Effect of Fe2O3–ZrO2 Catalyst Morphology on Sulfamethazine Degradation in the Fenton-Like Reaction
Abstract
1. Introduction
2. Results and Discussions
2.1. Characterization of the Catalysts
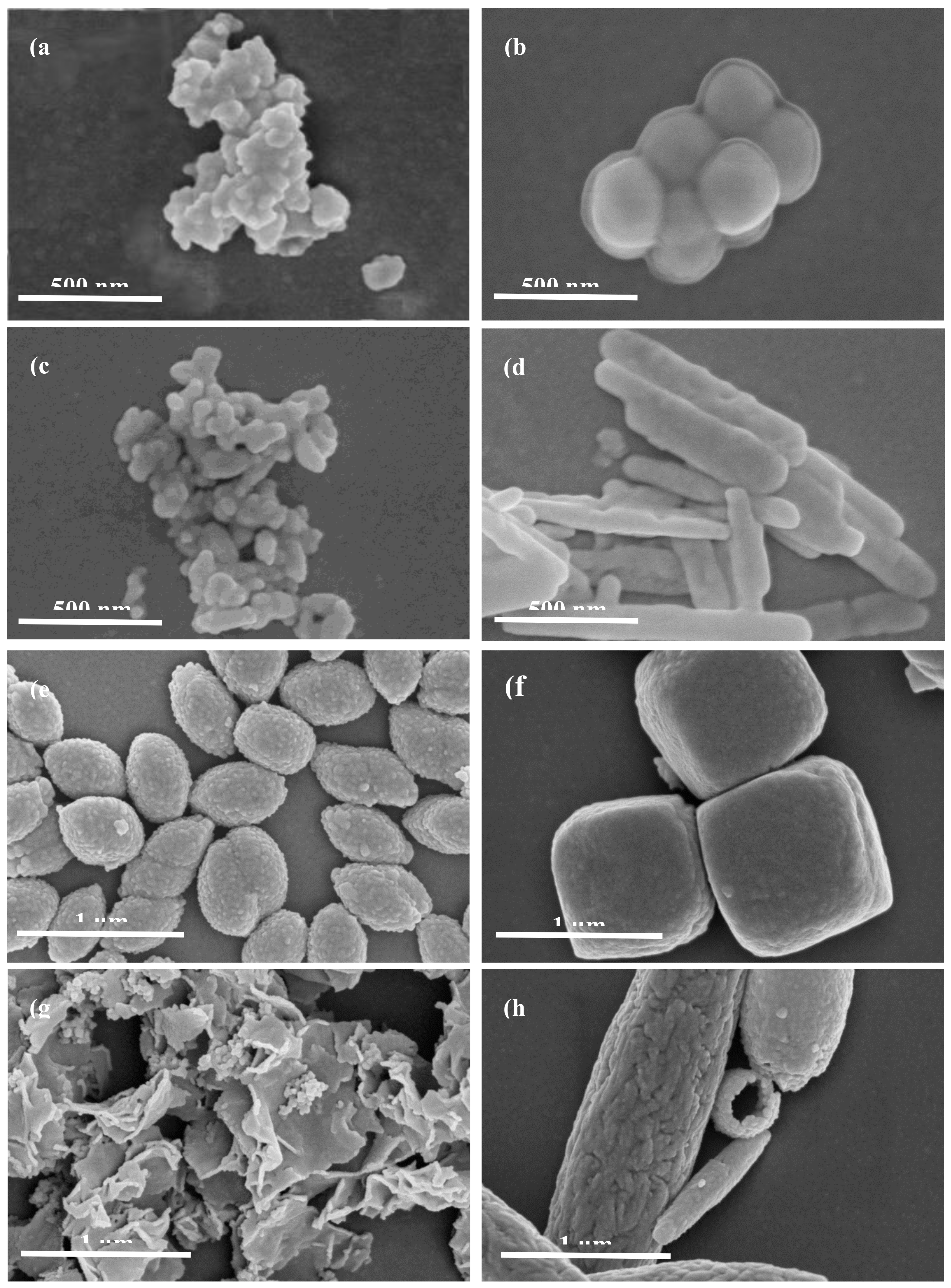
2.1.1. Scanning Electron Microscope (SEM) Analysis
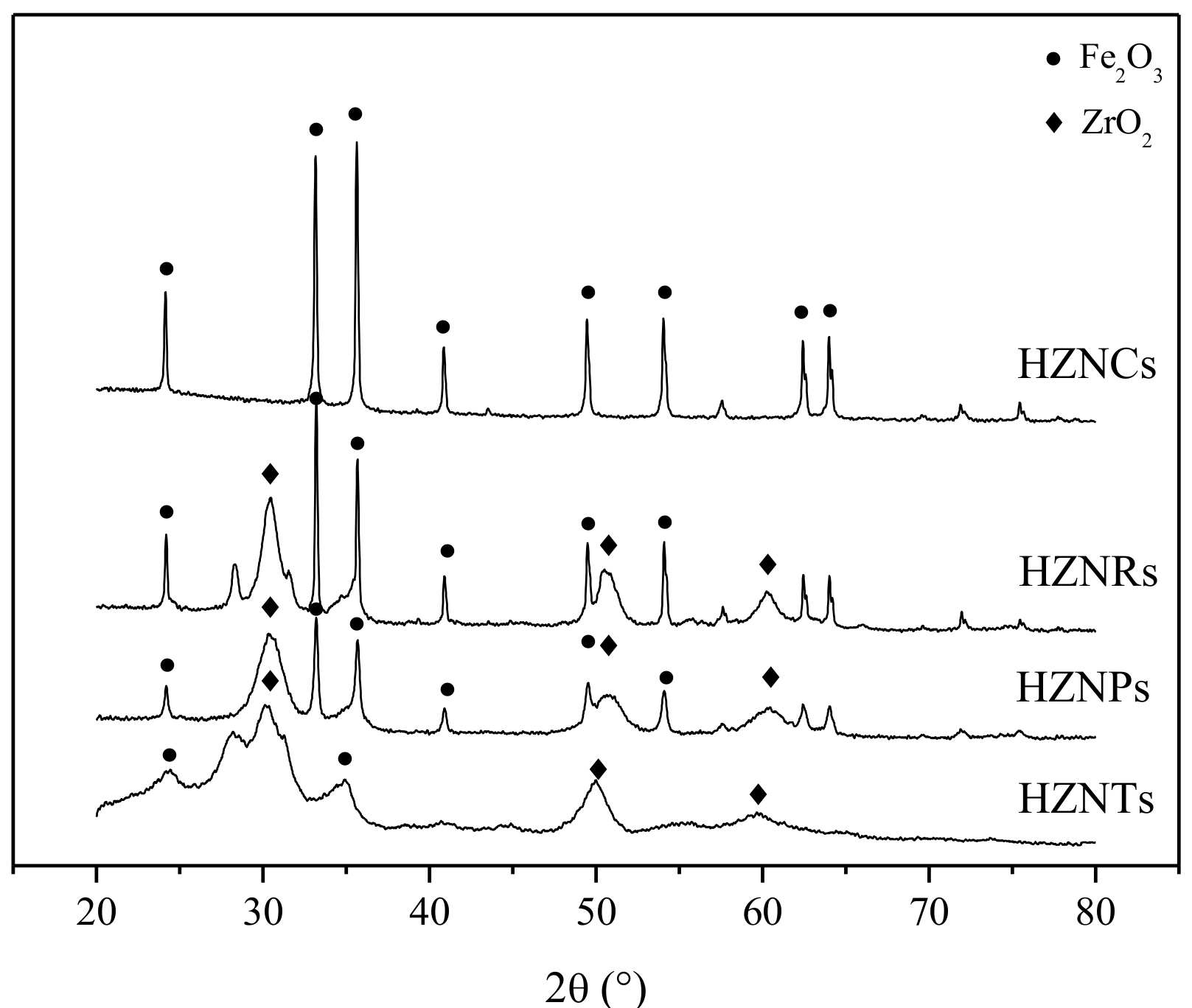
2.1.2. X-ray Diffraction (XRD) and Brunauer–Emmett-Teller (BET) Analysis
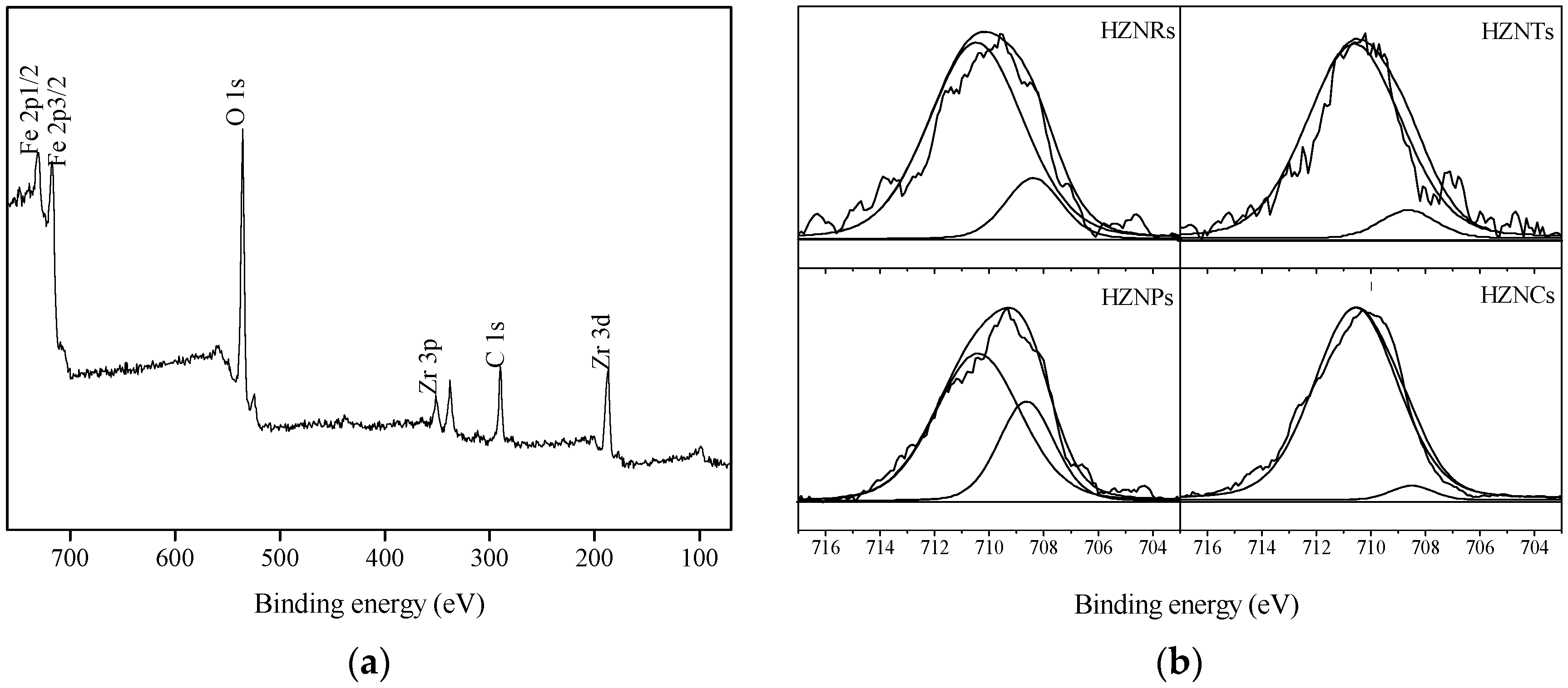
2.1.3. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.2. The Activity of the Fe2O3–ZrO2 Catalysts
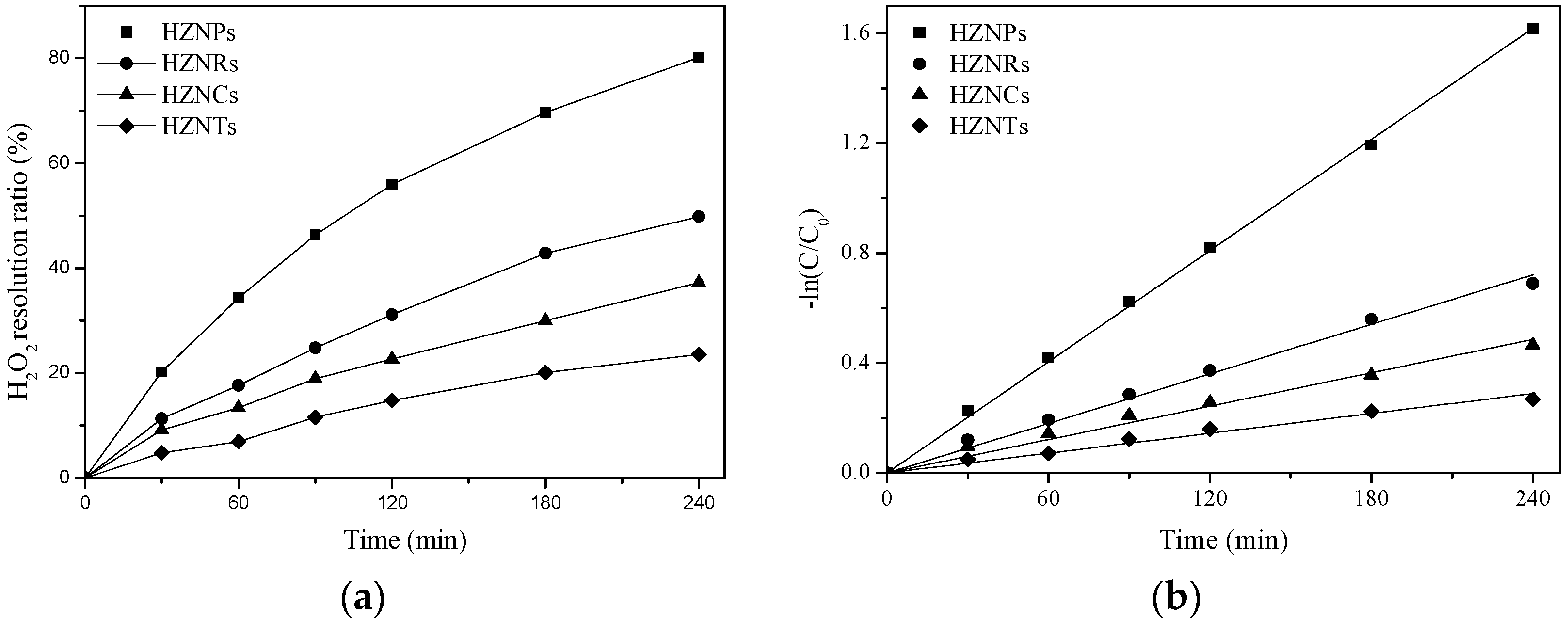
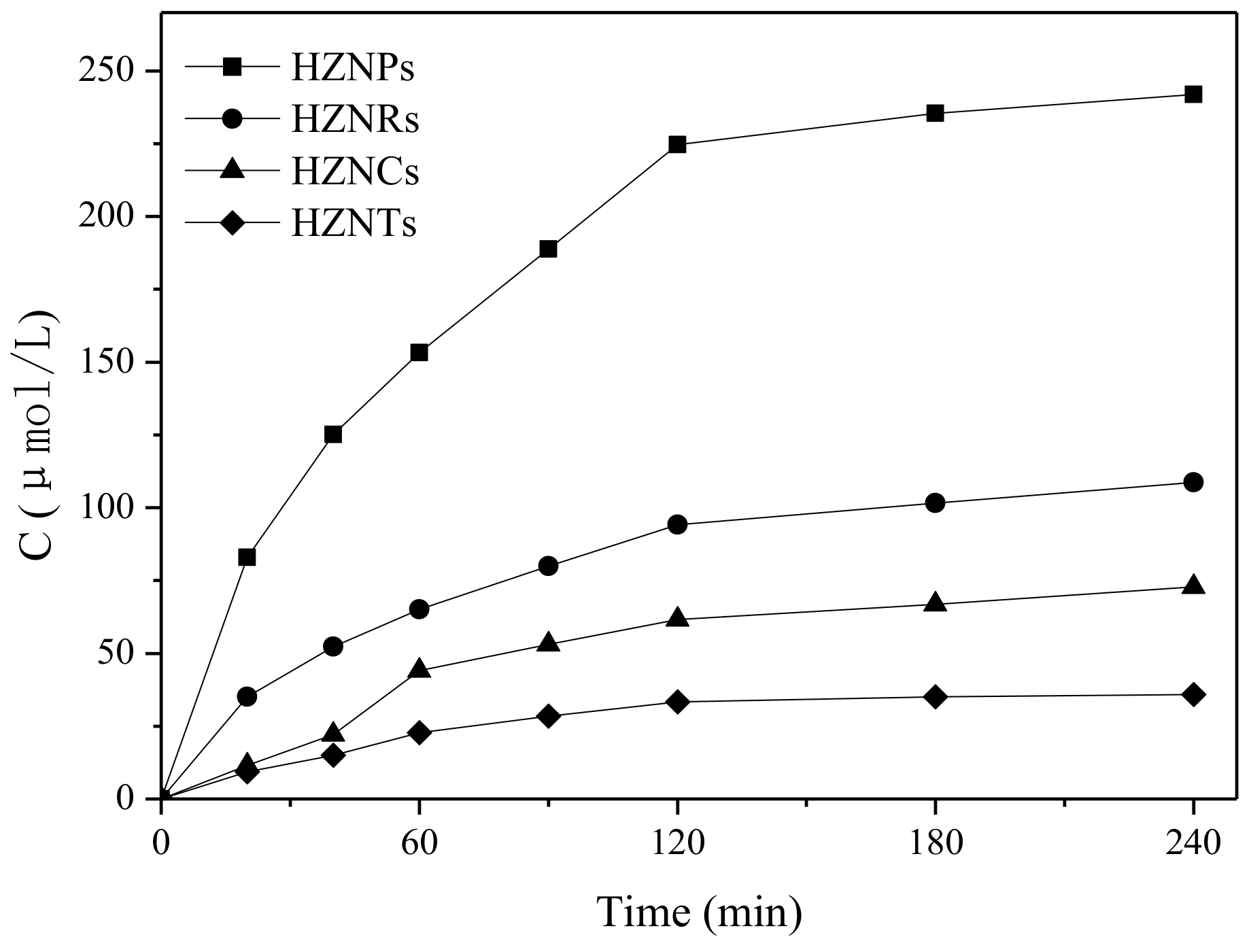
2.2.1. The Interaction of Hematite Facets with H2O2
2.2.2. The Activity of the Fe2O3–ZrO2 Catalysts
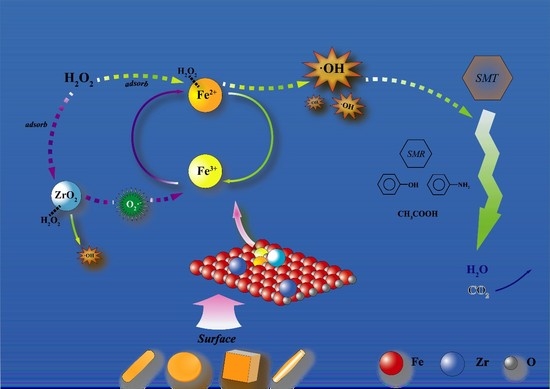
2.3. Mechanism Analysis
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis Methods
3.2.1. Preparation of Fe2O3–ZrO2 and Fe2O3 Nanoplates
3.2.2. Preparation of Fe2O3–ZrO2 and Fe2O3 Nanorods
3.2.3. Preparation of Fe2O3–ZrO2 and Fe2O3 Nanocubes
3.2.4. Preparation of Fe2O3–ZrO2 and Fe2O3 Nanotubes
3.3. Catalyst Characterization
3.4. H2O2 and ·OH Concentration Measurement
3.5. Fenton Degradation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.L.; Wang, S.Z. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Qian, L.; Chen, Y.; Wang, Q.N.; Zhao, G.H. Selective catalytic two-electron O2 reduction for onsite efficient oxidation reaction in heterogeneous electro-Fenton process. Chem. Eng. J. 2018, 332, 486–496. [Google Scholar] [CrossRef]
- Xiao, J.D.; Xie, Y.B.; Cao, H.B.; Wang, Y.X.; Guo, Z.; Chen, Y. Towards effective design of active nanocarbon materials for integrating visible-light photocatalysis with ozonation. Carbon 2016, 107, 658–666. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliverira, L.C.A.; Murad, E. Iron oxide catalysts: Fenton and Fenton-like reactions—A review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Alvaeo, M.; Garcia, H. Metal nanoparticles as heterogeneous Fenton catalysts. ChemSusChem 2012, 5, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Suanon, F.; Sun, Q.; Li, M.Y.; Cai, X.; Zhang, Y.C.; Yan, Y.J.; Yu, C.P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Zhao, H.Y.; Zhao, G.H. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B 2015, 164, 396–406. [Google Scholar] [CrossRef]
- Sun, J.Q.; Chen, Y.L.; Chen, J.G. Morphology effect of one-dimensional iron oxide nanocatalysts on Fischer-Tropsch synthesis. Catal. Sci. Technol. 2016, 6, 7505–7511. [Google Scholar] [CrossRef]
- Wang, B.H.; Sun, J.Q.; Abbas, M.; Liu, Y.T.; Kong, F.H.; Xiao, H.C.; Chen, J.G. A Novel Hydrothermal Approach for the Synthesis of Flower-Like Fe2O3/Fe Foam Nanocrystals and Their Superior Performance in Fisher-Tropsch Synthesis. Catal. Lett. 2017, 147, 1153–1161. [Google Scholar] [CrossRef]
- Su, M.H.; He, C.; Shih, K. Facile synthesis of morphology and size-controlled α-Fe2O3 and Fe3O4 nano-and microstructures by hydrothermal/solvothermal process: The roles of reaction medium and urea dose. Ceram. Int. 2016, 42, 14793–14804. [Google Scholar] [CrossRef]
- Hou, L.W.; Zhang, Q.H.; Jérôme, F.; Duprez, D.; Zhang, H.; Royer, S. Shape-controlled nanostructured magnetite-type materials as highly efficient Fenton catalysts. Appl. Catal. B 2014, 144, 739–749. [Google Scholar] [CrossRef]
- Huang, X.P.; Hou, X.J.; Zhao, J.C.; Zhang, L.Z. Hematite facet confined ferrous ions as high efficient Fenton catalysts to degrade organic contaminants by lowering H2O2 decomposition energetic span. Appl. Catal. B. 2016, 181, 127–137. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J.L. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; He, Z.S.; Zhong, Y.H.; Tan, W.; He, H.P.; Yuan, P.; Zhu, J.X.; Zhang, J. The effect of transition metal substitution on the catalytic activity of magnetite in heterogeneous Fenton reaction: In interfacial view. Coll. Surf. A 2013, 435, 28–35. [Google Scholar] [CrossRef]
- Gao, P.; Song, Y.; Hao, M.J.; Zhu, A.N.; Yang, H.W.; Yang, S.X. An effective and magnetic Fe2O3-ZrO2 catalyst for phenol degradation under neutral pH in the heterogeneous Fenton-like reaction. Sep. Purif. Technol. 2018, 201, 238–243. [Google Scholar] [CrossRef]
- Gao, Q.X.; Wang, X.F.; Di, J.L.; Wu, X.C.; Tao, Y.R. Enhanced catalytic activity of α-Fe2O3 nanorods enclosed with {110} and {001} planes for methane combustion and CO oxidation. Catal. Sci. Technol. 2011, 1, 574–577. [Google Scholar] [CrossRef]
- Chen, L.Q.; Yang, X.F.; Chen, J.; Wu, J.H.; Zhan, H.Q.; Liang, C.L.; Wu, M.M. Continuous shape- and spectroscopy-tuning of hematite nanocrystals. Inorg. Chem. 2010, 49, 8411–8420. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, S.L.; Pan, J.; Sun, L.L.; Zhou, J.; Dai, Z.G.; Xiao, X.H.; Zhang, H.B.; Jiang, C.Z. Metal ion-mediated synthesis and shape-dependent magnetic properties of single-crystalline α-Fe2O3 nanoparticles. CrystEngComm 2014, 16, 5566–5572. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Francés, A.; King, D.M.; Liang, X.H.; Branch, B.A.; Cavanagh, A.S.; George, S.M.; Weimer, A.W. Atomic layer deposition of iron(III) oxide on zirconia nanoparticles in a fluidized bed reactor using ferrocene and oxygen. Thin Solid Films 2009, 517, 1874–1879. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J.L. Degradation of sulfamethazine antibiotics using Fe3O4–Mn3O4 nanocomposite as a Fenton-like catalyst. J. Chem. Technol. Biotechnol. 2017, 92, 874–883. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yin, P.; Zhu, X.; OuYang, C.Z.; Xie, Y. Synthesis of hematite (alpha-Fe2O3) nanorods: Diameter-size and shape effects on their applications in magnetism, lithium ion battery, and gas sensors. J. Phys. Chem. B 2006, 110, 17806–17812. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Aschengrau, D.; Diamant, Y.; Rabani, J. Photolysis of aqueous H2O2: Quantum yield and applications for polychromatic UV actinometry in photoreactors. Environ. Sci. Technol. 2007, 41, 7486–7490. [Google Scholar] [CrossRef] [PubMed]


| Shape | dFe [nm] | Surface Area [m2/g] | ωFe a [wt.%] | SDIAs b [Fe/nm2] | τ Fe2+ c [at.%] | Density Site d [Fe2+/nm2] |
|---|---|---|---|---|---|---|
| HZNPs | 47 | 107 | 0.21 | 21.10 | 0.28 | 5.91 |
| HZNRs | 66 | 97 | 0.26 | 28.81 | 0.18 | 5.18 |
| HZNCs | 75 | 66 | 0.25 | 40.72 | 0.06 | 2.44 |
| HZNTs | 35 | 193 | 0.19 | 10.03 | 0.09 | 0.90 |
| Sample | AH2O2a [mmol/L] | A’H2O2b [mg/m2] | BH2O2c [%] | kH2O2d [min−1] | kH2O2/ QH2O2 [g/(mg·min)] |
|---|---|---|---|---|---|
| HZNPs | 0.67 | 0.43 | 80.1 | 6.70 × 10−3 | 1.47 × 10−4 |
| HZNRs | 0.36 | 0.25 | 49.8 | 3.00 × 10−3 | 1.23 × 10−4 |
| HZNCs | 0.21 | 0.25 | 37.2 | 2.00 × 10−3 | 1.23 × 10−4 |
| HZNTs | 0.24 | 0.07 | 23.5 | 1.20 × 10−3 | 8.40 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Hao, M.; He, Y.; Song, Y.; Yang, S. Effect of Fe2O3–ZrO2 Catalyst Morphology on Sulfamethazine Degradation in the Fenton-Like Reaction. Catalysts 2019, 9, 85. https://doi.org/10.3390/catal9010085
Gao P, Hao M, He Y, Song Y, Yang S. Effect of Fe2O3–ZrO2 Catalyst Morphology on Sulfamethazine Degradation in the Fenton-Like Reaction. Catalysts. 2019; 9(1):85. https://doi.org/10.3390/catal9010085
Chicago/Turabian StyleGao, Pan, Mengjie Hao, Yue He, Yuan Song, and Shaoxia Yang. 2019. "Effect of Fe2O3–ZrO2 Catalyst Morphology on Sulfamethazine Degradation in the Fenton-Like Reaction" Catalysts 9, no. 1: 85. https://doi.org/10.3390/catal9010085
APA StyleGao, P., Hao, M., He, Y., Song, Y., & Yang, S. (2019). Effect of Fe2O3–ZrO2 Catalyst Morphology on Sulfamethazine Degradation in the Fenton-Like Reaction. Catalysts, 9(1), 85. https://doi.org/10.3390/catal9010085