Development of a Novel Magnetic Reactor Based on Nanostructured Fe3O4@PAA as Heterogenous Fenton Catalyst
Abstract
1. Introduction
2. Results and Discussion
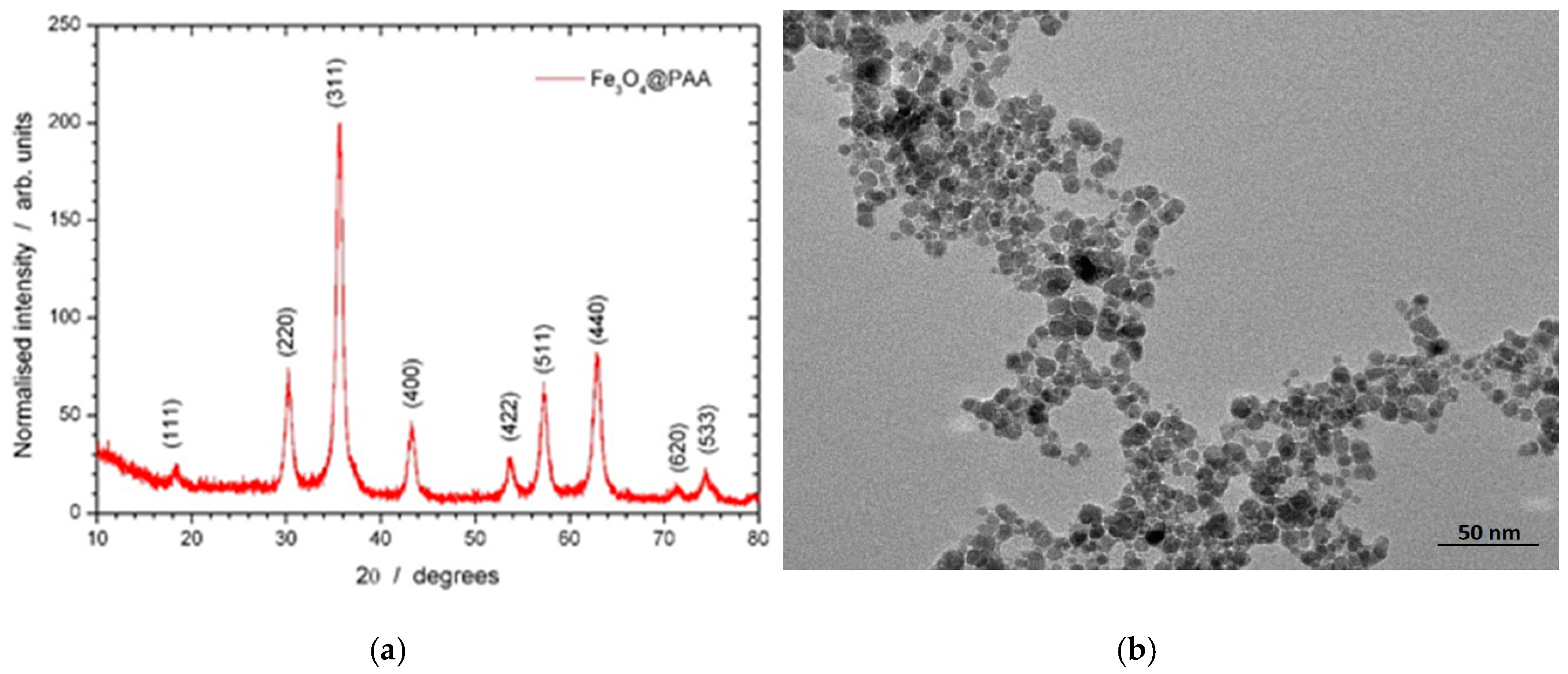
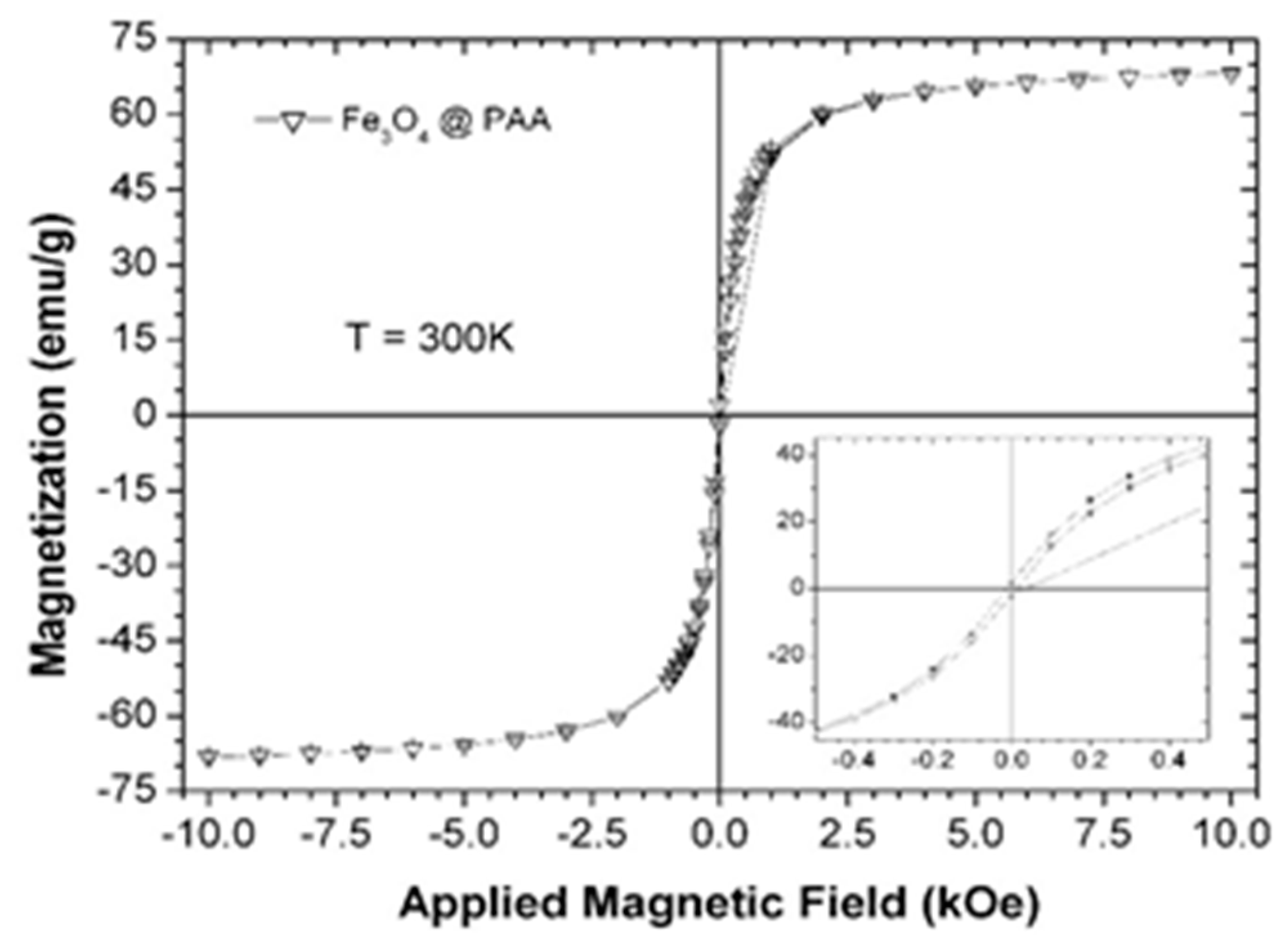
2.1. Characterization of Fe3O4@PAA
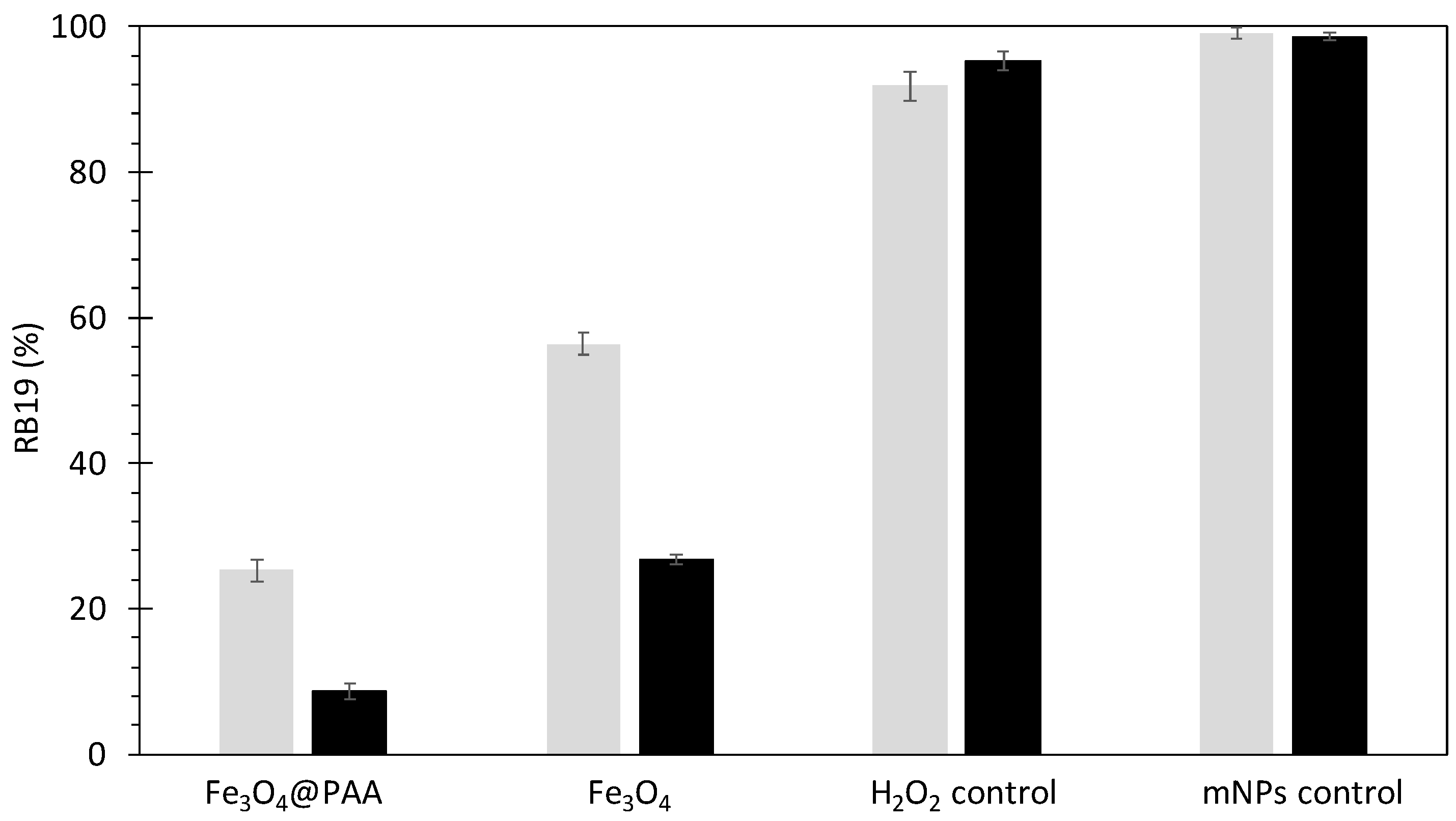
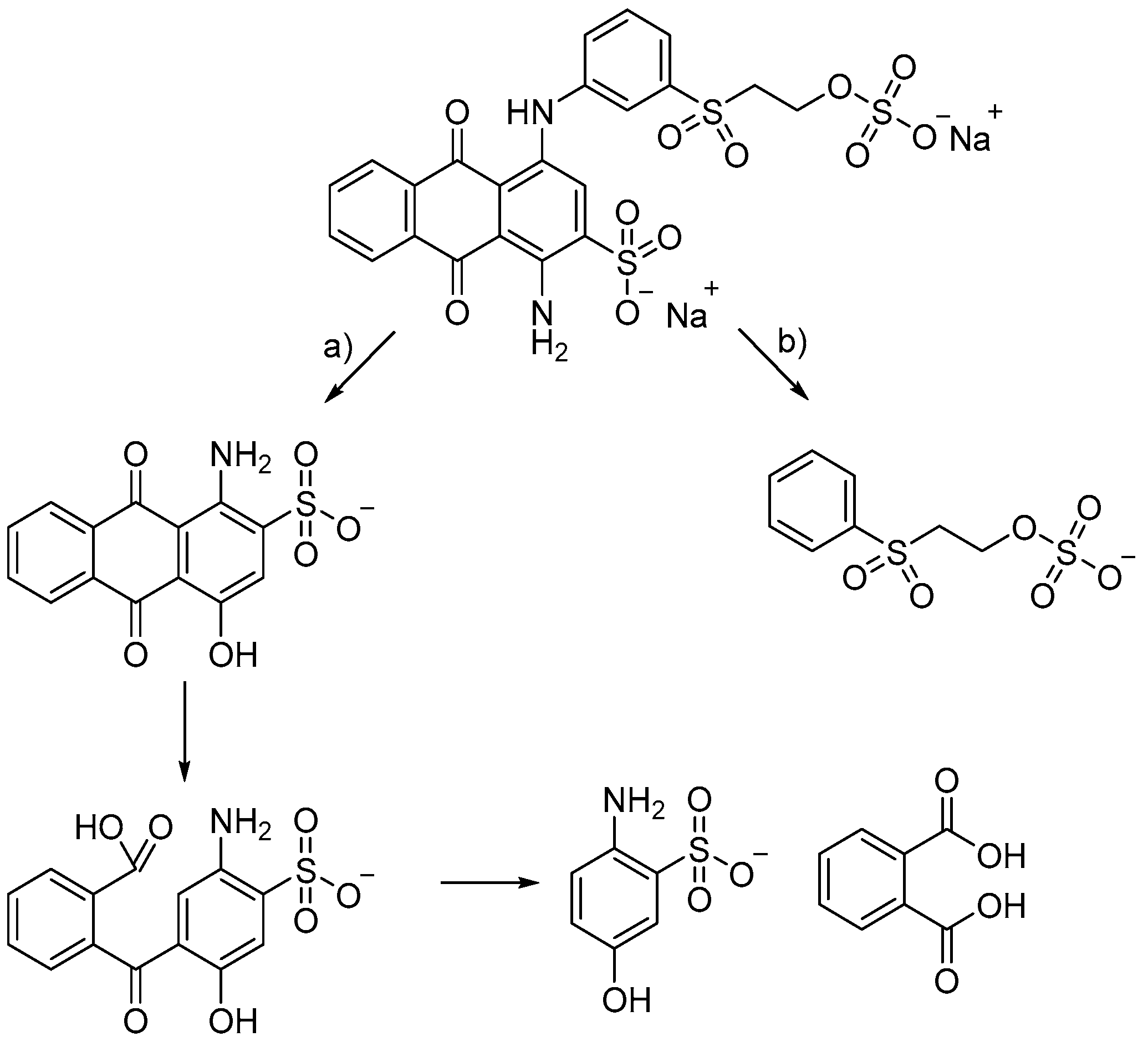
2.2. Searching for Optimal Fenton Parameters for Dye Decolorization
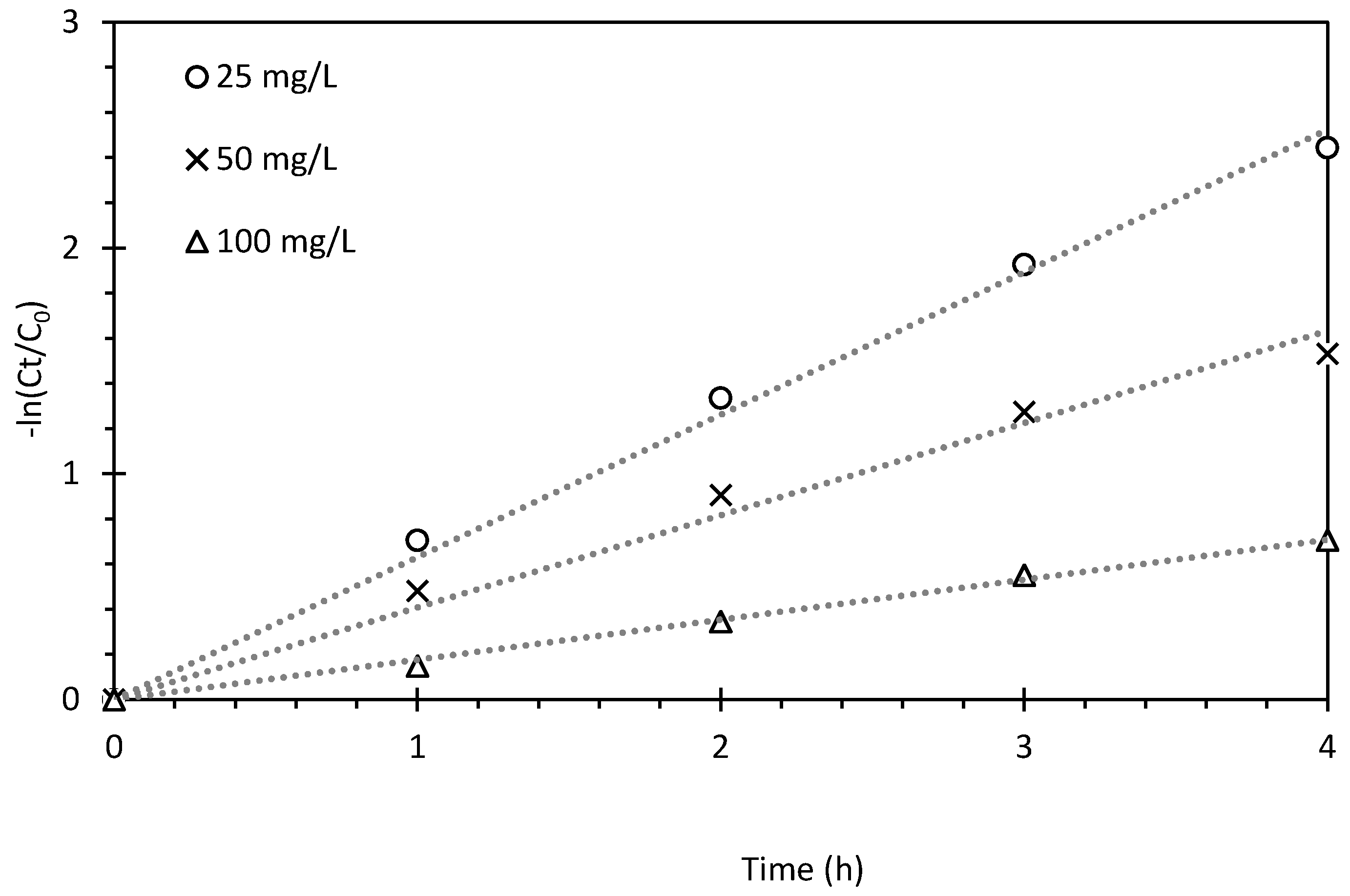
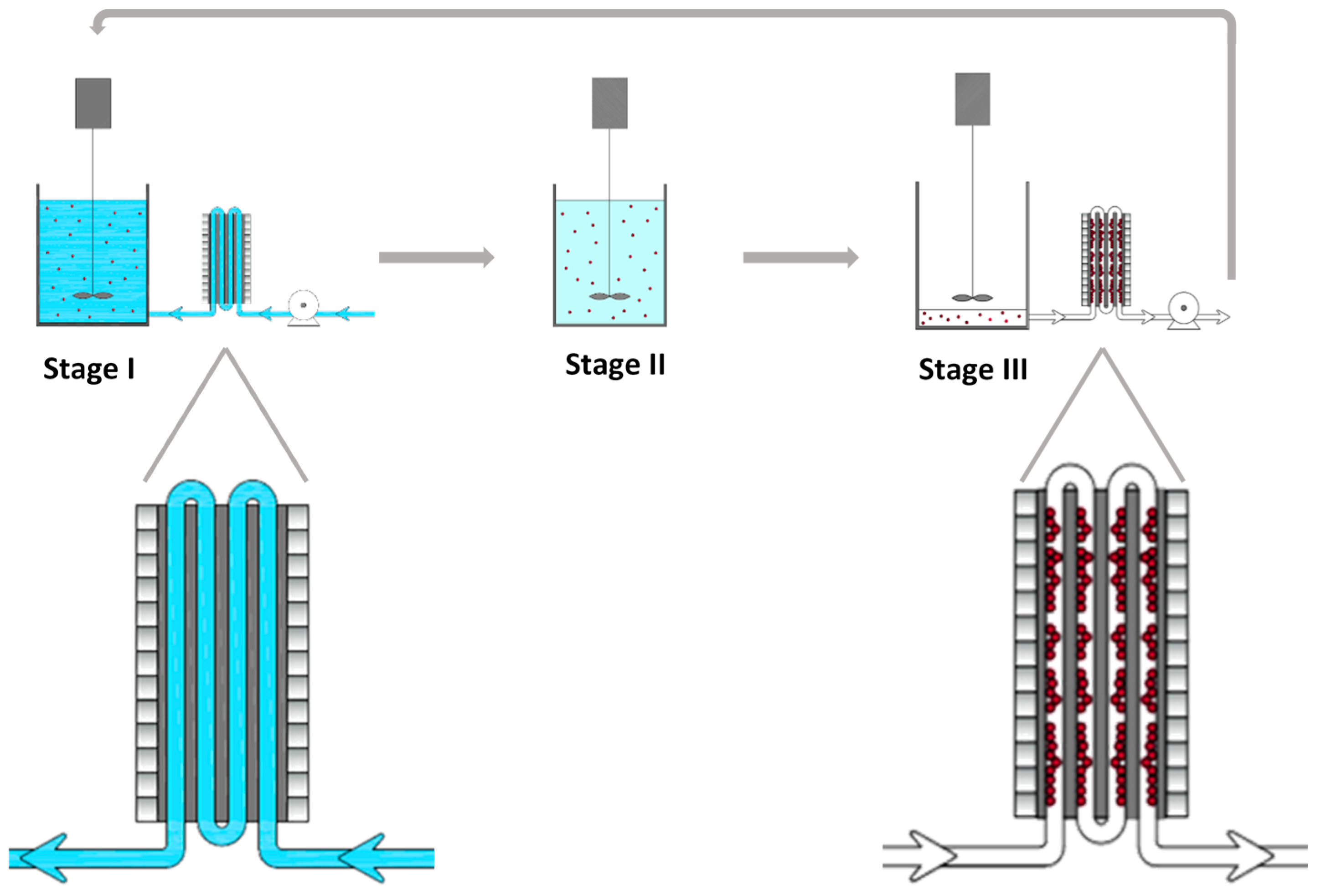
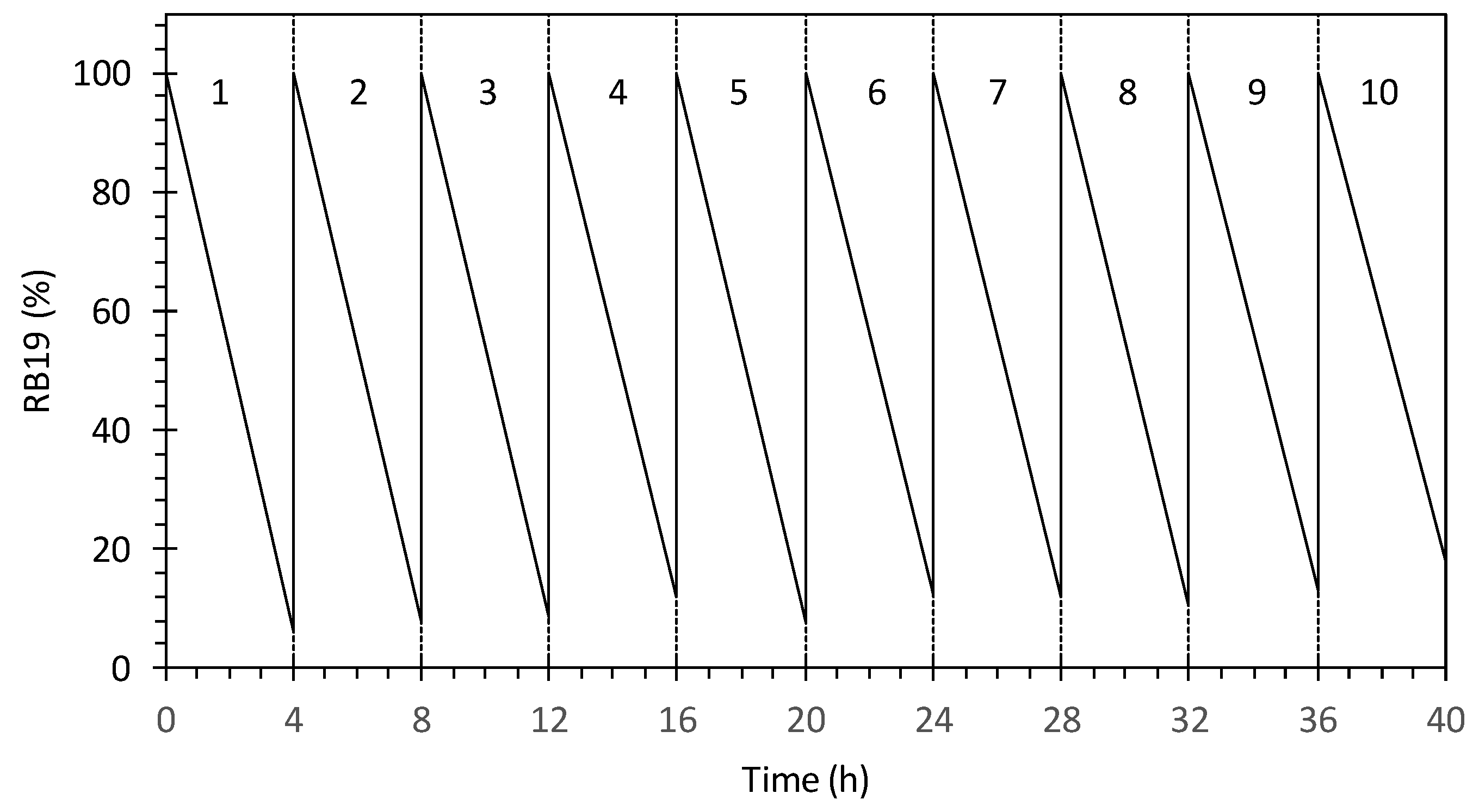
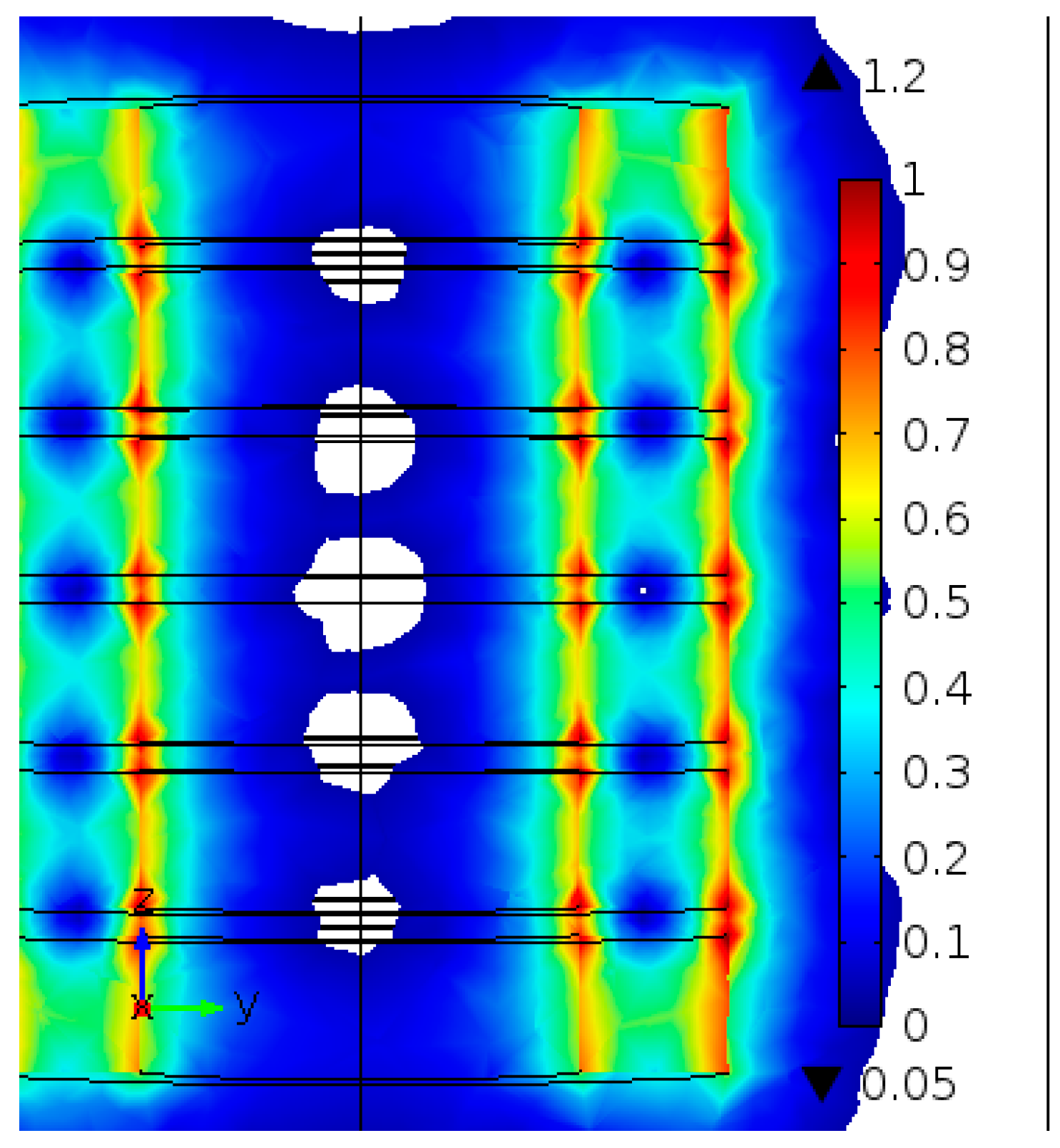
2.3. Sequential Batch Reactor (SBR) with External Magnetic Separation System
3. Materials and Methods
3.1. Chemicals and Magnetic Nanoparticles
3.2. Characterization of Fe3O4@PAA Magnetic Nanoparticles (mNPs)
3.3. Experimental Set-Up for Dye Removal by Heterogeneous Fenton Catalysis
- where C0 and Ct represent the dye concentration (mg L−1) before and after the reaction time, respectively. To clarify the influence of mNPs on dye removal, control experiments containing RB19 and H2O2 were conducted in parallel experiments lacking catalyst.
- where kobs is the observed rate constant (h−1) and t is the time (h). A total organic carbon (TOC) analyser with autosampler (Shimadzu TOC 5000, Kyoto, Japan) was used to determine TOC concentrations in samples at mg L−1 levels and to calculate the percentage of mineralization after treatment.
3.4. Experimental Design and Optimization
3.5. Sequential Batch Reactor with Magnetic Separation System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Andreozzi, R.; Campanella, L.; Fraysse, B.; Garric, J.; Gonnella, A.; Lo Giudice, R.; Marotta, R.; Pinto, G.; Pollio, A. Effects of advanced oxidation processes (AOPs) on the toxicity of a mixture of pharmaceuticals. Water Sci. Technol. 2004, 50, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tayo, L.L.; Caparanga, A.R.; Doma, B.T.; Liao, C.-H. A Review on the Removal of Pharmaceutical and Personal Care Products (PPCPs) using Advanced Oxidation Processes. J. Adv. Oxid. Technol. 2018, 21, 196–214. [Google Scholar] [CrossRef]
- Ning, B.; Graham, N.; Zhang, Y.; Nakonechny, M.; Gamal El-Din, M. Degradation of Endocrine Disrupting Chemicals by Ozone/AOPs. Ozone Sci. Eng. 2007, 29, 153–176. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Anandan, S. Review on the recent improvements in sonochemical and combined sonochemical oxidation processes—A powerful tool for destruction of environmental contaminants. Renew. Sus. Energ. Rev. 2016, 55, 426–454. [Google Scholar] [CrossRef]
- Ayoub, K.; van Hullebusch, E.D.; Cassir, M.; Bermond, A. Application of advanced oxidation processes for TNT removal: A review. J. Hazard. Mater. 2010, 178, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Sable, S.S.; Ghute, P.P.; Álvarez, P.; Beltrán, F.J.; Medina, F.; Contreras, S. FeOOH and derived phases: Efficient heterogeneous catalysts forclofibric acid degradation by advanced oxidation processes (AOPs). Catal. Today 2015, 240, 46–54. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Beijing, China, 24–27 October 2017; p. 012089. [Google Scholar]
- Álvarez, P.M.; Jaramillo, J.; López-Piñeiro, F.; Plucinski, P.K. Preparation and characterization of magnetic TiO2 nanoparticles and their utilization for the degradation of emerging pollutants in water. Appl. Catal. B 2010, 100, 338–345. [Google Scholar] [CrossRef]
- Munoz, M.; Zahara, M.D.P.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Bae, S.; Kim, D.; Lee, W. Degradation of diclofenac by pyrite catalyzed Fenton oxidation. Appl. Catal. B 2013, 134–135, 93–102. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Miao, C.; Shang, J.; Liu, Y.; Liu, J. Structural effects on the catalytic activity of carbon-supported magnetite nanocomposites in heterogeneous Fenton-like reactions. RSC Adv. 2018, 8, 16193–16201. [Google Scholar] [CrossRef]
- Velichkova, F.; Julcour-Lebigue, C.; Koumanova, B.; Dlmas, H. Heterogeneous Fenton oxidation of paracetamol using iron oxide (nano)particles. J. Environ. Chem. Eng. 2013, 1, 1214–1222. [Google Scholar] [CrossRef]
- Mehdaoui, R.; El Ghali, A.; Cheikhrouhou, W.; Beyou, E.; Baouab, M.H.V. Fe3O4 nanoparticles coated by new functionalized tetraaza-2,3 dialdehyde micro-crystalline cellulose: Synthesis, characterization, and catalytic application for degradation of Acid Yellow 17. Iran. Polym. J. 2017, 26, 597–613. [Google Scholar] [CrossRef]
- Kim, D.K.; Mikhaylova, M.; Zhang, Y.; Muhammed, M. Protective Coating of Superparamagnetic Iron Oxide Nanoparticles. Chem. Mater. 2003, 15, 1617–1627. [Google Scholar] [CrossRef]
- Umar, I.A.; Giwa, A.; Salisu, B.; Sallahudeen, M.; Mustapha, A. Kinetics, equilibrium and thermodynamics studies of C.I. Reactive Blue 19 dye adsorption on coconut shell based activated carbon. Int. Biodeterior. Biodegrad. 2015, 102, 265–273. [Google Scholar]
- Apostol, L.C.; Pereira, L.; Pereira, R.; Gavrilescu, M.; Alves, M.M. Biological decolorization of xanthene dyes by anaerobic granular biomass. Biodegradation 2012, 23, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Regina Mary, R.; Sekaran, G. A novel method for the extraction of prodigiosin from bacterial fermenter integrated with sequential batch extraction reactor using magnetic iron oxide. Process Biochem. 2016, 51, 1731–1737. [Google Scholar]
- Chen, G.; Liu, J.; Yao, J.; Qi, Y.; Yan, B. Biodiesel production from waste cooking oil in a magnetically fluidized bed reactor using whole-cell biocatalysts. Energy Convers. Manag. 2017, 138, 556–564. [Google Scholar] [CrossRef]
- Liang, X.; He, Z.; Zhong, Y.; Tan, W.; He, H.; Yuan, P.; Zhu, J.; Zhang, J. The effect of transition metal substitution on the catalytic activity of magnetite in heterogeneous Fenton reaction: In interfacial view. Colloids Surf. A 2013, 435, 28–35. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Naimi, I.; Bellakhal, N. Removal of 17β-Estradiol by Electro-Fenton Process. Mater. Sci. Appl. 2012, 3, 880–886. [Google Scholar] [CrossRef]
- Siddique, M.; Farooq, R.; Price, G.J. Synergistic effects of combining ultrasound with the Fenton process in the degradation of Reactive Blue 19. Ultrason. Sonochem. 2014, 21, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.; Li, C.; Wang, H.; Hu, H.; Wang, W.; Zhang, X. Photocatalytic degradation, toxicological assessment and degradation pathway of CI Reactive Blue 19 dye. Chem. Eng. Res. Des. 2018, 129, 384–390. [Google Scholar] [CrossRef]
- Fanchiang, J.-M.; Dyi-Hwa, T. Degradation of anthraquinone dye C.I. Reactive Blue 19 in aqueous solution by ozonation. Chemosphere 2009, 77, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
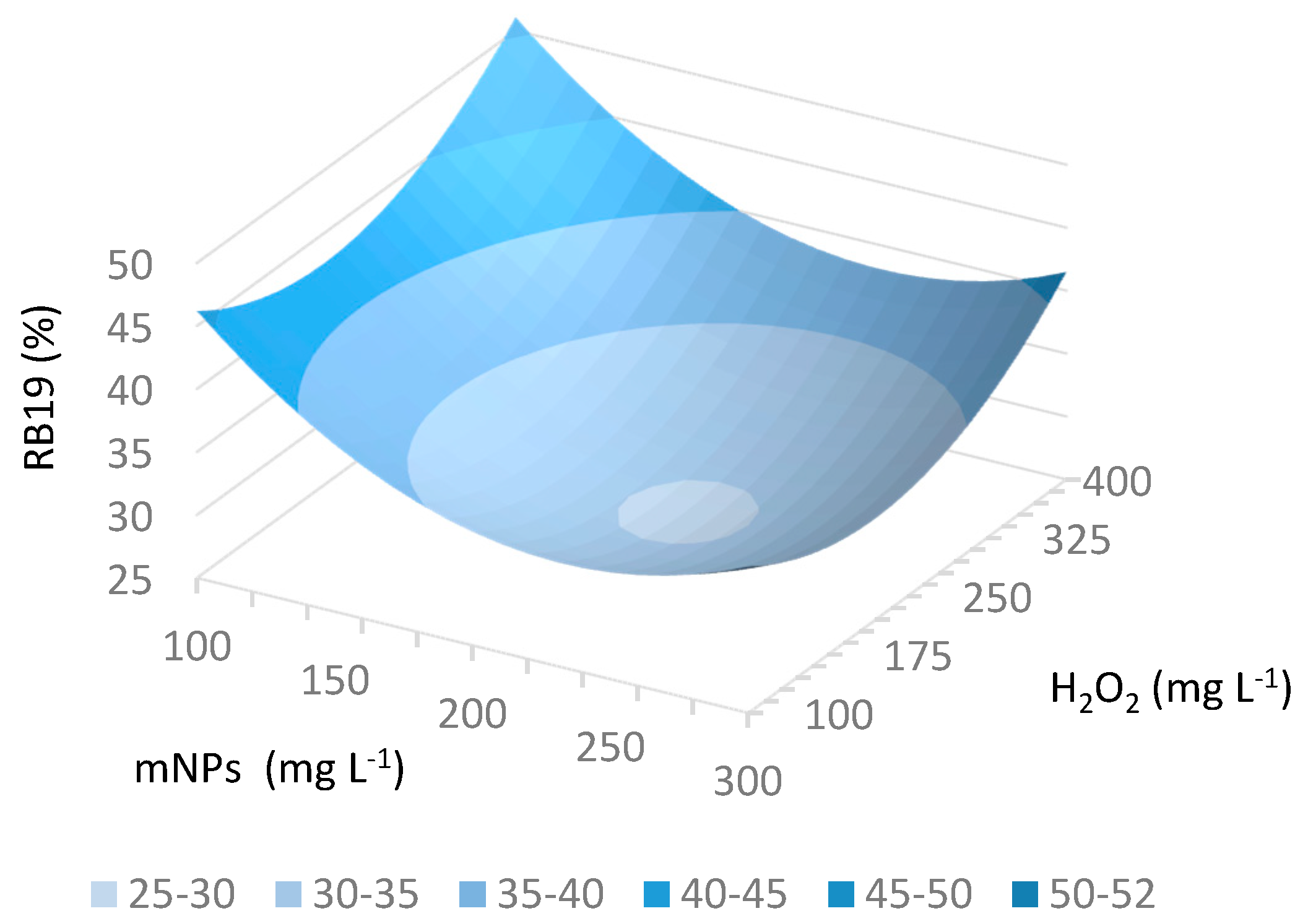
| Exp No. | Dimensional Independent Variables | Dimensionless Independent Variables | Dependent Variable | ||
|---|---|---|---|---|---|
| H2O2 (mg L−1) | mNPs (mg L−1) | x1 | x2 | y1 or RB19 (%) | |
| 1 | 100 | 100 | −1 | −1 | 44.49 |
| 2 | 400 | 100 | 1 | −1 | 37.79 |
| 3 | 100 | 300 | −1 | 1 | 52.20 |
| 4 | 400 | 300 | 1 | 1 | 41.23 |
| 5 | 250 | 200 | 0 | 0 | 28.95 |
| 6 | 250 | 200 | 0 | 0 | 31.17 |
| 7 | 250 | 200 | 0 | 0 | 31.92 |
| 8 | 37.9 | 200 | −1.414 | 0 | 52.69 |
| 9 | 250 | 341.4 | 0 | 1.414 | 45.04 |
| 10 | 462.1 | 200 | 1.414 | 0 | 38.06 |
| 11 | 250 | 58.6 | 0 | −1.414 | 39.10 |
| Dye (mg L−1) | R2 | kobs (h−1) | t1/2 (h) |
|---|---|---|---|
| 25 | 0.995 | 0.631 | 1.098 |
| 50 | 0.983 | 0.408 | 1.698 |
| 100 | 0.996 | 0.177 | 3.909 |
| Dye | Classification | Structure | λmax | MW (g mol−1) |
|---|---|---|---|---|
| Reactive Blue 19 (RB19) | Anthraquinone | 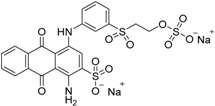 | 592 | 626.54 |
| Levels | |||||
|---|---|---|---|---|---|
| Variables | −1.414 | −1 | 0 | 1 | 1.414 |
| Fe3O4@PAA mNPs (mg L−1) | 58.6 | 100 | 200 | 300 | 341.4 |
| H2O2 (mg L−1) | 250 | 100 | 250 | 400 | 462.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamallo, M.; Fernández, L.; Vázquez-Vázquez, C.; Fondado, A.; Mira, J.; Feijoo, G.; Moreira, M.T. Development of a Novel Magnetic Reactor Based on Nanostructured Fe3O4@PAA as Heterogenous Fenton Catalyst. Catalysts 2019, 9, 18. https://doi.org/10.3390/catal9010018
Gamallo M, Fernández L, Vázquez-Vázquez C, Fondado A, Mira J, Feijoo G, Moreira MT. Development of a Novel Magnetic Reactor Based on Nanostructured Fe3O4@PAA as Heterogenous Fenton Catalyst. Catalysts. 2019; 9(1):18. https://doi.org/10.3390/catal9010018
Chicago/Turabian StyleGamallo, María, Lucía Fernández, Carlos Vázquez-Vázquez, Alfonso Fondado, Jorge Mira, Gumersindo Feijoo, and María Teresa Moreira. 2019. "Development of a Novel Magnetic Reactor Based on Nanostructured Fe3O4@PAA as Heterogenous Fenton Catalyst" Catalysts 9, no. 1: 18. https://doi.org/10.3390/catal9010018
APA StyleGamallo, M., Fernández, L., Vázquez-Vázquez, C., Fondado, A., Mira, J., Feijoo, G., & Moreira, M. T. (2019). Development of a Novel Magnetic Reactor Based on Nanostructured Fe3O4@PAA as Heterogenous Fenton Catalyst. Catalysts, 9(1), 18. https://doi.org/10.3390/catal9010018








