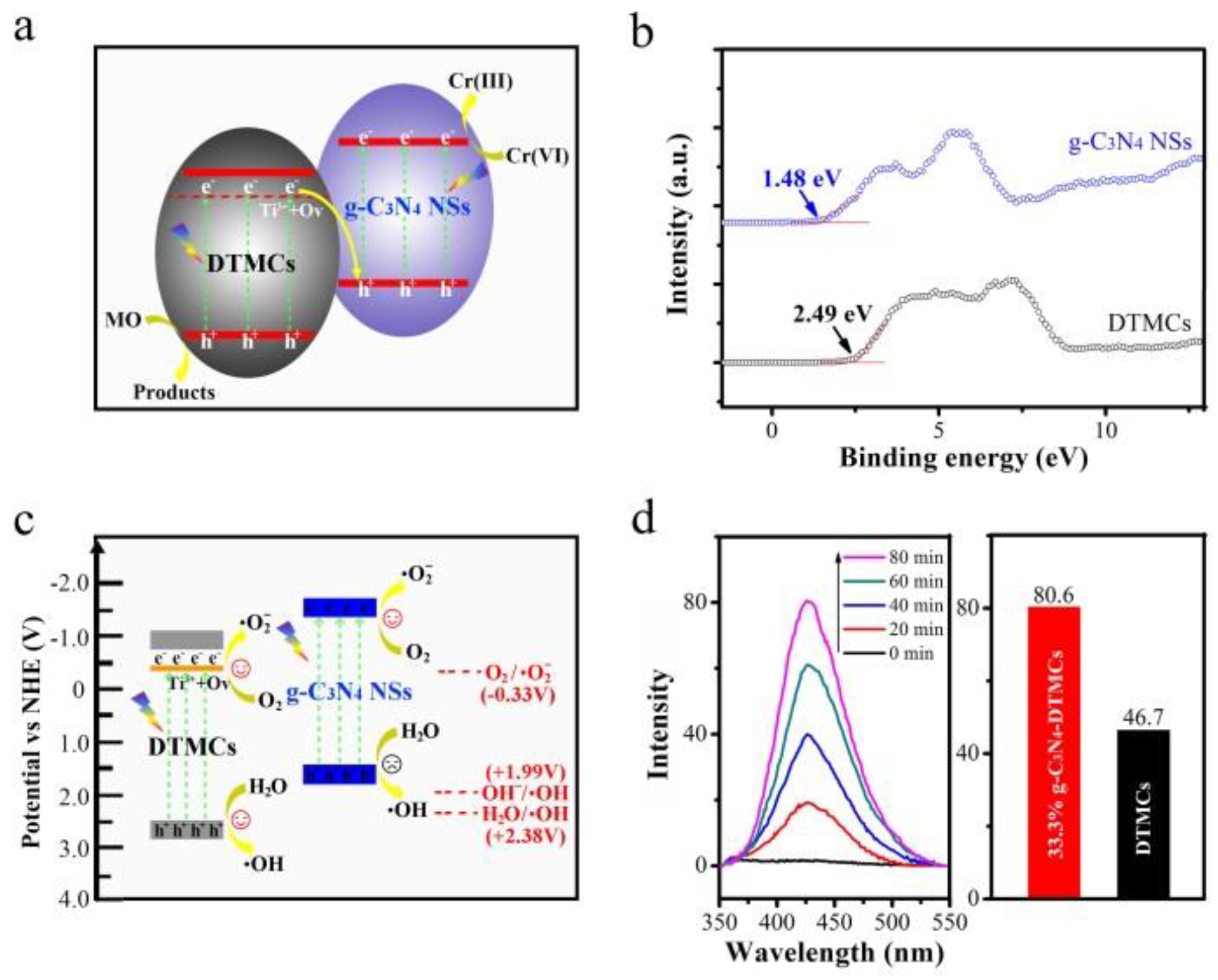
- Tan, B.; Ye, X.; Li, Y.; Ma, X.; Wang, Y.; Ye, J. Defective anatase TiO2-x mesocrystal growth in situ on g-C3N4 nanosheets: Construction of 3D/2D Z-scheme heterostructures for highly efficient visible-light photocatalysis. Chem. Eur. J. 2018, 24, 13311–13321. [Google Scholar] [CrossRef]
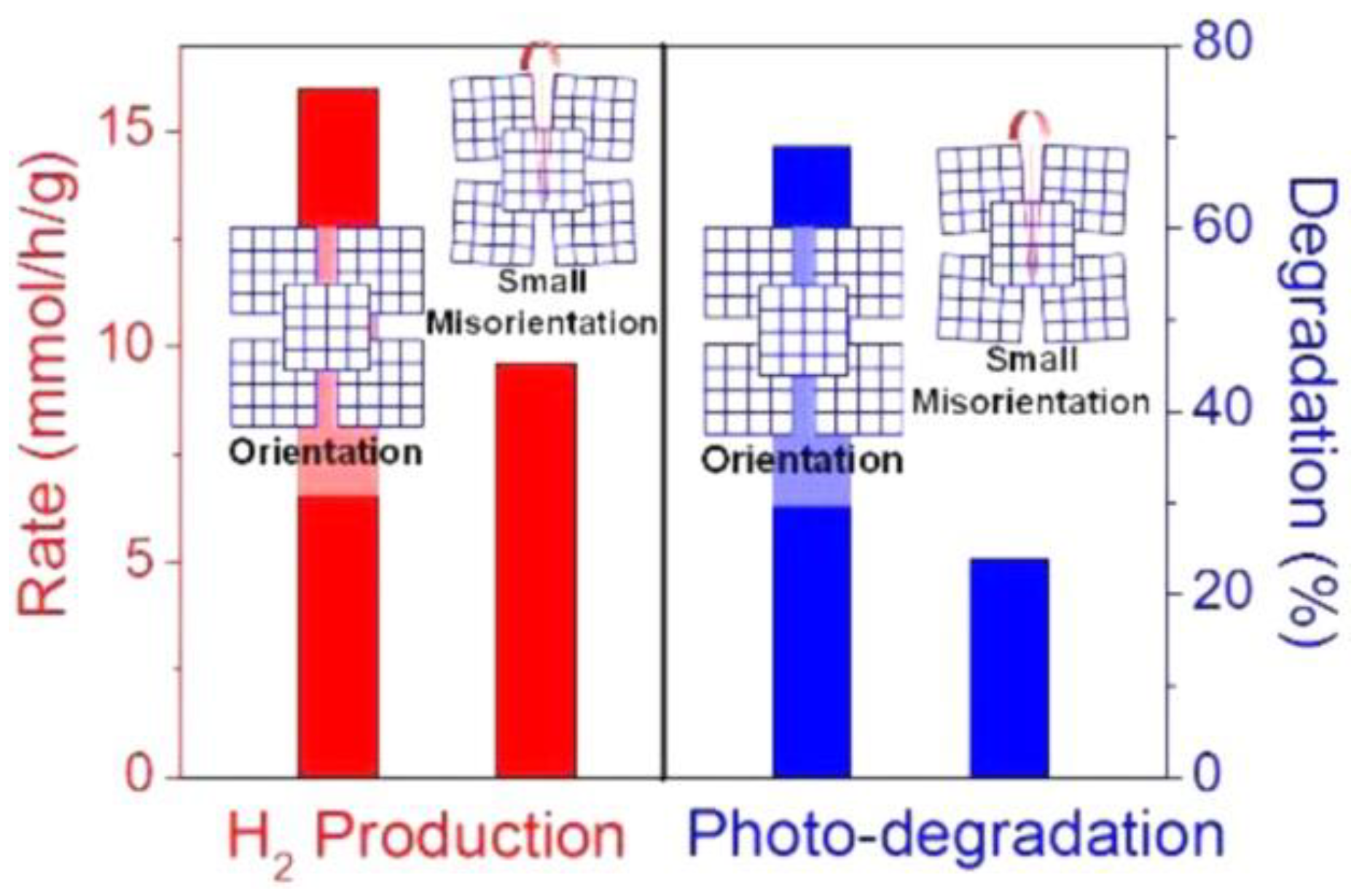
- Chen, F.; Cao, F.; Li, H.; Bian, Z. Exploring the important role of nanocrystals orientation in TiO2 superstructure on photocatalytic performances. Langmuir 2015, 31, 3494–3499. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Cao, S.; Du, M.; Ye, X.; Wang, Y.; Ye, J. Titanium Dioxide (TiO2) Mesocrystals: Synthesis, Growth Mechanisms and Photocatalytic Properties. Catalysts 2019, 9, 91. https://doi.org/10.3390/catal9010091
Zhang B, Cao S, Du M, Ye X, Wang Y, Ye J. Titanium Dioxide (TiO2) Mesocrystals: Synthesis, Growth Mechanisms and Photocatalytic Properties. Catalysts. 2019; 9(1):91. https://doi.org/10.3390/catal9010091
Chicago/Turabian StyleZhang, Boxue, Shengxin Cao, Meiqi Du, Xiaozhou Ye, Yun Wang, and Jianfeng Ye. 2019. "Titanium Dioxide (TiO2) Mesocrystals: Synthesis, Growth Mechanisms and Photocatalytic Properties" Catalysts 9, no. 1: 91. https://doi.org/10.3390/catal9010091
APA StyleZhang, B., Cao, S., Du, M., Ye, X., Wang, Y., & Ye, J. (2019). Titanium Dioxide (TiO2) Mesocrystals: Synthesis, Growth Mechanisms and Photocatalytic Properties. Catalysts, 9(1), 91. https://doi.org/10.3390/catal9010091





