The Role of Surface Texture on the Photocatalytic H2 Production on TiO2
Abstract
1. Introduction
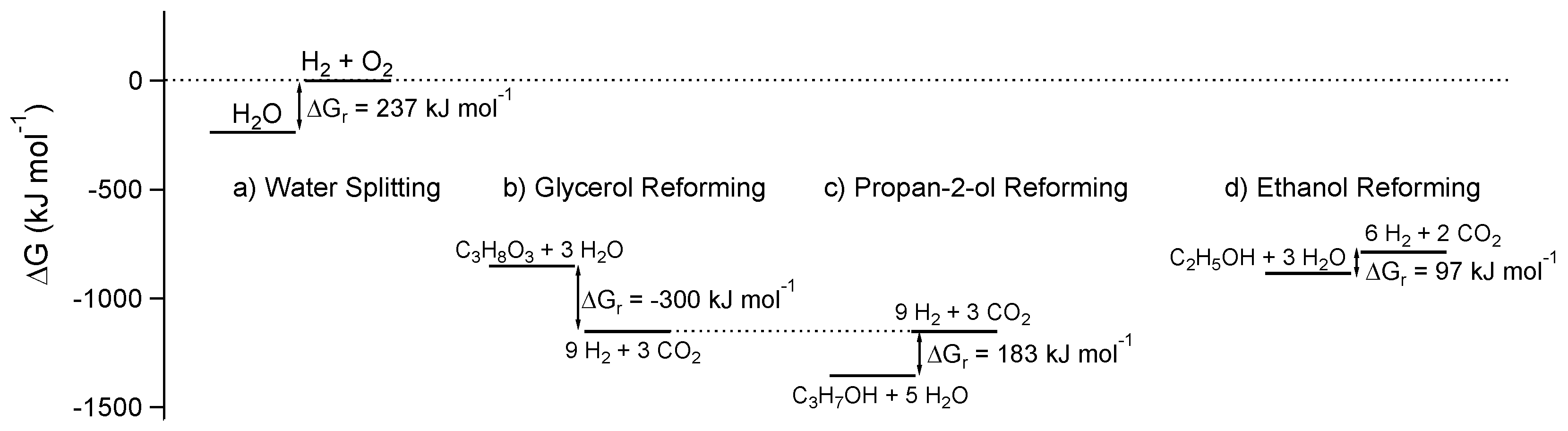
1.1. Semantics and Thermodynamics
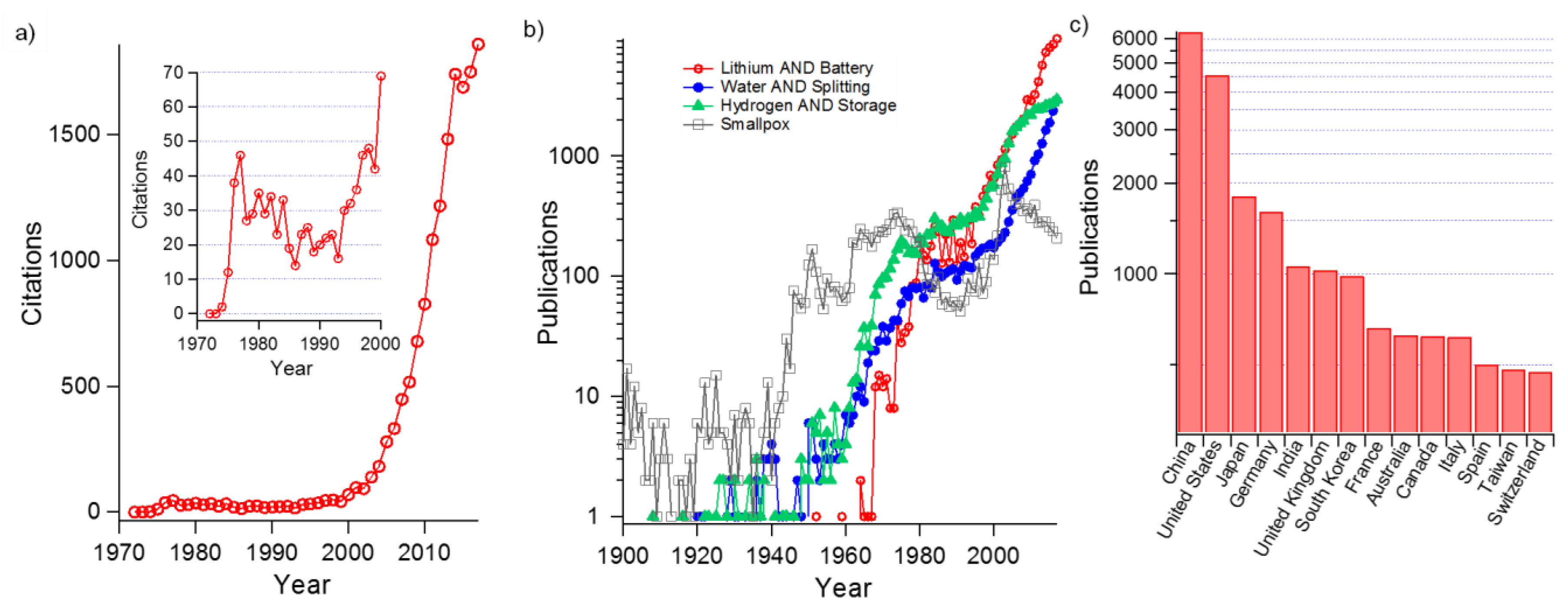
1.2. History and Developments
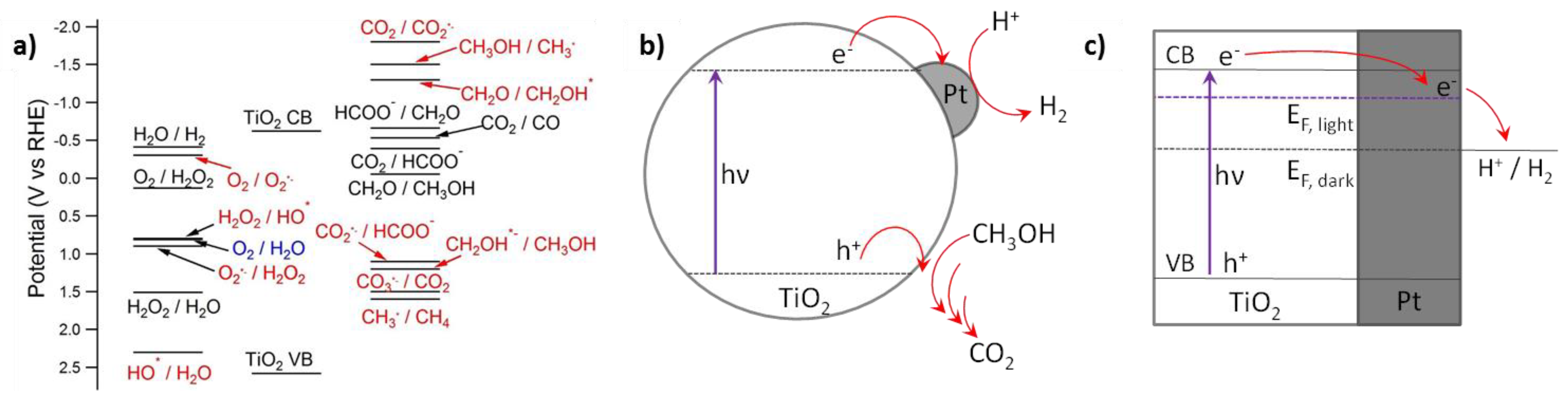
1.3. Mechanistic Considerations
2. Shape Controlled TiO2 Structures
3. Photocatalytic Hydrogen Production from Anatase TiO2
3.1. Effect of Crystal Shape and Structure
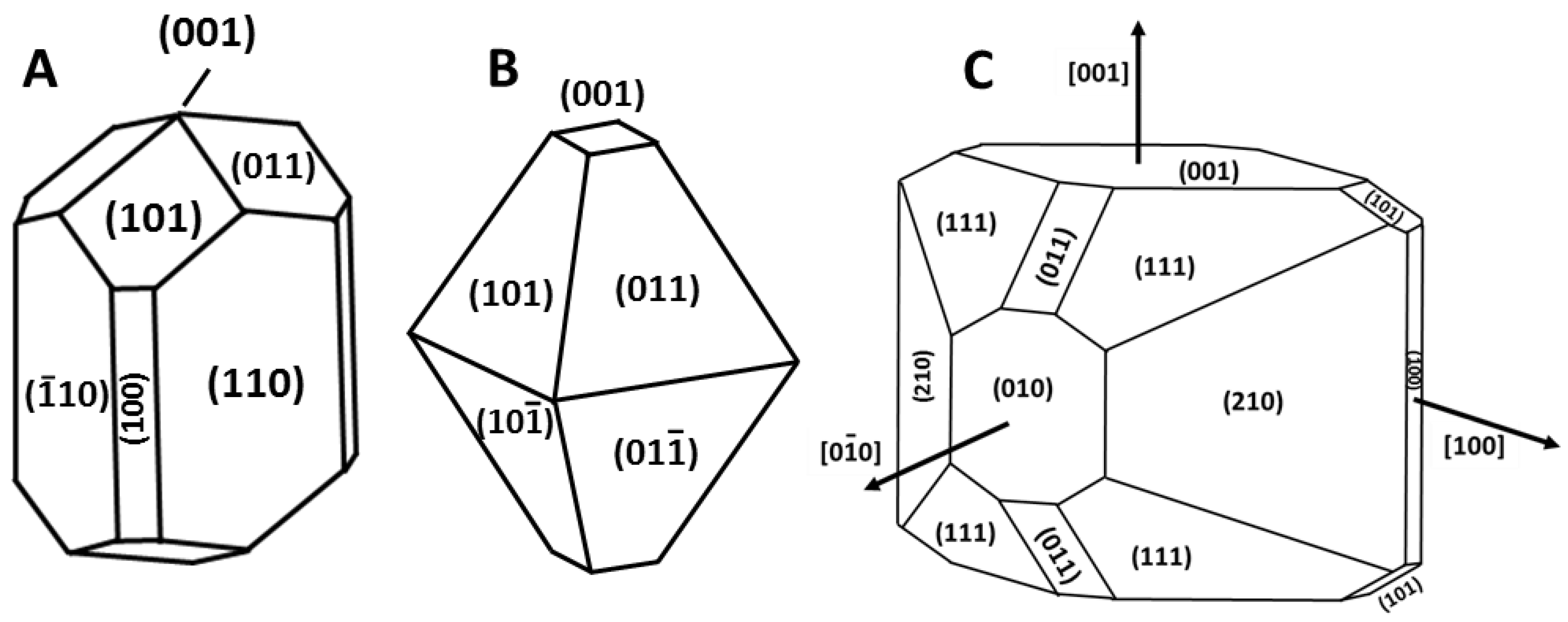
3.2. Effect of Exposed Surfaces
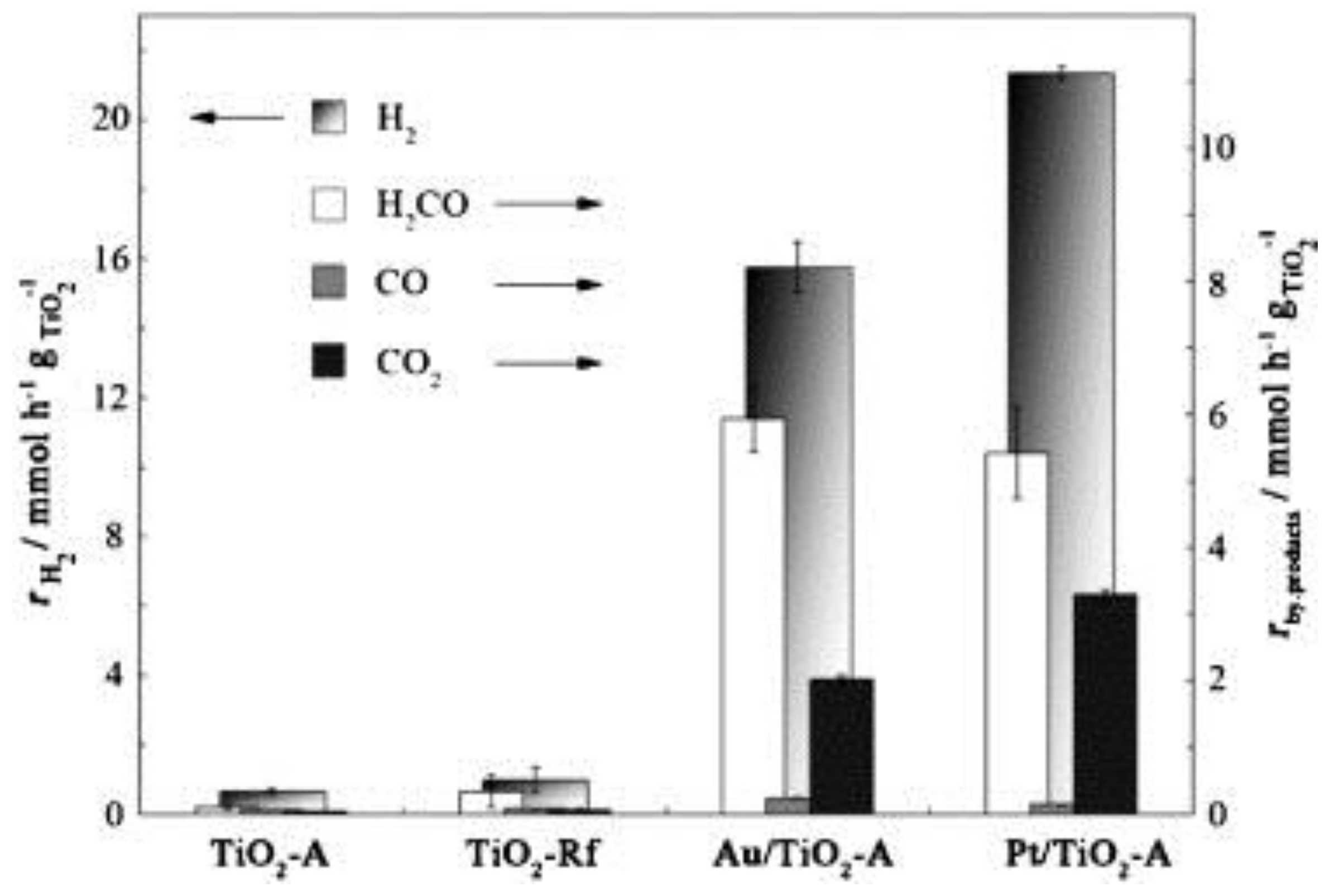
3.3. Effect of the Cocatalyst
3.4. Effect of the Sacrificial Agent
- (1)
- Ethanol, which is produced in a large number of fermentation processes of second-generation biomasses, as well as by the transformation of cellulose and lignocellulose [152]. The mechanism of reaction for the ethanol photoreforming was investigated on Pt/TiO2 catalyst. The mechanism proposed followed the scheme described above where protons are reduced by electrons cumulating into Pt particles, while the chemisorbed or physisorbed ethanol is stepwise oxidized by holes [153]. The stepwise oxidation of the substrate gives predominantly acetaldehyde, acetic acid and CO2, but also compounds with larger number of carbon atoms, such as acetone and 2-butenal. 2-butenal (crotonaldehyde) can be formed by the aldol condensation of two acetaldehyde molecules or through the formation of the ∙CH3CHO radicals. In a similar way a possible path for the production of acetone is the reaction of acetaldehyde (or acetic acid) with ∙CH3 radicals [154]. The accumulation of byproducts can give a partial poisoning of the catalyst with its progressive deactivation [155].
- (2)
- Glycerol, which is the main byproduct of the biodiesel industry, is alternatively produced through the sorbitol hydrogenolysis or the fermentation of glucose [156,157]. Considering that (i) the total reforming of one molecule of glycerol gives 7 molecules of H2 and (ii) the raw glycerol obtained as byproduct of the biodiesel industry is a complex mixture—containing methanol, water, inorganic salts, free fatty acids, triglycerides, and methyl esters—that requires expensive treatments to be purified, the energetic valorization of glycerol is a very promising path. The mechanism of transformation of glycerol on bare TiO2 was investigated by Minero et al. [140] Glyceraldehyde (GAD), dihydroxyacetone (DHA), formaldehyde (FORM) and glycolaldehyde (GLY) were identified as the main byproducts obtained through two different reaction paths. GAD and DHA are the products formed from the OH radical mediated oxidation, while FORM and GLY are produced through a direct electron transfer (hole transfer from TiO2 to the chemisorbed glycerol). Interestingly, the ratio between the produced byproducts is strongly affected by the surface properties of the catalyst. The direct hole transfer is favored on catalysts with more defective surfaces able to strongly adsorb the substrate on the more defective (and more reactive) sites.
- (3)
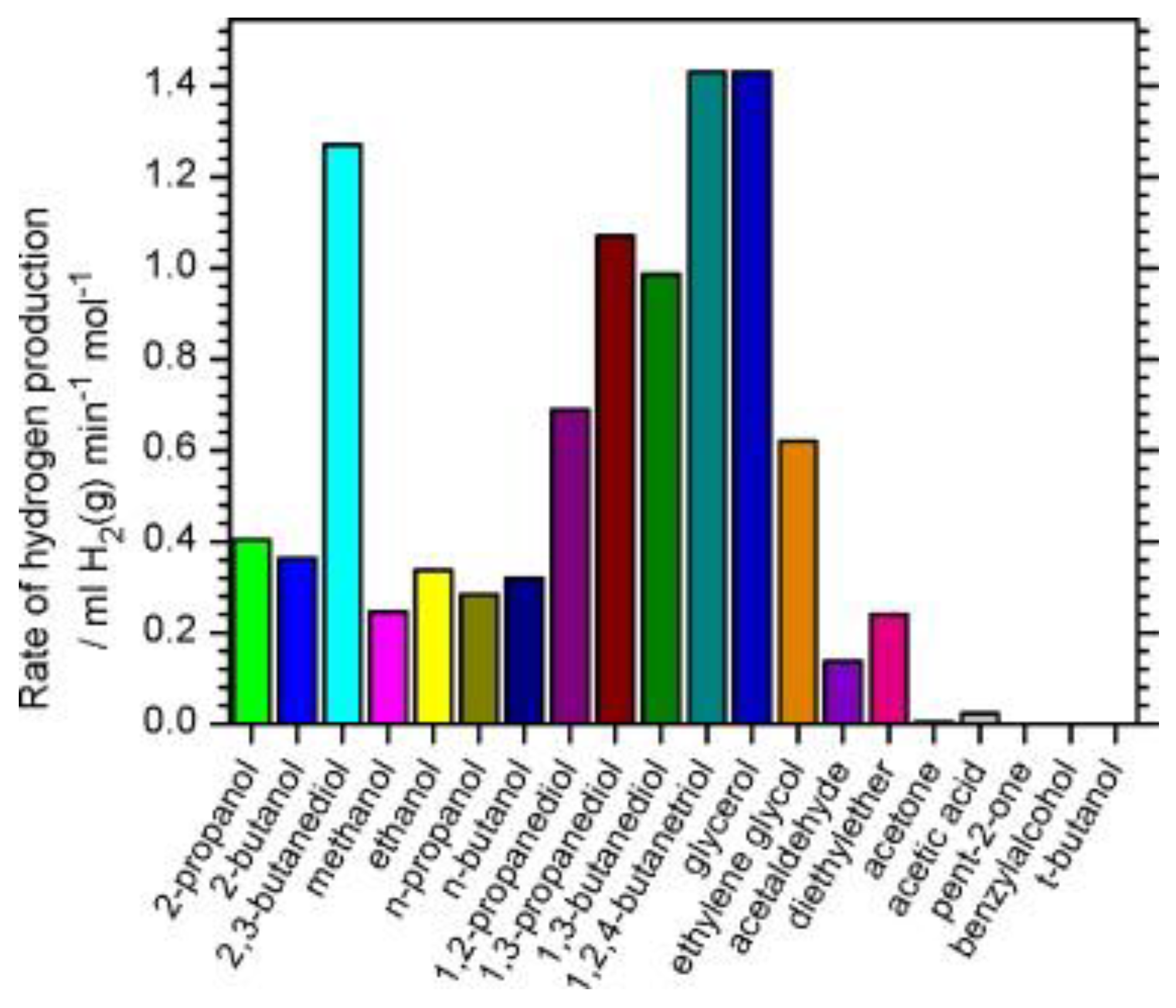
- Glucose, and other simple or complex sugar, which are mainly obtained from the degradation of cellulose materials [158,159]. The photoreforming of sugars, starches and cellulose was demonstrated for the first time on the ternary photocatalyst RuO2/TiO2/Pt [155]. As reported above, these substrates are ideal candidates to promote an efficient photoreforming because of the presence in the sugar molecules of numerous hydroxyl groups with hydrogen atom in α position. The more complex the carbohydrates, the lower is the efficiency. Fu and coworkers investigated systematically the glucose photoreforming under irradiated TiO2 decorated with different metal cocatalysts in the absence of oxygen, and observed the following reaction rate: Pd > Pt > Au ≈ Rh > Ag ≈ Ru. The presence of O2—as expected—and the decrement of the pH inhibited the hydrogen production [159]. Recently, Kennedy et al. compared the hydrogen production with C2-C5 polyols, cyclic alcohols and mono and di-saccharides under irradiated Pd-TiO2. For the compounds in the first class the hydrogen evolution rate is directly related to the number of OH groups and the availability of hydrogen atoms in α position to the hydroxyl. This rule is not followed by the cyclic alcohols and especially sugar (glucose, fructose and sucrose). For the reforming of cyclic alcohols it was also observed the dehydrogenation and decarbonylation of α CC bond [107].
4. Environmental Implications
5. Conclusions
- TiO2 crystal morphology:
- Structuration. The crystal shape and size can be modulated in order to maximize the light absorption and, therefore, the charge carrier density under irradiation. The same effect can be achieved with photonic crystal structures, which can increase the performance of TiO2, as discussed in the Section 3.1.
- Exposed Facets. Besides light absorption, morphology can also decrease the recombination of the charge carriers, accelerating their interfacial transfer. This was highlighted by several works, demonstrating the different behavior of the exposed facets for TiO2 anatase and rutile. In the case of hydrogen evolution on anatase, the surface {101} has a prominent role due to his reductive nature. In the presence of a cocatalyst as Pt, the higher is the amount of the {101} surface the higher is the rate of H2 photoproduction. Conversely, on pristine TiO2 surface, a synergistic effect results between the different surfaces of anatase crystals, indicating the formation of a “surface heterojunction” that increases the efficiency of nanoparticles exposing considerable amount of oxidative surface.
- Cocatalyst. The role of the cocatalyst is crucial for improving the TiO2 activity in the H2 photoproduction. The common characteristics of a good cocatalyst in HER are the ability to drain photoelectrons from the conduction band and then decrease the required overpotential for reducing the proton. Noble metals are still in a predominant position, and especially Pt shows the best activity, followed by Pd, while Au and Ag perform relatively worse. Other metals, such as Rh, Ni, Mn, Ru, Cr and Co, were recently proposed. The emergence of cheaper alternatives, such as Cu and carbonaceous materials (graphene, C3N4, etc.) could substantially decrease the costs of the H2 production, especially in the prospect of large-scale production.
- Substrates and Sacrificial Agents: usually, the role of the hole scavenger goes into the background compared to the reductive part in photocatalytic reforming. However, the sacrificial agent largely affects the H2 photoproduction. While a wide range of alcohols (methanol, ethanol, propanol, etc.) can be exploited mainly for research purposes, to better understand the mechanisms involved in the process, the coupling with biomass-derived substrates, such as glycerol, glucose and sugars, or recalcitrant pollutants, could be a useful way for producing H2 in a greener manner and to fully exploit the positive valence band potential of TiO2 and other wide band gap semiconductors.
Author Contributions
Funding
Conflicts of Interest
References
- Smil, V. Energy Transitions: Global and National Perspectives, 2nd ed.; ABC-CLIO: Santa Barbara, CA, USA, 2017. [Google Scholar]
- Raupach, M.R.; Davis, S.J.; Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Ciais, P.; Friedlingstein, P.; Jotzo, F.; van Vuuren, D.P.; Le Quéré, C. Sharing a quota on cumulative carbon emissions. Nat. Clim. Chang. 2014, 4, 873. [Google Scholar] [CrossRef]
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.B.; Frieler, K.; Knutti, R.; Frame, D.J.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 °C. Nature 2009, 458, 1158. [Google Scholar] [CrossRef] [PubMed]
- Yen Kheng, T. Review of Energy Harvesting Technologies for Sustainable WSN. In Sustainable Wireless Sensor Networks; Sanjib Kumar Panda, E.D.W.S.E.D.Y.K.T., Ed.; Charpter 2; IntechOpen: Rijeka, Croatia, 2010. [Google Scholar]
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M. Photocatalytic Hydrogen Production and Oxygenate Photoreforming. Catal. Lett. 2012, 142, 923–929. [Google Scholar] [CrossRef]
- Bockris, J. Modern Electrochemistry 1, 2A, and 2B; Springer: New York, NY, USA, 2007; p. 2158. [Google Scholar]
- Hammes, G.G.; Hammes-Schiffer, S. Physical Chemistry for the Biological Sciences, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Noufi, R.N.; Kohl, P.A.; Bard, A.J. Semiconductor Electrodes XV. Photoelectrochemical Cells with Mixed Polycrystalline n-Type CdS - CdSe Electrodes. J. Electrochem. Soc. 1978, 125, 375–379. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Rush, J.D. Reduction potential of the carbon dioxide/carbon dioxide radical anion: A comparison with other C1 radicals. J. Phys. Chem. 1987, 91, 4429–4430. [Google Scholar] [CrossRef]
- Wardman, P. Reduction Potentials of One-Electron Couples Involving Free Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Green Emission to Probe Photoinduced Charging Events in ZnO−Au Nanoparticles. Charge Distribution and Fermi-Level Equilibration. J. Phys. Chem. B 2003, 107, 7479–7485. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Coehn, A. Studien über photochemische Gleichgewichte. IV. Das Lichtgleichgewicht Knallgas-Wasserdampf. Berichte der Deutschen Chemischen Gesellschaft 1910, 43, 880–884. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K.I.; Kikuchi, S.I. Photosensitized Electrolytic Oxidation on Semiconducting n-Type TiO2 Electrode. J. Soc. Chem. Ind. Jpn. 1969, 72, 108–113. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Evidence of Photosensitized Electrolytic Oxidation on TiO2 Electrode from pH Change of Electrolyte Solution. J. Soc. Chem. Ind. Jpn. 1971, 74, 355–358. [Google Scholar] [CrossRef]
- Fujishima, A.; Sugiyama, E.; Honda, K. Photosensitized Electrolytic Oxidation of Iodide Ions on Cadmium Sulfide Single Crystal Electrode. Bull. Chem. Soc. Jpn. 1971, 44, 304. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K.J. Seisan Kenkyu; Institute of Industrial Science, University of Tokyo: Tokyo, Japan, 1970; Volume 22, p. 478. [Google Scholar]
- Available online: https://www.scopus.com/record/pubmetrics.uri?eid=2-s2.0-35348875044&origin=recordpage (accessed on 25 October 2018).
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Frank, S.N.; Bard, A.J. Semiconductor electrodes. 12. Photoassisted oxidations and photoelectrosynthesis at polycrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1977, 99, 4667–4675. [Google Scholar] [CrossRef]
- Tooru, I.; Tadashi, W.; Akira, F.; Kenichi, H. Competitive photosensitized oxidation at tio2 photoanode. Chem. Lett. 1977, 6, 1073–1076. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Photoelectrosynthesis of ethane from acetate ion at an n-type titanium dioxide electrode. The photo-Kolbe reaction. J. Am. Chem. Soc. 1977, 99, 7729–7731. [Google Scholar] [CrossRef]
- Dutoit, E.C.; Cardon, F.; Gomes, W.P. Electrochemical Properties of the Semiconducting TiO2 (Rutile) Single Crystal Electrode. Berichte Bunsenges. Phys. Chem. 1976, 80, 475–481. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Grätzel, M. Cyclic Cleavage of Water into H2 and O2 by Visible Light with Coupled Redox Catalysts. Angew. Chem. Int. Ed. Engl. 1979, 18, 701–702. [Google Scholar] [CrossRef]
- Borgarello, E.; Kiwi, J.; Pelizzetti, E.; Visca, M.; Grätzel, M. Photochemical cleavage of water by photocatalysis. Nature 1981, 289, 158. [Google Scholar] [CrossRef]
- Graetzel, M. Artificial photosynthesis: Water cleavage into hydrogen and oxygen by visible light. Accounts Chem. Res. 1981, 14, 376–384. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Guth, T.D. Photocatalytic reactions. 1. Photolysis of water and photoreduction of nitrogen on titanium dioxide. J. Am. Chem. Soc. 1977, 99, 7189–7193. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (Version 45). Prog. Photovolt. Res. Appl. 2014, 23, 1–9. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at titanium dioxide powder. J. Am. Chem. Soc. 1977, 99, 303–304. [Google Scholar] [CrossRef]
- Minero, C.; Lorenzi, E.; Pramauro, E.; Pelizzetti, E. Dioxygen evolution from inorganic systems. Water oxidation mediated by RuO2 and TiO2-RuO2 Colloids. Inorganica Chim. Acta 1984, 91, 301–305. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.-a.; Onishi, H. Electron- and Hole-Capture Reactions on Pt/TiO2 Photocatalyst Exposed to Methanol Vapor Studied with Time-Resolved Infrared Absorption Spectroscopy. J. Phys. Chem. B 2002, 106, 9122–9125. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.-A.; Onishi, H. Effects of accumulated electrons on the decay kinetics of photogenerated electrons in Pt/TiO2 photocatalyst studied by time-resolved infrared absorption spectroscopy. J. Photochem. Photobiol. A Chem. 2003, 160, 33–36. [Google Scholar] [CrossRef]
- Baba, R.; Nakabayashi, S.; Fujishima, A.; Honda, K. Investigation of the mechanism of hydrogen evolution during photocatalytic water decomposition on metal-loaded semiconductor powders. J. Phys. Chem. 1985, 89, 1902–1905. [Google Scholar] [CrossRef]
- Yamakata, A.; Ishibashi, T.-a.; Onishi, H. Effects of Water Addition on the Methanol Oxidation on Pt/TiO2 Photocatalyst Studied by Time-Resolved Infrared Absorption Spectroscopy. J. Phys. Chem. B 2003, 107, 9820–9823. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Morris, J.R. Surface chemistry of Au/TiO2: Thermally and photolytically activated reactions. Surf. Sci. Rep. 2016, 71, 77–271. [Google Scholar] [CrossRef]
- Yang, X.; Tamai, N. How fast is interfacial hole transfer? In situ monitoring of carrier dynamics in anatase TiO2 nanoparticles by femtosecond laser spectroscopy. Phys. Chem. Chem. Phys. 2001, 3, 3393–3398. [Google Scholar] [CrossRef]
- Chen, T.; Feng, Z.; Wu, G.; Shi, J.; Ma, G.; Ying, P.; Li, C. Mechanistic Studies of Photocatalytic Reaction of Methanol for Hydrogen Production on Pt/TiO2 by in situ Fourier Transform IR and Time-Resolved IR Spectroscopy. J. Phys. Chem. C 2007, 111, 8005–8014. [Google Scholar] [CrossRef]
- Iwata, K.; Takaya, T.; Hamaguchi, H.-O.; Yamakata, A.; Ishibashi, T.-A.; Onishi, H.; Kuroda, H. Carrier Dynamics in TiO2 and Pt/TiO2 Powders Observed by Femtosecond Time-Resolved Near-Infrared Spectroscopy at a Spectral Region of 0.9−1.5 μm with the Direct Absorption Method. J. Phys. Chem. B 2004, 108, 20233–20239. [Google Scholar] [CrossRef]
- Fang, Y.-H.; Wei, G.-F.; Liu, Z.-P. Catalytic Role of Minority Species and Minority Sites for Electrochemical Hydrogen Evolution on Metals: Surface Charging, Coverage, and Tafel Kinetics. J. Phys. Chem. C 2013, 117, 7669–7680. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar] [CrossRef]
- Zeradjanin, A.R.; Grote, J.-P.; Polymeros, G.; Mayrhofer, K.J.J. A Critical Review on Hydrogen Evolution Electrocatalysis: Re-exploring the Volcano-relationship. Electroanalysis 2016, 28, 2256–2269. [Google Scholar] [CrossRef]
- Santos, E.; Quaino, P.; Schmickler, W. Theory of electrocatalysis: Hydrogen evolution and more. Phys. Chem. Chem. Phys. 2012, 14, 11224–11233. [Google Scholar] [CrossRef]
- Calza, P.; Minella, M.; Demarchis, L.; Sordello, F.; Minero, C. Photocatalytic rate dependence on light absorption properties of different TiO2 specimens. Catal. Today 2018. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous Synthesis of TiO2 Nanocrystals Using TiF4 to Engineer Morphology, Oxygen Vacancy Concentration, and Photocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Habas, S.E.; Lee, H.; Radmilovic, V.; Somorjai, G.A.; Yang, P. Shaping binary metal nanocrystals through epitaxial seeded growth. Nat. Mater. 2007, 6, 692–697. [Google Scholar] [CrossRef]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Kipp, T.; Cingolani, R.; Cozzoli, P.D. Nonhydrolytic synthesis of high-quality anisotropically shaped brookite TiO2 nanocrystals. J. Am. Chem. Soc. 2008, 130, 11223–11233. [Google Scholar] [CrossRef] [PubMed]
- Tsung, C.K.; Kuhn, J.N.; Huang, W.; Aliaga, C.; Hung, L.I.; Somorjai, G.A.; Yang, P. Sub-10 nm platinum nanocrystals with size and shape control: Catalytic study for ethylene and pyrrole hydrogenation. J. Am. Chem. Soc. 2009, 131, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Sinsermsuksakul, P.; Yang, P. Polyhedral silver nanocrystals with distinct scattering signatures. Angew. Chem. Int. Ed. Engl. 2006, 45, 4597–4601. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Yang, P. Semiconductor nanowires for energy conversion. Chem. Rev. 2010, 110, 527–546. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. Engl. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Lim, B.; Kobayashi, H.; Yu, T.; Wang, J.; Kim, M.J.; Li, Z.Y.; Rycenga, M.; Xia, Y. Synthesis of Pd-Au bimetallic nanocrystals via controlled overgrowth. J. Am. Chem. Soc. 2010, 132, 2506–2507. [Google Scholar] [CrossRef]
- Yin, Y.; Erdonmez, C.; Aloni, S.; Alivisatos, A.P. Faceting of nanocrystals during chemical transformation: From solid silver spheres to hollow gold octahedra. J. Am. Chem. Soc. 2006, 128, 12671–12673. [Google Scholar] [CrossRef]
- Feng, X.; Zhai, J.; Jiang, L. The fabrication and switchable superhydrophobicity of TiO2 nanorod films. Angew. Chem. Int. Ed. Engl. 2005, 44, 5115–5118. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Gong, X.Q.; Selloni, A.; Batzill, M.; Diebold, U. Steps on anatase TiO2(101). Nat Mater 2006, 5, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Setvin, M.; Hulva, J.; Wang, H.H.; Simschitz, T.; Schmid, M.; Parkinson, G.S.; Di Valentin, C.; Selloni, A.; Diebold, U. Formaldehyde Adsorption on the Anatase TiO2(101) Surface: Experimental and Theoretical Investigation. J. Phys. Chem. C 2017, 121, 8914–8922. [Google Scholar] [CrossRef]
- Selcuk, S.; Selloni, A. Facet-dependent trapping and dynamics of excess electrons at anatase TiO2 surfaces and aqueous interfaces. Nat. Mater. 2016, 15, 1107. [Google Scholar] [CrossRef] [PubMed]
- Setvin, M.; Aschauer, U.; Hulva, J.; Simschitz, T.; Daniel, B.; Schmid, M.; Selloni, A.; Diebold, U. Following the Reduction of Oxygen on TiO2 Anatase (101) Step by Step. J. Am. Chem. Soc. 2016, 138, 9565–9571. [Google Scholar] [CrossRef] [PubMed]
- Setvin, M.; Buchholz, M.; Hou, W.Y.; Zhang, C.; Stoger, B.; Hulva, J.; Simschitz, T.; Shi, X.; Pavelec, J.; Parkinson, G.S.; et al. A Multitechnique Study of CO Adsorption on the TiO2 Anatase (101) Surface. J. Phys. Chem. C 2015, 119, 21044–21052. [Google Scholar] [CrossRef]
- Dulub, O.; Batzilln, M.; Solovev, S.; Loginova, E.; Alchagirov, A.; Madey, T.E.; Diebold, U. Electron-induced oxygen desorption from the TiO2(011)-2x1 surface leads to self-organized vacancies. Science 2007, 317, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-C.; Chu, L.-N.; Gong, X.-Q.; Diebold, U. Hydrogen bonding controls the dynamics of catechol adsorbed on a TiO2(110) surface. J. Mater. Chem. 2010, 20, 10319. [Google Scholar] [CrossRef]
- Kavan, L.; Gratzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and photoelectrochemical investigation of single-crystal anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Jang, E.S.; Won, J.H.; Hwang, S.J.; Choy, J.H. Fine Tuning of the Face Orientation of ZnO Crystals to Optimize Their Photocatalytic Activity. Adv. Mater. 2006, 18, 3309–3312. [Google Scholar] [CrossRef]
- Mino, L.; Pellegrino, F.; Rades, S.; Radnik, J.; Hodoroaba, V.-D.; Spoto, G.; Maurino, V.; Martra, G. Beyond Shape Engineering of TiO2 Nanoparticles: Post-Synthesis Treatment Dependence of Surface Hydration, Hydroxylation, Lewis Acidity and Photocatalytic Activity of TiO2 Anatase Nanoparticles with Dominant {001} or {101} Facets. ACS Appl. Nano Mater. 2018. [Google Scholar] [CrossRef]
- Gong, X.Q.; Selloni, A. Reactivity of anatase TiO2 nanoparticles: The role of the minority (001) surface. J. Phys. Chem. B 2005, 109, 19560–19562. [Google Scholar] [CrossRef] [PubMed]
- Vittadini, A.; Selloni, A.; Rotzinger, F.P.; Gratzel, M. Structure and energetics of water adsorbed at TiO2 anatase (101) and (001) surfaces. Phys. Rev. Lett. 1998, 81, 2954–2957. [Google Scholar] [CrossRef]
- Selloni, A. Crystal growth—Anatase shows its reactive side. Nat. Mater. 2008, 7, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Vittadini, A.; Casarin, M.; Selloni, A. Chemistry of and on TiO2-anatase surfaces by DFT calculations: A partial review. Theor. Chem. Accounts 2007, 117, 663–671. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.; Cheng, H.M. On the true photoreactivity order of {001}, {010}, and {101} facets of anatase TiO2 crystals. Angew. Chem. Int. Ed. Engl. 2011, 50, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. Zur Frage der Geschwindigkeit des Wachstums und der Auflösung der Krystallflächen. Z. Kryst. Miner. 1901, 449–530. [Google Scholar]
- Ramamoorthy, M.; Vanderbilt, D.; King-Smith, R.D. First-principles calculations of the energetics of stoichiometric TiO2 surfaces. Phys. Rev. B Condens Matter 1994, 49, 16721–16727. [Google Scholar] [CrossRef]
- Gong, X.-Q.; Selloni, A. First-principles study of the structures and energetics of stoichiometric brookite TiO2 surfaces. Phys. Rev. B 2007, 76. [Google Scholar] [CrossRef]
- Barnard, A.S.; Zapol, P.; Curtiss, L.A. Modeling the Morphology and Phase Stability of TiO2 Nanocrystals in Water. J. Chem. Theo. Comput. 2005, 1, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Van de Hulst, H.C. Light scattering by small particles. Q. J. R. Meteorol. Soc. 1958, 84, 198–199. [Google Scholar] [CrossRef]
- Mackowski, D.W.; Mishchenko, M.I. Calculation of the T matrix and the scattering matrix for ensembles of spheres. J. Opt. Soc. of Am. Opt. Image Sci. Vis. 1996, 13, 2266–2278. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, H.; Jool, J.B.; Kim, N.D.; Yi, J. Effect of TiO2 nanoparticle shape on hydrogen evolution via water splitting. J. Nanosci. Nanotechnol. 2011, 11, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Chen, Z.B.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X.L. Branched TiO2 Nanorods for Photoelectrochemical Hydrogen Production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Tang, Y.; Tay, Q.; Zhang, Y.; Malyi, O.I.; Wang, D.; Deng, J.; Lai, Y.; Zhou, H.; Chen, X.; et al. Understanding the Role of Nanostructures for Efficient Hydrogen Generation on Immobilized Photocatalysts. Adv. Energy Mater. 2013, 3, 1368–1380. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Johnson, S.G.; Winn, J.N.; Meade, R.D. Photonic Crystals Molding the Flow of Light, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Baba, T. Slow light in photonic crystals. Nat. Photonics 2008, 2, 465–473. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.-W.; Zhang, X.-Y.; Yuan, B.; Zhang, S. New mechanisms of slow light and their applications. Opt. Laser Technol. 2009, 41, 517–525. [Google Scholar] [CrossRef]
- Sordello, F.; Minero, C. Photocatalytic hydrogen production on Pt-loaded TiO2 inverse opals. Appl. Catal. B Environ. 2015, 163, 452–458. [Google Scholar] [CrossRef]
- Chen, J.I.L.; von Freymann, G.; Choi, S.Y.; Kitaev, V.; Ozin, G.A. Slow photons in the fast lane in chemistry. J. Mater. Chem. 2008, 18, 369–373. [Google Scholar] [CrossRef]
- Sordello, F.; Duca, C.; Maurino, V.; Minero, C. Photocatalytic metamaterials: TiO2 inverse opals. Chem. Commun. 2011, 47, 6147–6149. [Google Scholar] [CrossRef] [PubMed]
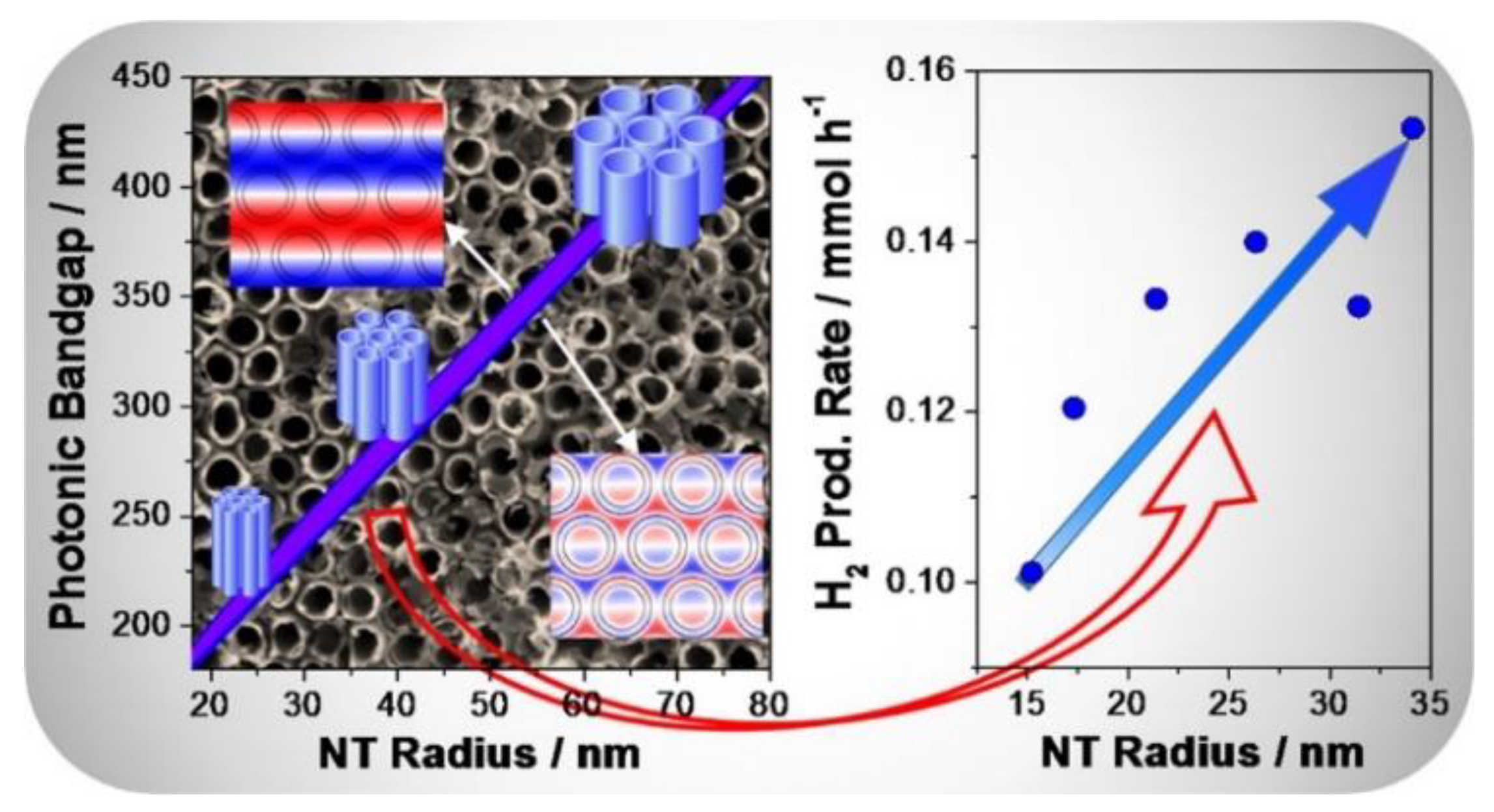
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the Photonic Crystal Properties of TiO2 Nanotube Arrays To Enhance Photocatalytic Hydrogen Production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
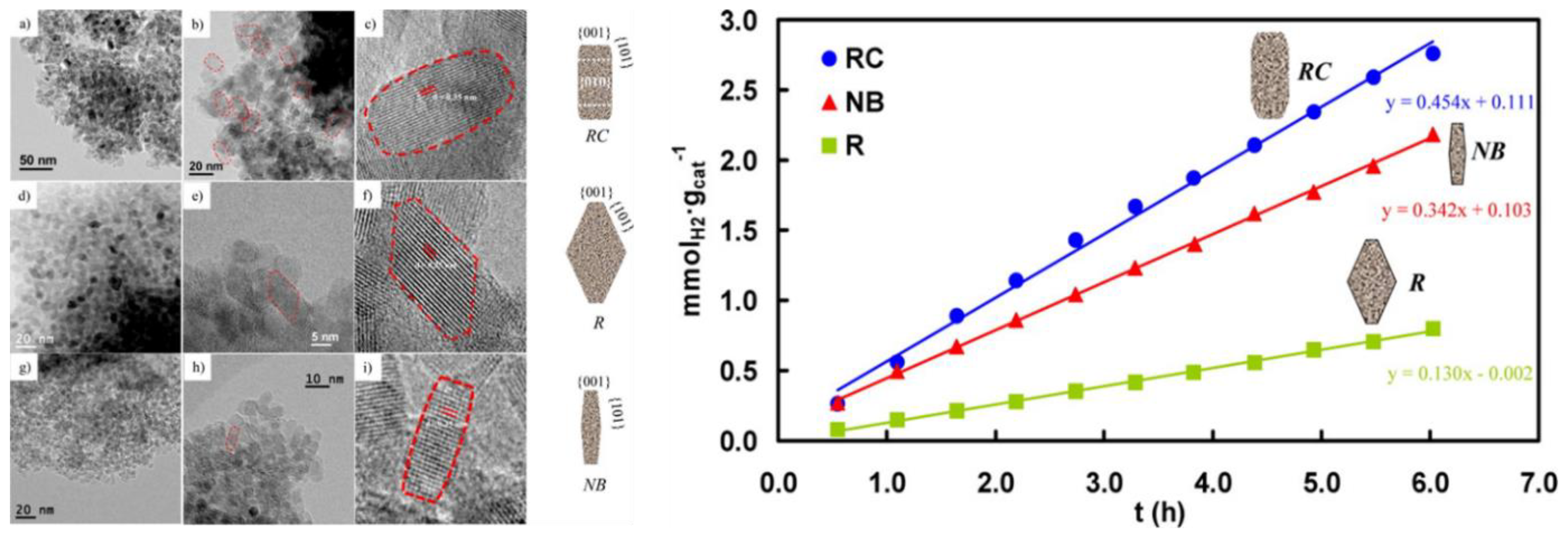
- D’Arienzo, M.; Dozzi, M.V.; Redaelli, M.; Di Credico, B.; Morazzoni, F.; Scotti, R.; Polizzi, S. Crystal Surfaces and Fate of Photogenerated Defects in Shape-Controlled Anatase Nanocrystals: Drawing Useful Relations to Improve the H2 Yield in Methanol Photosteam Reforming. J. Phys. Chem. C 2015, 119, 12385–12393. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Olds, D.; Peng, R.; Yu, L.; Foo, G.S.; Qian, S.; Keum, J.; Guiton, B.S.; Wu, Z.L.; Page, K. Quantitative Analysis of the Morphology of {101} and {001} Faceted Anatase TiO2 Nanocrystals and Its Implication on Photocatalytic Activity. Chem. Mater. 2017, 29, 5591–5604. [Google Scholar] [CrossRef]
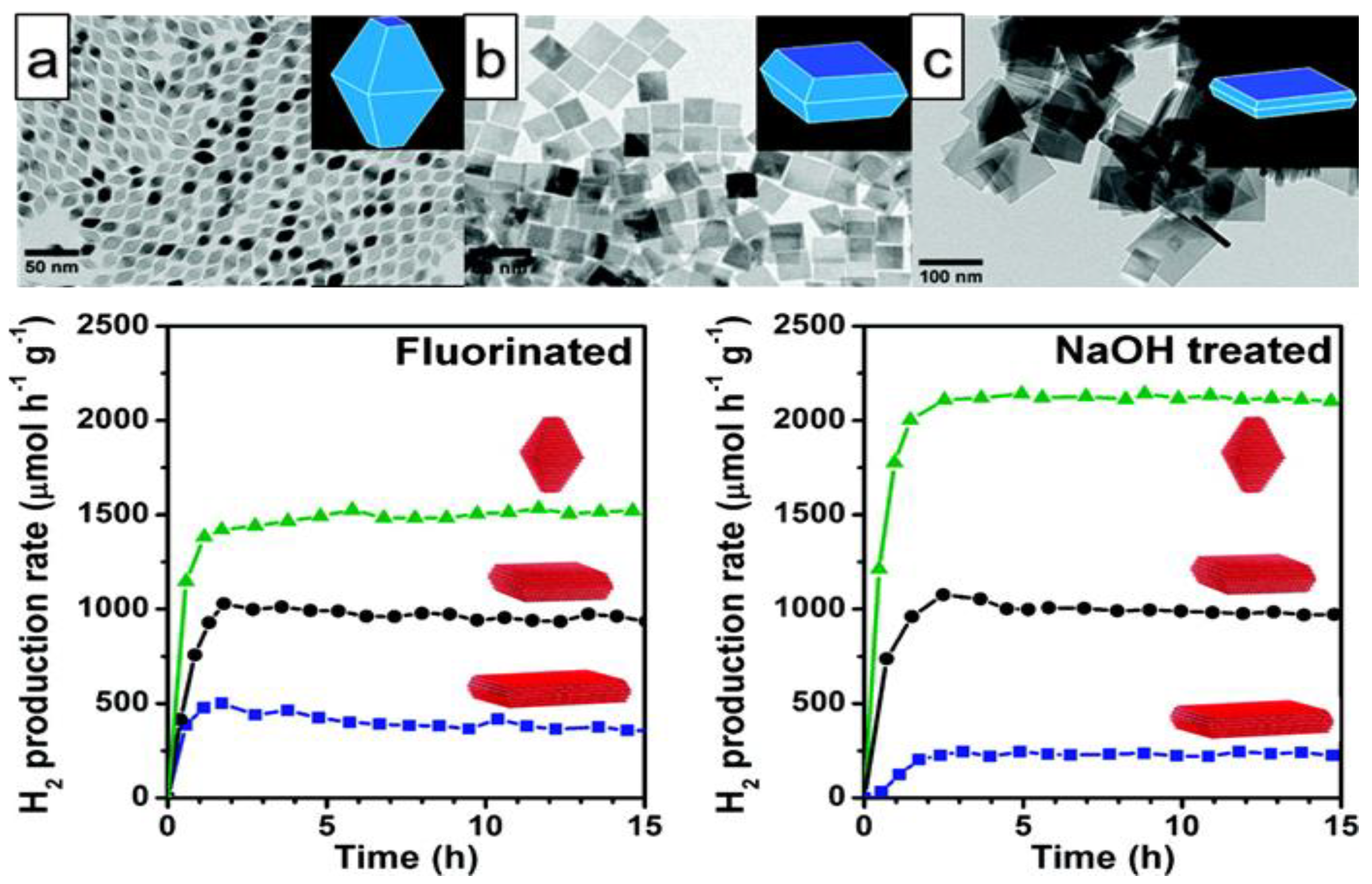
- Chiarello, G.L.; Dozzi, M.V.; Scavini, M.; Grunwaldt, J.D.; Selli, E. One step flame-made fluorinated Pt/TiO2 photocatalysts for hydrogen production. Appl. Catal. B Environ. 2014, 160–161, 144–151. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Amano, F.; Prieto-Mahaney, O.-O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral Single-Crystalline Particles of Anatase Titanium(IV) Oxide with High Photocatalytic Activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Selli, E.; Forni, L. Photocatalytic hydrogen production over flame spray pyrolysis-synthesised TiO2 and Au/TiO2. Appl. Catal. B Environ. 2008, 84, 332–339. [Google Scholar] [CrossRef]
- Naldoni, A.; D’Arienzo, M.; Altomare, M.; Marelli, M.; Scotti, R.; Morazzoni, F.; Selli, E.; Dal Santo, V. Pt and Au/TiO2 photocatalysts for methanol reforming: Role of metal nanoparticles in tuning charge trapping properties and photoefficiency. Appl. Catal. B Environ. 2013, 130–131, 239–248. [Google Scholar] [CrossRef]
- Jones, W.; Martin, D.J.; Caravaca, A.; Beale, A.M.; Bowker, M.; Maschmeyer, T.; Hartley, G.; Masters, A. A comparison of photocatalytic reforming reactions of methanol and triethanolamine with Pd supported on titania and graphitic carbon nitride. Appl. Catal. B Environ. 2017, 240, 373–379. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Kondarides, D.I. Efficient production of hydrogen by photo-induced reforming of glycerol at ambient conditions. Catal. Today 2009, 144, 75–80. [Google Scholar] [CrossRef]
- López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Montes, V.; Marinas, A.; Urbano, F.J.; Marinas, J.M.; Ilieva, L.; Tabakova, T.; Reid, F. A comparative study of hydrogen photocatalytic production from glycerol and propan-2-ol on M/TiO2 systems (M = Au, Pt, Pd). Catal. Today 2017, 280, 58–64. [Google Scholar] [CrossRef]
- Melchionna, M.; Beltram, A.; Montini, T.; Monai, M.; Nasi, L.; Fornasiero, P.; Prato, M. Highly efficient hydrogen production through ethanol photoreforming by a carbon nanocone/Pd@TiO2 hybrid catalyst. Chem. Commun. 2016, 52, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, J.J.; Fernández-González, R.; Díaz, L.; Pulido Melián, E.; Rodríguez, V.D.; Núñez, P. Effect of reaction temperature and sacrificial agent on the photocatalytic H2-production of Pt-TiO2. J. Alloys Compd. 2017, 721, 405–410. [Google Scholar] [CrossRef]
- López, C.R.; Melián, E.P.; Ortega Méndez, J.A.; Santiago, D.E.; Doña Rodríguez, J.M.; González Díaz, O. Comparative study of alcohols as sacrificial agents in H2 production by heterogeneous photocatalysis using Pt/TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2015, 312, 45–54. [Google Scholar] [CrossRef]
- Kennedy, J.; Bahruji, H.; Bowker, M.; Davies, P.R.; Bouleghlimat, E.; Issarapanacheewin, S. Hydrogen generation by photocatalytic reforming of potential biofuels: Polyols, cyclic alcohols, and saccharides. J. Photochem. Photobiol. A Chem. 2018, 356, 451–456. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Pedrono, F. New insights into the mechanism of photocatalytic reforming on Pd/TiO2. Appl. Catal. B Environ. 2011, 107, 205–209. [Google Scholar] [CrossRef]
- Taylor, S.; Mehta, M.; Samokhvalov, A. Production of hydrogen by glycerol photoreforming using binary nitrogen-metal-promoted N-M-TiO2 photocatalysts. Chemphyschem 2014, 15, 942–949. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Zeng, S.; Su, Y.; Hao, H. Enhanced H2 evolution from photocatalytic cellulose conversion based on graphitic carbon layers on TiO2/NiOx. Green Chem. 2018, 20, 3008–3013. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Chen, Y.; Cheng, Z.; Luo, X.; Mo, S.; Jia, L.; Shu, R.; Lin, P.; Yang, Z.; et al. Efficient hydrogen production from glycerol photoreforming over Ag2O TiO2 synthesized by a sol–gel method. Int. J. Hydrogen Energy 2017, 42, 17063–17074. [Google Scholar] [CrossRef]
- Berto, T.F.; Sanwald, K.E.; Eisenreich, W.; Gutiérrez, O.Y.; Lercher, J.A. Photoreforming of ethylene glycol over Rh/TiO2 and Rh/GaN:ZnO. J. Catal. 2016, 338, 68–81. [Google Scholar] [CrossRef]
- Sanwald, K.E.; Berto, T.F.; Eisenreich, W.; Gutiérrez, O.Y.; Lercher, J.A. Catalytic routes and oxidation mechanisms in photoreforming of polyols. J. Catal. 2016, 344, 806–816. [Google Scholar] [CrossRef]
- Caravaca, A.; Jones, W.; Hardacre, C.; Bowker, M. H2 production by the photocatalytic reforming of cellulose and raw biomass using Ni, Pd, Pt and Au on titania. Proc. Math. Phys. Eng. Sci. 2016, 472, 20160054. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Sakata, T. Photocatalytic decomposition of gaseous water over TiO2 and TiO2—RuO2 surfaces. Chem. Phys. Lett. 1980, 72, 87–89. [Google Scholar] [CrossRef]
- Nie, Y.-C.; Yu, F.; Wang, L.-C.; Xing, Q.-J.; Liu, X.; Pei, Y.; Zou, J.-P.; Dai, W.-L.; Li, Y.; Suib, S.L. Photocatalytic degradation of organic pollutants coupled with simultaneous photocatalytic H2 evolution over graphene quantum dots/Mn-N-TiO2 /g-C3N4 composite catalysts: Performance and mechanism. Appl. Catal. B Environ. 2018, 227, 312–321. [Google Scholar] [CrossRef]
- Yu, X.; Fan, X.; An, L.; Liu, G.; Li, Z.; Liu, J.; Hu, P. Mesocrystalline Ti3+TiO2 hybridized g-C3N4 for efficient visible-light photocatalysis. Carbon 2018, 128, 21–30. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.; Liu, C.; Gong, Y.; Wang, R.; He, B.; Wang, H. Rational Design and Fabrication of Noble-metal-free NixP Cocatalyst Embedded 3D N-TiO2/g-C3N4 Heterojunctions with Enhanced Photocatalytic Hydrogen Evolution. ChemCatChem 2018, 10, 3069–3077. [Google Scholar] [CrossRef]
- Yang, C.; Qin, J.; Xue, Z.; Ma, M.; Zhang, X.; Liu, R. Rational design of carbon-doped TiO2 modified g-C3N4 via in-situ heat treatment for drastically improved photocatalytic hydrogen with excellent photostability. Nano Energy 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Mao, J.; Li, K.; Zan, L. Graphitic carbon nitride (g-C3N4)-Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys. Chem. Chem. Phys. 2012, 14, 16745–16752. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Minella, M.; Sordello, F.; Minero, C. Photocatalytic process in TiO2/graphene hybrid materials. Evidence of charge separation by electron transfer from reduced graphene oxide to TiO2. Catal. Today 2017, 281, 29–37. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M.T. Advanced nanostructured photocatalysts based on reduced graphene oxide–TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Qianqian, Z.; Tang, B.; Guoxin, H. High photoactive and visible-light responsive graphene/titanate nanotubes photocatalysts: Preparation and characterization. J. Hazard. Mater. 2011, 198, 78–86. [Google Scholar] [CrossRef]
- Long, R.; English, N.J.; Prezhdo, O.V. Photo-induced Charge Separation across the Graphene–TiO2 Interface Is Faster than Energy Losses: A Time-Domain ab Initio Analysis. J. Am. Chem. Soc. 2012, 134, 14238–14248. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, R.; Lin, J.; Zhu, Y. Significant photocatalytic enhancement in methylene blue degradation of TiO2 photocatalysts via graphene-like carbon in situ hybridization. Appl. Catal. B Environ. 2010, 100, 179–183. [Google Scholar] [CrossRef]
- Minella, M.; Fabbri, D.; Calza, P.; Minero, C. Selected hybrid photocatalytic materials for the removal of drugs from water. Curr. Opin. Green Sustain. Chem. 2017, 6, 11–17. [Google Scholar] [CrossRef]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene Modified TiO2 Composite Photocatalysts: Mechanism, Progress and Perspective. Nano Mater. 2018, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, R.; Siciliano, A.; Clarizia, L.; Russo, D.; Di Somma, I.; Di Natale, F.; Guida, M.; Andreozzi, R.; Marotta, R. Sacrificial photocatalysis: Removal of nitrate and hydrogen production by nano-copper-loaded P25 titania. A kinetic and ecotoxicological assessment. Environ. Sci. Pollut. Res. Int. 2017, 24, 5898–5907. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Liu, S.; Gao, L.; Mao, L.; Fan, Z.; Shangguan, W.; Jiang, Z. Non-noble metal Cu as a cocatalyst on TiO2 nanorod for highly efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2018, 445, 527–534. [Google Scholar] [CrossRef]
- Segovia-Guzmán, M.O.; Román-Aguirre, M.; Verde-Gomez, J.Y.; Collins-Martínez, V.H.; Zaragoza-Galán, G.; Ramos-Sánchez, V.H. Green Cu2O/TiO2 heterojunction for glycerol photoreforming. Catal. Today 2018. [Google Scholar] [CrossRef]
- Ampelli, C.; Passalacqua, R.; Genovese, C.; Perathoner, S.; Centi, G.; Montini, T.; Gombac, V.; Fornasiero, P. Solar Energy and Biowaste Conversion into H2 on CuOx/TiO2 Nanocomposites. Chem. Eng. Trans. 2013, 35. [Google Scholar] [CrossRef]
- Praveen Kumar, D.; Shankar, M.V.; Kumari, M.M.; Sadanandam, G.; Srinivas, B.; Durgakumari, V. Nano-size effects on CuO/TiO2 catalysts for highly efficient H2 production under solar light irradiation. Chem. Commun. 2013, 49, 9443–9445. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ran, J. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364. [Google Scholar] [CrossRef]
- He, Z.; Fu, J.; Cheng, B.; Yu, J.; Cao, S. Cu2(OH)2CO3 clusters: Novel noble-metal-free cocatalysts for efficient photocatalytic hydrogen production from water splitting. Appl. Catal. B Environ. 2017, 205, 104–111. [Google Scholar] [CrossRef]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Bonomo, M.; Dini, D.; Decker, F. Electrochemical and Photoelectrochemical Properties of Nickel Oxide (NiO) With Nanostructured Morphology for Photoconversion Applications. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol. Ullmann’s Encycl. Ind. Chem. 2012. [Google Scholar] [CrossRef]
- Bowker, M.; Davies, P.R.; Al-Mazroai, L.S. Photocatalytic Reforming of Glycerol over Gold and Palladium as an Alternative Fuel Source. Catal. Lett. 2009, 128, 253–255. [Google Scholar] [CrossRef]
- Matthews, R. Photocatalytic oxidation of chlorobenzene in aqueous suspensions of titanium dioxide. J. Catal. 1986, 97, 565–568. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kawai, T.; Sakata, T. Photocatalytic Reactions of Hydrocarbons and Fossil Fuels with Water. Hydrogen Production and Oxidation. J. Phys. Chem. 1984, 88, 4083–4088. [Google Scholar] [CrossRef]
- Serpone, N.; Borgarello, E.; Harris, R.; Cahill, P.; Borgarello, M.; Pelizzetti, E. Photocatalysis over TiO2 supported on a glass substrate. Sol. Energy Mater. 1986, 14, 121–127. [Google Scholar] [CrossRef]
- Barbeni, M.; Pramauro, E.; Pelizzetti, E.; Borgarello, E.; Serpone, N.; Jamieson, M.A. Photochemical degradation of chlorinated dioxins, biphenyls phenols and benzene on semiconductor dispersion. Chemosphere 1986, 15, 1913–1916. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Borgarello, M.; Minero, C.; Pramauro, E.; Borgarello, E.; Serpone, N. Photocatalytic degradation of polychlorinated dioxins and polychlorinated biphenyls in aqueous suspensions of semiconductors irradiated with simulated solar light. Chemosphere 1988, 17, 499–510. [Google Scholar] [CrossRef]
- Pelizzetti, E.; Minero, C. Mechanism of the photo-oxidative degradation of organic pollutants over TiO2 particles. Electrochim. Acta 1993, 38, 47–55. [Google Scholar] [CrossRef]
- Maurino, V.; Bedini, A.; Minella, M.; Rubertelli, F.; Pelizzetti, E.; Minero, C. Glycerol Transformation Through Photocatalysis: A Possible Route to Value Added Chemicals. J. Adv. Oxid. Technol. 2008, 11, 184–192. [Google Scholar] [CrossRef]
- Minero, C.; Bedini, A.; Maurino, V. Glycerol as a probe molecule to uncover oxidation mechanism in photocatalysis. Appl. Catal. B Environ. 2012, 128, 135–143. [Google Scholar] [CrossRef]
- Pellegrino, F.; Pellutiè, L.; Sordello, F.; Minero, C.; Ortel, E.; Hodoroaba, V.-D.; Maurino, V. Influence of agglomeration and aggregation on the photocatalytic activity of TiO2 nanoparticles. Appl. Catal. B Environ. 2017, 216, 80–87. [Google Scholar] [CrossRef]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Z.; Chang, C.H.; Idriss, H. Photo-catalytic production of hydrogen form ethanol over M/TiO2 catalysts (M = Pd, Pt or Rh). Appl. Catal. B Environ. 2006, 67, 217–222. [Google Scholar] [CrossRef]
- Antoniadou, M.; Kondarides, D.I.; Lianos, P. Photooxidation Products of Ethanol During Photoelectrochemical Operation Using a Nanocrystalline Titania Anode and a Two Compartment Chemically Biased Cell. Catal. Lett. 2009, 129, 344–349. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic applications in the production of biodiesel from vegetable oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Arechederra, R.L.; Treu, B.L.; Minteer, S.D. Development of glycerol/O2 biofuel cell. J. Power Sources 2007, 173, 156–161. [Google Scholar] [CrossRef]
- Zhang, Y.H.P. A sweet out-of-the-box solution to the hydrogen economy: Is the sugar-powered car science fiction? Energy Environ. Sci. 2009, 2, 272–282. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, D.Y.C.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrogen Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Cargnello, M.; Gasparotto, A.; Gombac, V.; Montini, T.; Barreca, D.; Fornasiero, P. Photocatalytic H2 and Added-Value By-Products—The Role of Metal Oxide Systems in Their Synthesis from Oxygenates. Eur. J. Inorg. Chem. 2011. [Google Scholar] [CrossRef]
- Rossetti, I. Hydrogen Production by Photoreforming of Renewable Substrates. ISRN Chem. Eng. 2012, 2012, 1–21. [Google Scholar] [CrossRef]
- Speltini, A.; Sturini, M.; Maraschi, F.; Dondi, D.; Serra, A.; Profumo, A.; Buttafava, A.; Albini, A. Swine sewage as sacrificial biomass for photocatalytic hydrogen gas production: Explorative study. Int. J. Hydrogen Energy 2014, 39, 11433–11440. [Google Scholar] [CrossRef]
- Liu, C.; Lei, Z.; Yang, Y.; Zhang, Z. Preliminary trial on degradation of waste activated sludge and simultaneous hydrogen production in a newly-developed solar photocatalytic reactor with AgX/TiO2-coated glass tubes. Water Res. 2013, 47, 4986–4992. [Google Scholar] [CrossRef] [PubMed]
- Hans Wedepohl, K. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Huang, N.M.; Lim, H.N.; Chia, C.H.; Yarmo, M.A.; Muhamad, M.R. Simple room-temperature preparation of high-yield large-area graphene oxide. Int. J. Nanomed. 2011, 6, 3443–3448. [Google Scholar] [CrossRef]
- Van Ruijven, B.; Lamarque, J.-F.; van Vuuren, D.P.; Kram, T.; Eerens, H. Emission scenarios for a global hydrogen economy and the consequences for global air pollution. Glob. Environ. Chang. 2011, 21, 983–994. [Google Scholar] [CrossRef]
- Prather, M.J. Atmospheric science. An environmental experiment with H2? Science 2003, 302, 581–582. [Google Scholar] [CrossRef]
- Sanderson, M.G.; Collins, W.J.; Derwent, R.G.; Johnson, C.E. Simulation of global hydrogen levels using a lagrangian three-dimensional model. J. Atmos. Chem. 2003, 46, 15–28. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, F.; Sordello, F.; Minella, M.; Minero, C.; Maurino, V. The Role of Surface Texture on the Photocatalytic H2 Production on TiO2. Catalysts 2019, 9, 32. https://doi.org/10.3390/catal9010032
Pellegrino F, Sordello F, Minella M, Minero C, Maurino V. The Role of Surface Texture on the Photocatalytic H2 Production on TiO2. Catalysts. 2019; 9(1):32. https://doi.org/10.3390/catal9010032
Chicago/Turabian StylePellegrino, Francesco, Fabrizio Sordello, Marco Minella, Claudio Minero, and Valter Maurino. 2019. "The Role of Surface Texture on the Photocatalytic H2 Production on TiO2" Catalysts 9, no. 1: 32. https://doi.org/10.3390/catal9010032
APA StylePellegrino, F., Sordello, F., Minella, M., Minero, C., & Maurino, V. (2019). The Role of Surface Texture on the Photocatalytic H2 Production on TiO2. Catalysts, 9(1), 32. https://doi.org/10.3390/catal9010032







