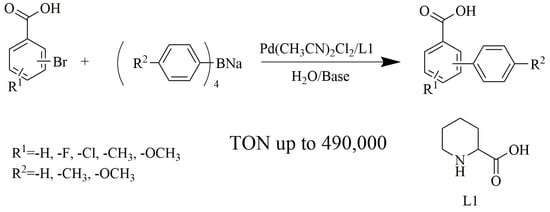
Pd(CH3CN)2Cl2/Pipecolinic Acid as a Highly Efficient Catalytic System for Suzuki-Miyaura Cross-coupling Reaction of Bromoaryl Carboxylic Acids in Water
Abstract
1. Introduction
2. Results and Discussions
2.1. Optimization of Catalytic Conditions
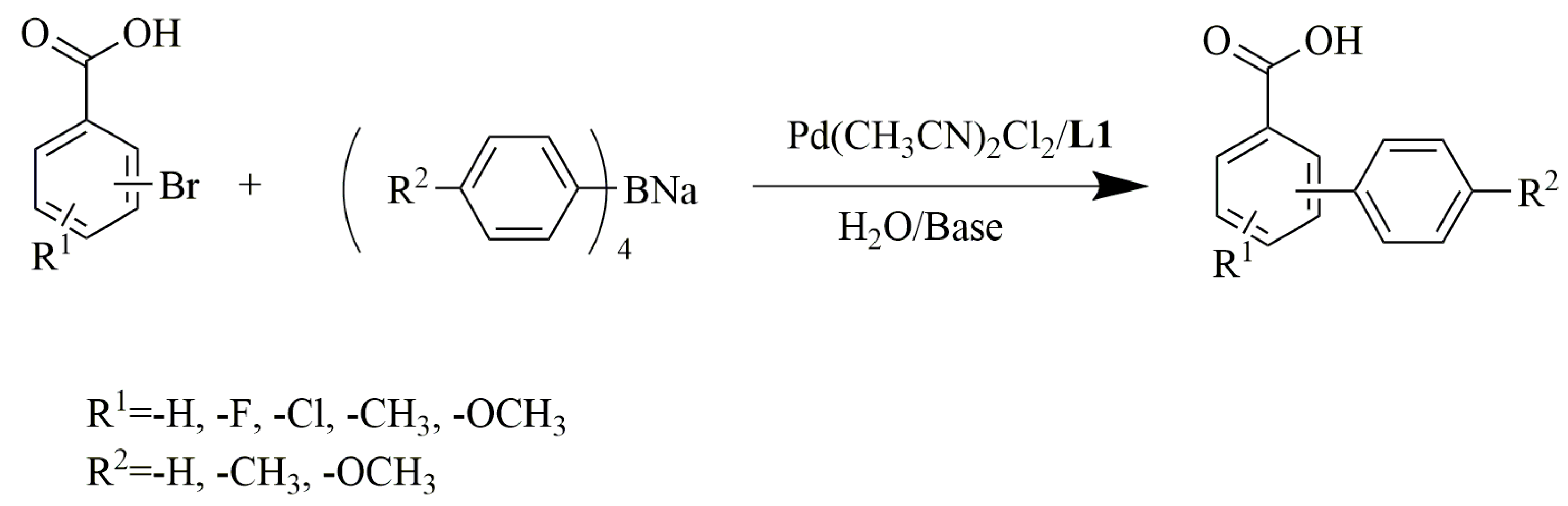
2.2. Evaluation of the Scope of Pd(CH3CN)2Cl2 /L1
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Witzel, S.; Xie, J.; Rudolph, M.; Hashmi, S.K. Photosensitizer-free, Gold-catalyzed C-C Cross-coupling of Boronic Acids and Diazonium Salts Enabled by Visible Light. Adv. Synth. Catal. 2017, 359, 1522–1528. [Google Scholar] [CrossRef]
- Xie, J.; Sekine, K.; Witzel, S.; Krämer, P.; Rudolph, M.; Romminger, F.; Hashmi, S.K. Light-Induced Gold-Catalyzed Hiyama Arylation: A Coupling Access to Biarylboranates. Angew. Chem. Int. Ed. 2018, 57, 16648–16653. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Linert, W. Schiff Base-derived Homogeneous and Heterogeneous Palladium Catalysts for the Suzuki–Miyaura Reaction. Coord. Chem. Rev. 2016, 311, 1–23. [Google Scholar] [CrossRef]
- Kumbhar, A. Functionalized. Nitrogen Ligands for Palladium Catalyzed Cross-coupling Reactions (part I). J. Organomet. Chem. 2017, 848, 22–28. [Google Scholar] [CrossRef]
- Tao, B.; Boykin, D.W. trans-Pd(OAc)2(Cy2NH)2 Catalyzed Suzuki Coupling Reactions and Its Temperature-dependent Activities toward Aryl Bromides. Tetrahedron Lett. 2003, 44, 7993–7996. [Google Scholar] [CrossRef]
- Tao, B.; Boykin, D.W. Simple Amine/Pd(OAc)2-Catalyzed Suzuki Coupling Reactions of Aryl Bromides under Mild Aerobic Conditions. J. Org. Chem. 2004, 69, 4330–4957. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Suresh, D.; Balakrishna, M.S.; Mague, J.T. An Inexpensive and Highly Stable Ligand 1,4-bis(2-hydroxy-3,5-di-tert-butylbenzyl)piperazine for Mizoroki-Heck and Room temperature Suzuki-Miyaura Cross-coupling Reactions. Tetrahedron 2008, 64, 240–247. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, W.J. Dabco as an Inexpensive and Highly Efficient Ligand for Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Org. Lett. 2004, 6, 2809–2811. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lv, M.Y.; Xiao, D.A.; Li, X.; Zhou, X.; Guo, M. A highly Efficient Catalyst of a Nitrogen-based Ligand for the Suzuki Coupling Reaction at Room Temperature under Air in Neat Water. Org. Biomol. Chem. 2014, 12, 4511–4516. [Google Scholar] [CrossRef] [PubMed]
- Karaca, E.O.; Akkoc, M.; Tahir, M.N.; Anici, C.; Imik, F.; Gurbuz, N.; Yasar, S.; Ozdemir, I. A Novel Ditopic Ring-expanded N-heterocyclic Carbene Ligand-assisted Suzuki-Miyaura Coupling Reaction in Aqueous Media. Tetrahedron Lett. 2017, 58, 3529–3532. [Google Scholar] [CrossRef]
- Liu, G.; Liu, C.H.; Han, F.; Wang, Z.H.; Wang, J. Highly Active Palladium Catalysts Containing a 1,10-Phenanthroline Analogue N-heterocyclic Carbene for Room Temperature Suzuki-Miyaura Coupling Reactions of Aryl Chlorides with Arylboronic Acids in Aqueous Media. Tetrahedron Lett. 2017, 58, 726–731. [Google Scholar] [CrossRef]
- Beigi, Z.; Kianfar, A.H.; Mohammadnezhad, G.; Gorls, H.; Plass, W. Palladium(II) Complexes with Diaminomaleonitrile-based Schiff-base Ligands: Synthesis, Characterization and Application as Suzuki–Miyaura Coupling Catalysts. Polyhedron 2017, 134, 65–72. [Google Scholar] [CrossRef]
- Luconi, L.; Gafurov, Z.; Rossin, A.; Tuci, G.; Sinyashin, O.; Yakhavarow, D.; Giambastiani, G. Palladium(II) Pyrazolyl-pyridyl Complexes Containing a Sterically Hindered N-heterocyclic Carbene Moiety for the Suzuki-Miyaura Cross-coupling Reaction. Inorg. Chim. Acta 2018, 470, 100–105. [Google Scholar] [CrossRef]
- Pioquinto-Mendoza, J.R.; Conelly-Espinosa, P.; Martinez, R.R.; Toscano, R.A.; German-Acacio, J.M.; Aorrosa, A.; Baldovino-Pantaleon, O.; Morales-Morales, D. A Simple and Facile to Prepare Pd(II) Complex Containing the Pyridyl Imine Ligand [C5H4N-2-CH3C=N-(CH2)3NH2]. Structural Characterization and Catalytic Evaluation in Suzuki-Miyaura C-C Couplings. J. Organomet. Chem. 2015, 797, 153–158. [Google Scholar] [CrossRef]
- Alvarez-Casao, Y.; Estepa, B.; Monge, D.; Ros, A.; Iglesias-Siguenza, J.; Alvarez, E.; Ferandez, R.; Lassaletta, J.M. Pyridine-hydrazone Ligands in Enantioselective Palladium-catalyzed Suzuki-Miyaura Cross-couplings. Tetrahedron 2016, 72, 5184–5190. [Google Scholar] [CrossRef]
- Gunawan, M.A.; Qiao, C.; Abrunhosa-Thomas, I.; Puget, B.; Roblin, J.P.; Prim, D.; Troin, Y. Simple Pyridylmethylamines: Efficient and Robust N,N-ligands for Suzuki-Miyaura Coupling Reactions. Tetrahedron Lett. 2010, 51, 5392–5394. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Liu, F.; Tan, Y.; Jiang, Y. A Simple and Novel Amide Lligand Based on Quinoline Derivative Used for Palladium-catalyzed Suzuki Coupling Reaction. J. Organomet. Chem. 2015, 794, 27–32. [Google Scholar] [CrossRef]
- Chen, Q.; Mao, Z.; Guo, F.; Liu, X. Indazolium Halides as Efficient Ligands for Pd-catalyzed Suzuki-Miyaura Cross-coupling of Aryl Bromides with Aarylboronic Acids. Tetrahedron Lett. 2016, 57, 3735–3738. [Google Scholar] [CrossRef]
- Sarmah, G.; Utpal, B. Simple Aminobenzoic Acid Ppromoted Palladium Catalyzed Room Temperature Suzuki-MiyauraCross-Coupling Reaction in Aqueous Media. Tetrahedron Lett. 2015, 56, 2906–2909. [Google Scholar] [CrossRef]
- Nagao, I.; Chatterjee, M.; Kawanami, H. Rapid Suzuli-Miyaura Couplings with ppm Level Palladium Catalyst in a High Pressure and High-Temperature Water System. Catalysts 2018, 8, 451–459. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, Q.; Ma, D.W. Amino Acid Promoted CuI-Catalyzed C-N Bond Formation Between Aryl Halide and Amine or N-Containing Heterocycles. J. Org. Chem. 2005, 70, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.P.; Liu, S.; Zhou, X.; Lv, M.; Chen, S.; Xiao, D.A. Simple Hydrophilic Palladium(II) Complex as a Highly Efficient Catalyst for Room Temperature Aerobic Suzuki Coupling Reactions in Aqueous Media. Molecules 2014, 19, 6524–6533. [Google Scholar] [CrossRef] [PubMed]
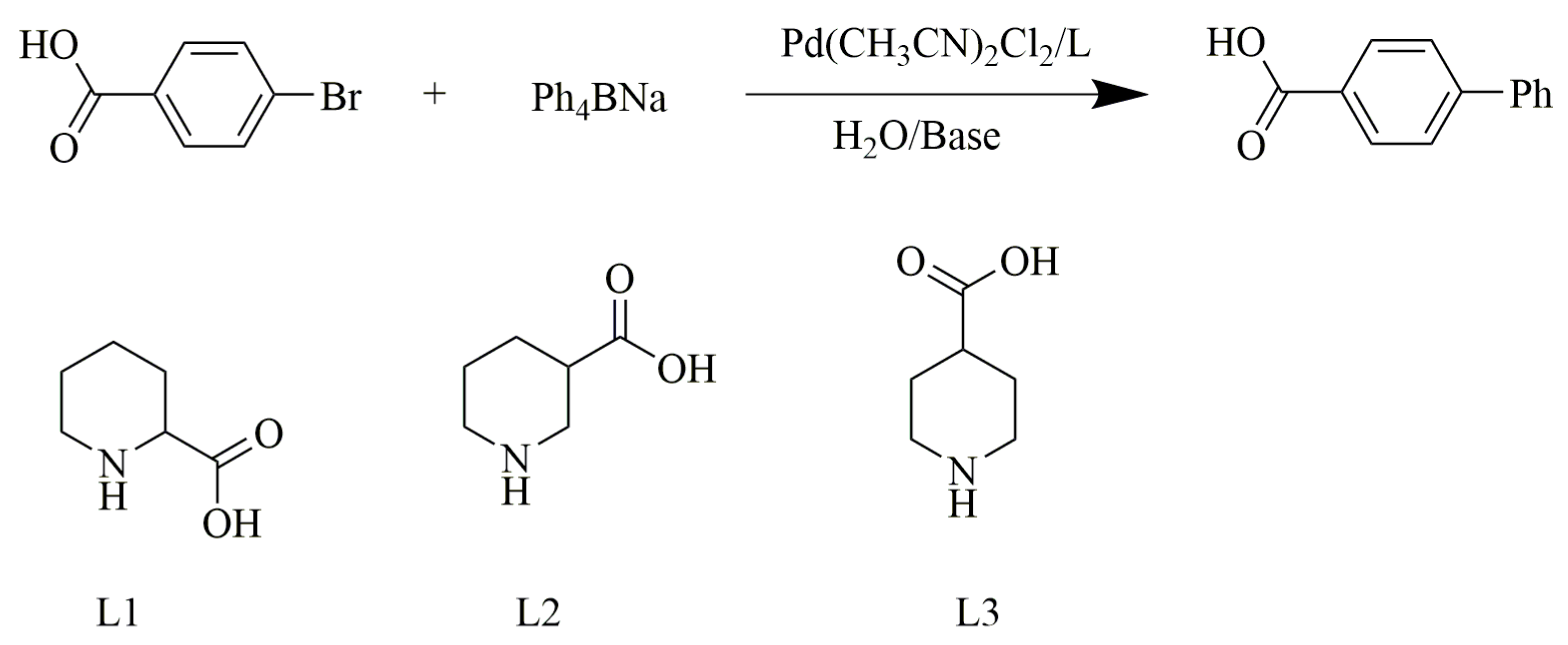
| Entry a | Ligand | Pd (mol%) | Base | Time (h) | Yield e (%) | TON f |
|---|---|---|---|---|---|---|
| 1 | - | 0.002 | Na2CO3 | 22 | 4.9 | - |
| 2 | L2 | 0.002 | Na2CO3 | 22 | 6.9 | - |
| 3 | L3 | 0.002 | Na2CO3 | 22 | 4.0 | - |
| 4 | L1 | 0.002 | Na2CO3 | 6 | 95 | 4.7 × 104 |
| 5 | L1 | 0.002 | NaHCO3 | 6 | 94 | 4.7 × 104 |
| 6 | L1 | 0.002 | NaF | 6 | 24 | 1.2 × 104 |
| 7 | L1 | 0.002 | NaOH | 6 | 84 | 4.2 × 104 |
| 8 | L1 | 0.004 | Na2CO3 | 6 | 99 | 2.5 × 104 |
| 9 | L1 | 0.0002 | Na2CO3 | 6 | 24 | 1.2 × 105 |
| 10 | L1 | 0.0002 | Na2CO3 | 22 | 97 | 4.9 × 105 |
| 11 b | L1 | 0.0002 | Na2CO3 | 22 | 8.2 | - |
| 12 c | L1 | 0.0002 | Na2CO3 | 22 | 7.5 | - |
| 13 d | L1 | 0.0002 | Na2CO3 | 22 | <5 | - |
| Entry a | Halide Fragment | Tetraarylboron Sodium Fragment | Yield d (%) | TON e |
|---|---|---|---|---|
| 1 | 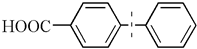 | 95(96) | 4.8 × 104 | |
| 2 b | 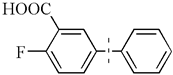 | 98(97) | 4.9 × 104 | |
| 3 | 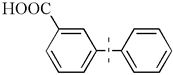 | 85(72) | 4.3 × 104 | |
| 4 | 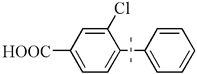 | 96(98) | 4.8 × 104 | |
| 5 | 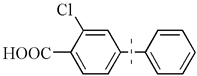 | 99(96) | 5.0 × 104 | |
| 6 | 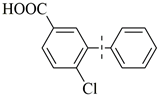 | 86(75) | 4.3 × 104 | |
| 7 | 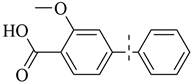 | 99(97) | 5.0 × 104 | |
| 8 | 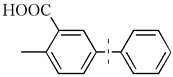 | 98(94) | 4.9 × 104 | |
| 9 | 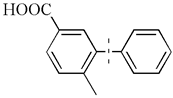 | 74(40) | 3.7 × 104 | |
| 10 | 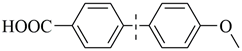 | 95(90) | 4.8 × 104 | |
| 11 b | 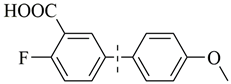 | 93(92) | 4.8 × 104 | |
| 12 | 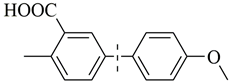 | 94(95) | 4.8 × 104 | |
| 13 | 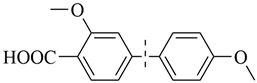 | 82(73) | 4.1 × 104 | |
| 14 | 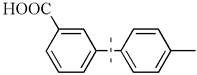 | 96(90) | 4.8 × 104 | |
| 15 c | 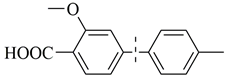 | 99(95) | 5.0 × 104 | |
| 16 b,c | 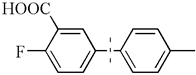 | 90(80) | 4.5 × 104 | |
| 17 c | 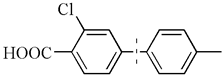 | 97(94) | 4.9 × 104 | |
| 18 c | 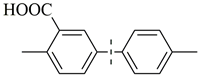 | 88(70) | 4.4 × 104 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, W.; Fu, L.; Guo, M. Pd(CH3CN)2Cl2/Pipecolinic Acid as a Highly Efficient Catalytic System for Suzuki-Miyaura Cross-coupling Reaction of Bromoaryl Carboxylic Acids in Water. Catalysts 2019, 9, 86. https://doi.org/10.3390/catal9010086
Li X, Zhang W, Fu L, Guo M. Pd(CH3CN)2Cl2/Pipecolinic Acid as a Highly Efficient Catalytic System for Suzuki-Miyaura Cross-coupling Reaction of Bromoaryl Carboxylic Acids in Water. Catalysts. 2019; 9(1):86. https://doi.org/10.3390/catal9010086
Chicago/Turabian StyleLi, Xiaogang, Wenbin Zhang, Leiqing Fu, and Mengping Guo. 2019. "Pd(CH3CN)2Cl2/Pipecolinic Acid as a Highly Efficient Catalytic System for Suzuki-Miyaura Cross-coupling Reaction of Bromoaryl Carboxylic Acids in Water" Catalysts 9, no. 1: 86. https://doi.org/10.3390/catal9010086
APA StyleLi, X., Zhang, W., Fu, L., & Guo, M. (2019). Pd(CH3CN)2Cl2/Pipecolinic Acid as a Highly Efficient Catalytic System for Suzuki-Miyaura Cross-coupling Reaction of Bromoaryl Carboxylic Acids in Water. Catalysts, 9(1), 86. https://doi.org/10.3390/catal9010086