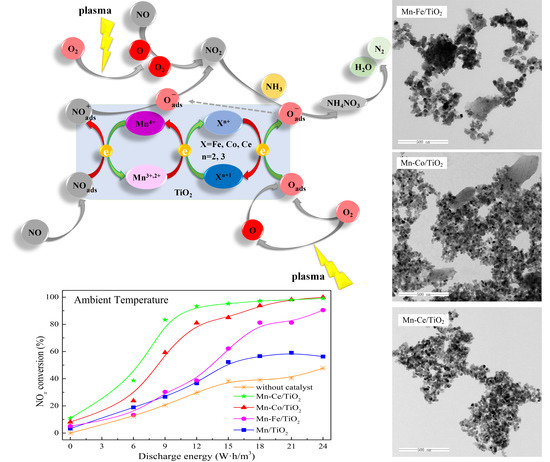
High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species
Abstract
:1. Introduction
2. Results and Discussion
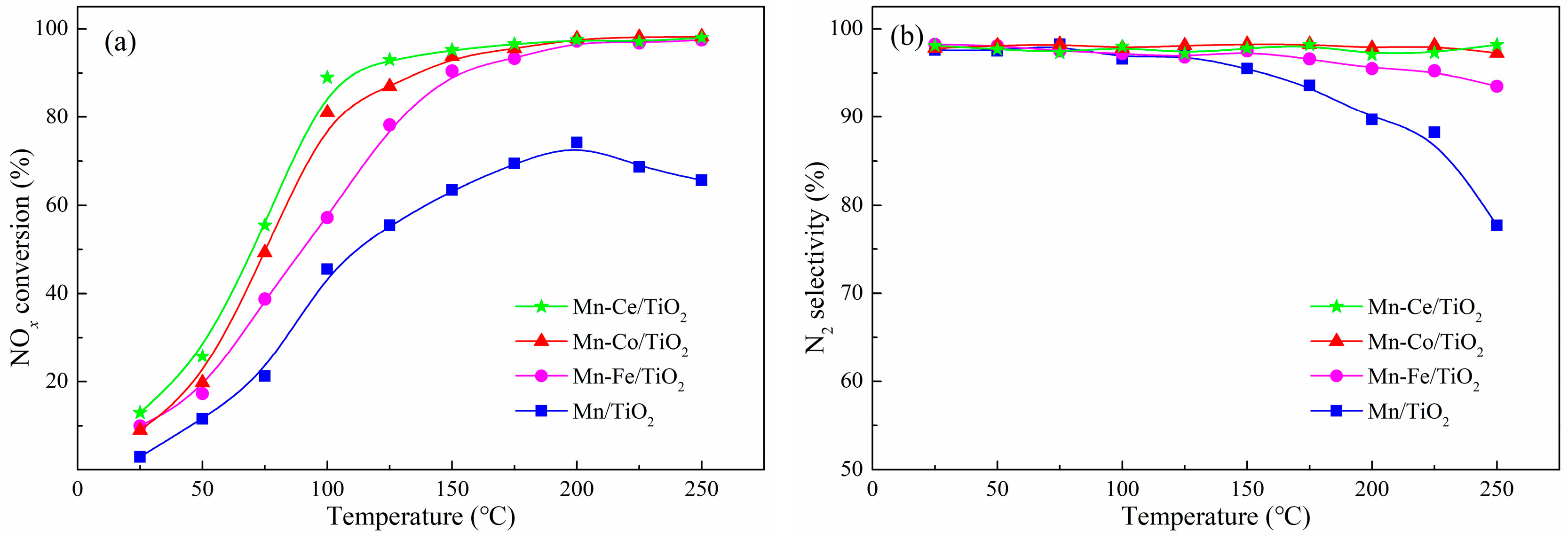
2.1. NOx Conversion of Catalyst Alone Catalytic Process
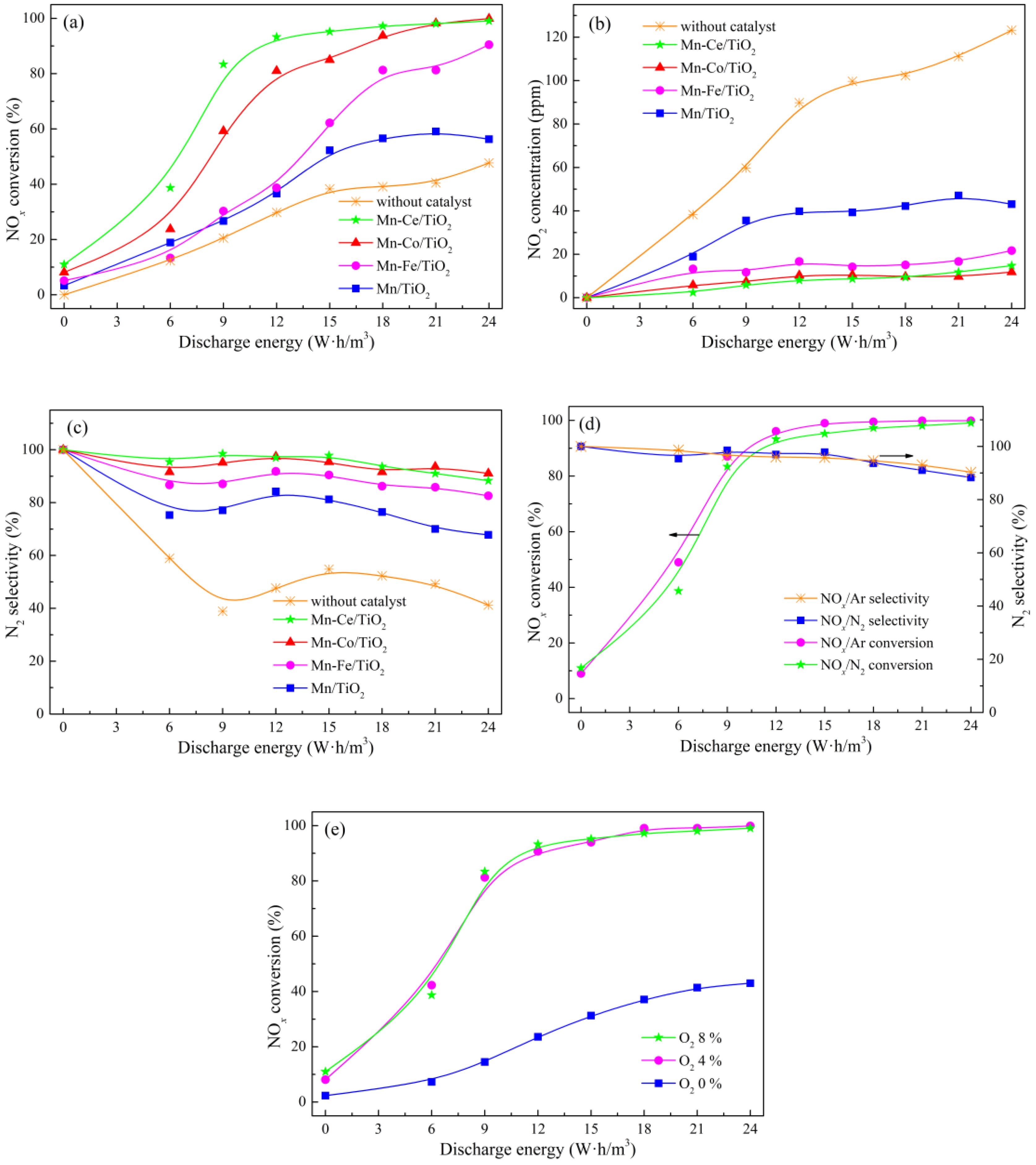
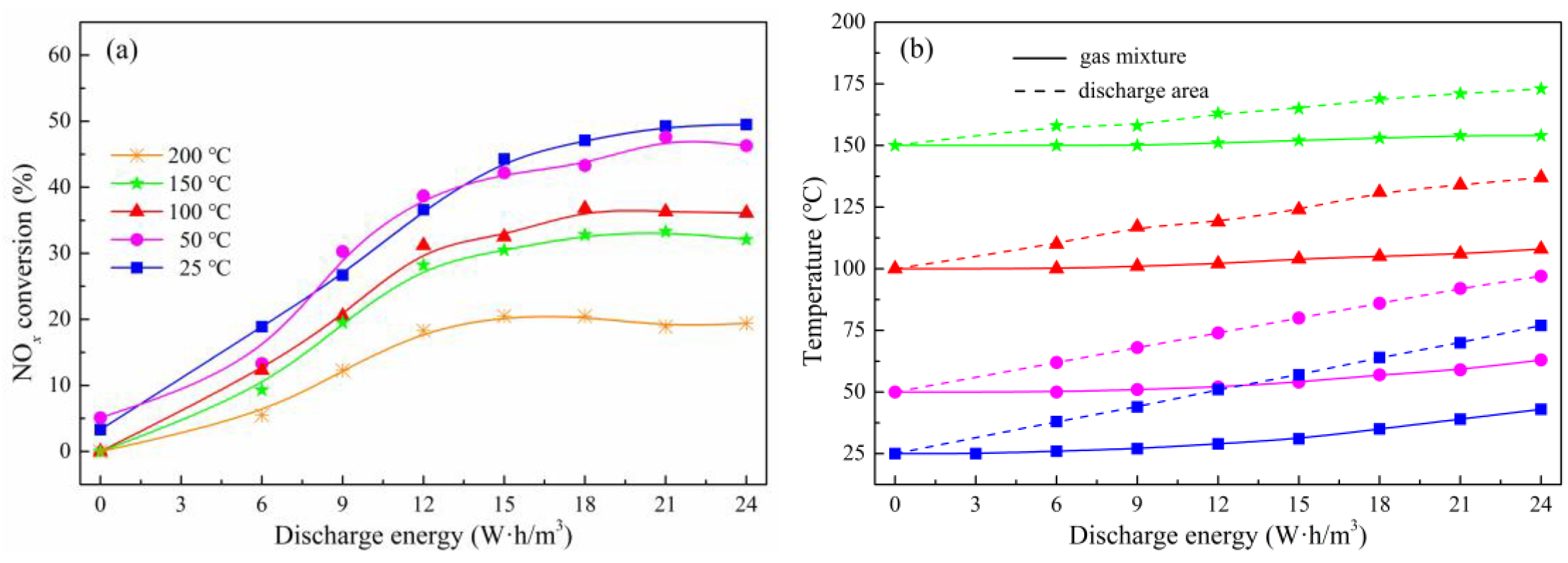
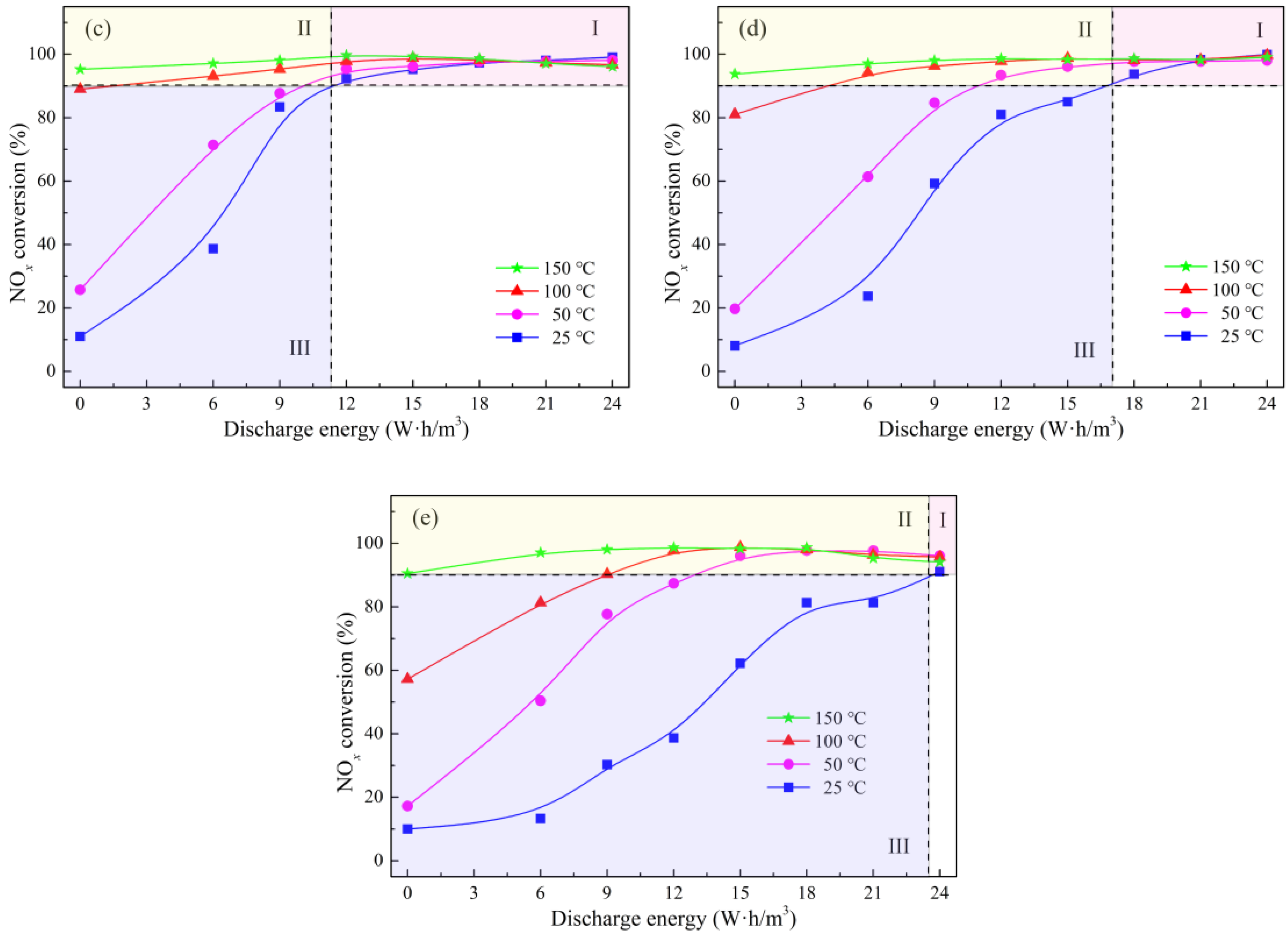
2.2. NOx Conversion of Plasma-Catalyst Hybrid Catalytic Process
2.3. Morphological Characterization
2.3.1. BET Measurements
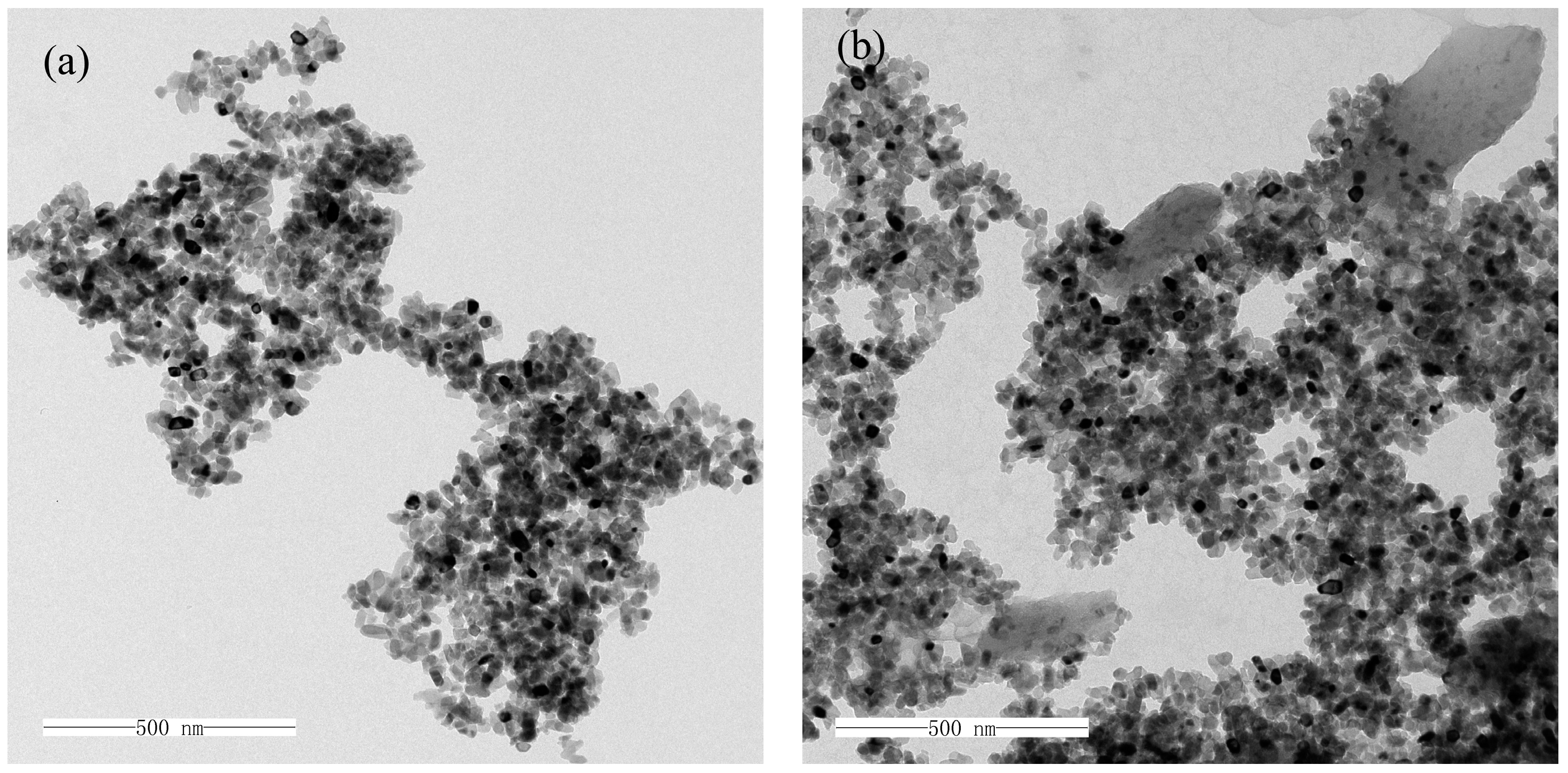
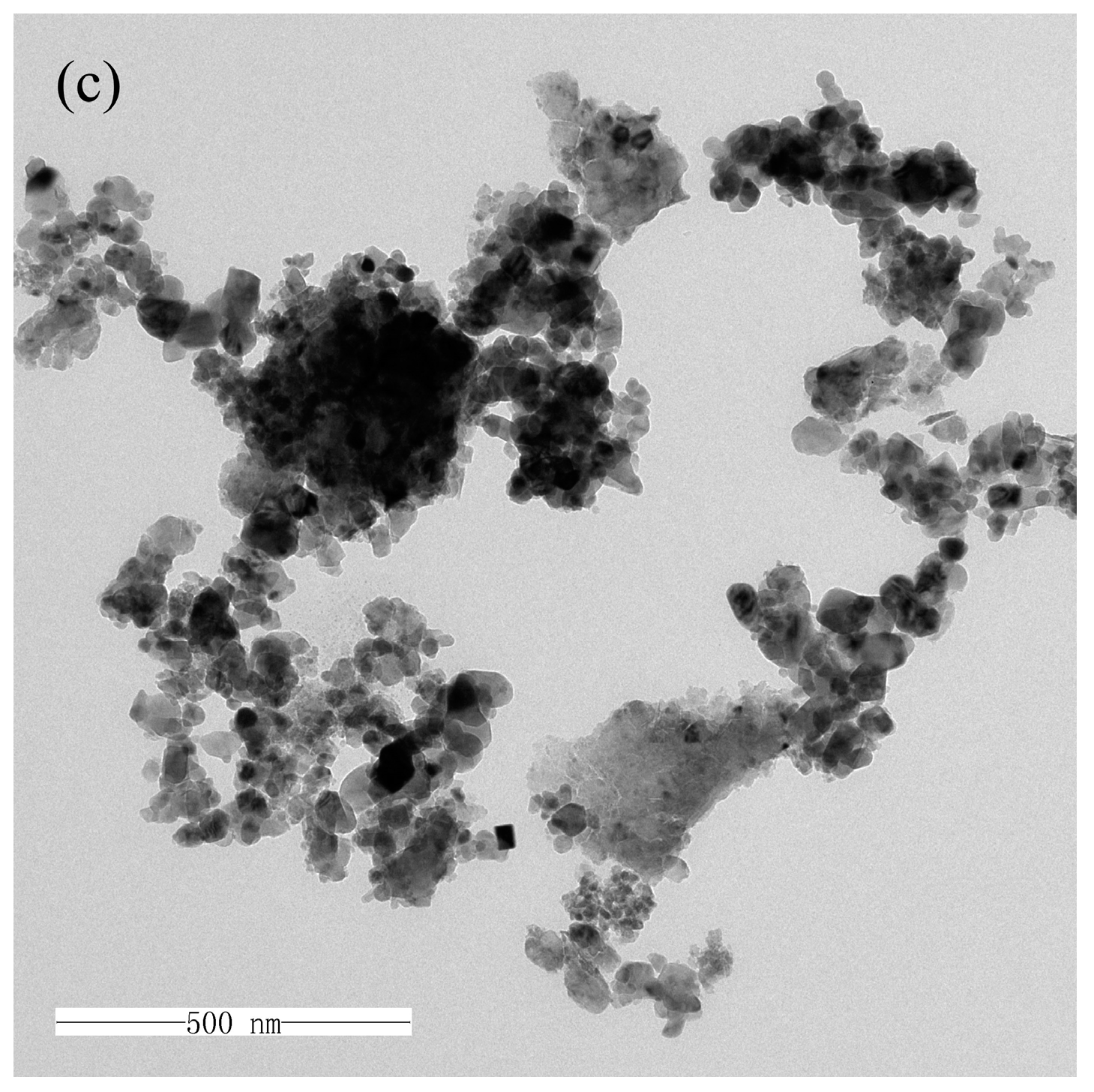
2.3.2. TEM Analysis
2.4. Structural Characterization
2.4.1. Textural Properties
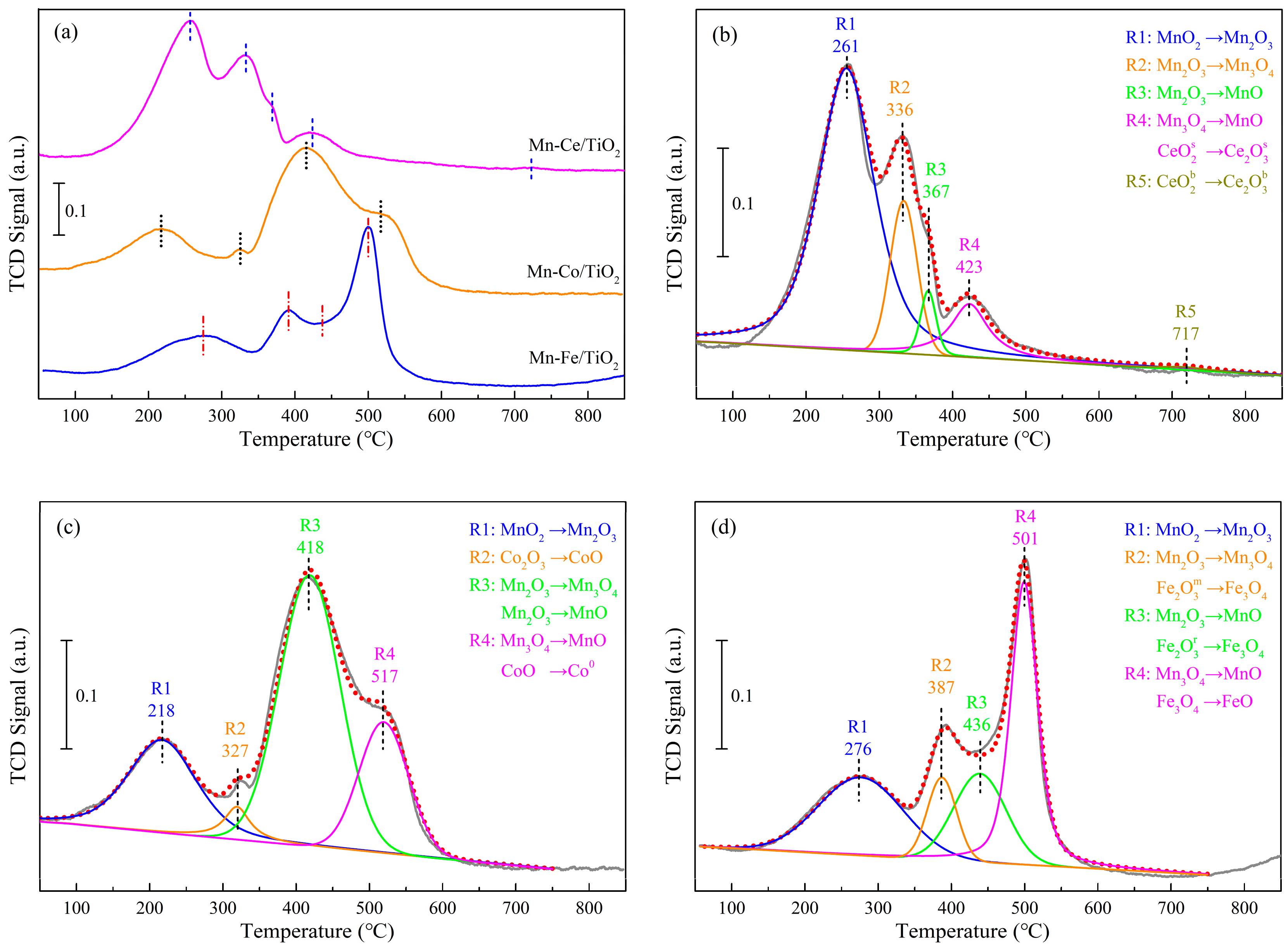
2.4.2. Reducibility Properties
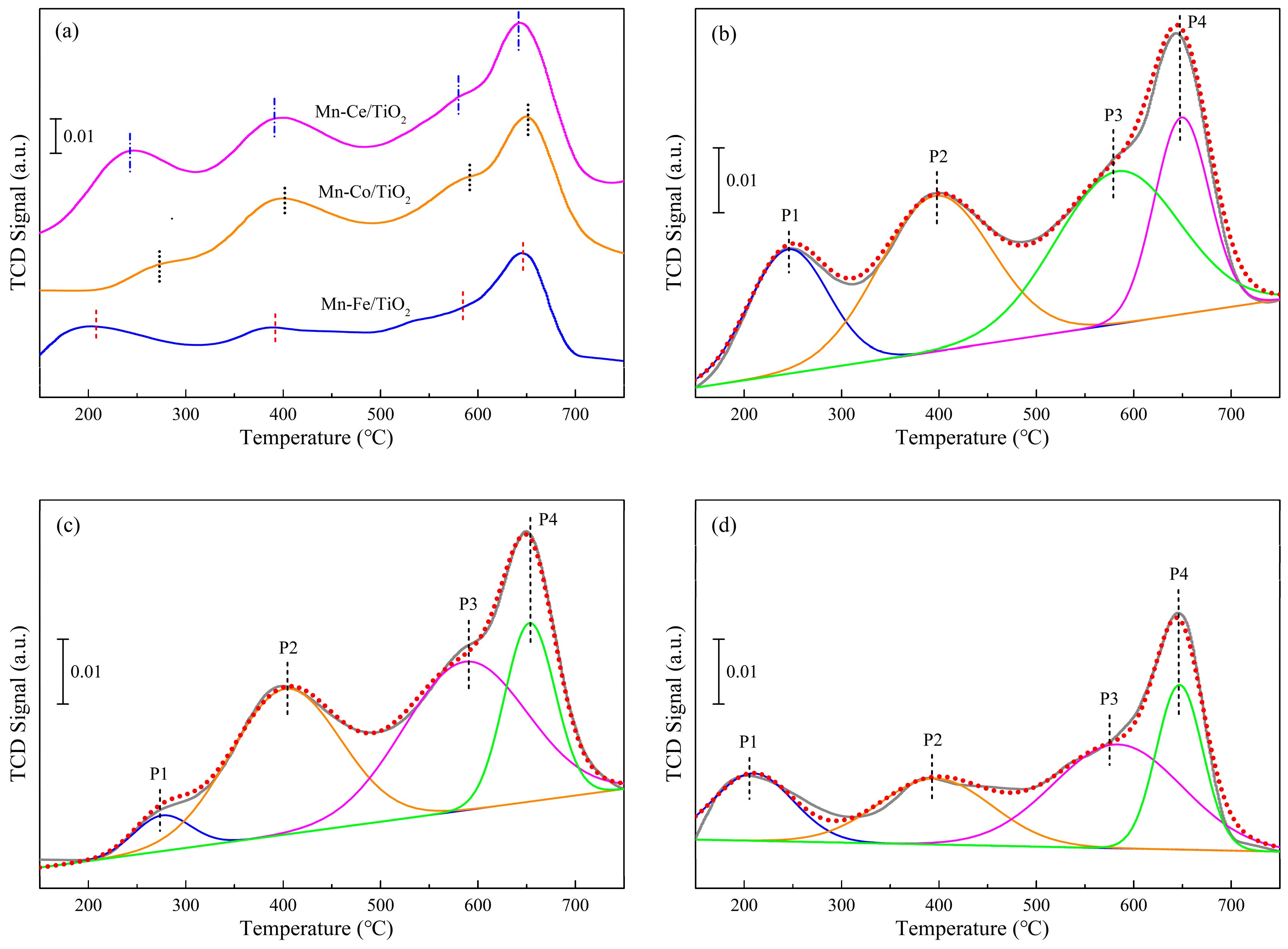
2.4.3. Ammonia Adsorption Properties
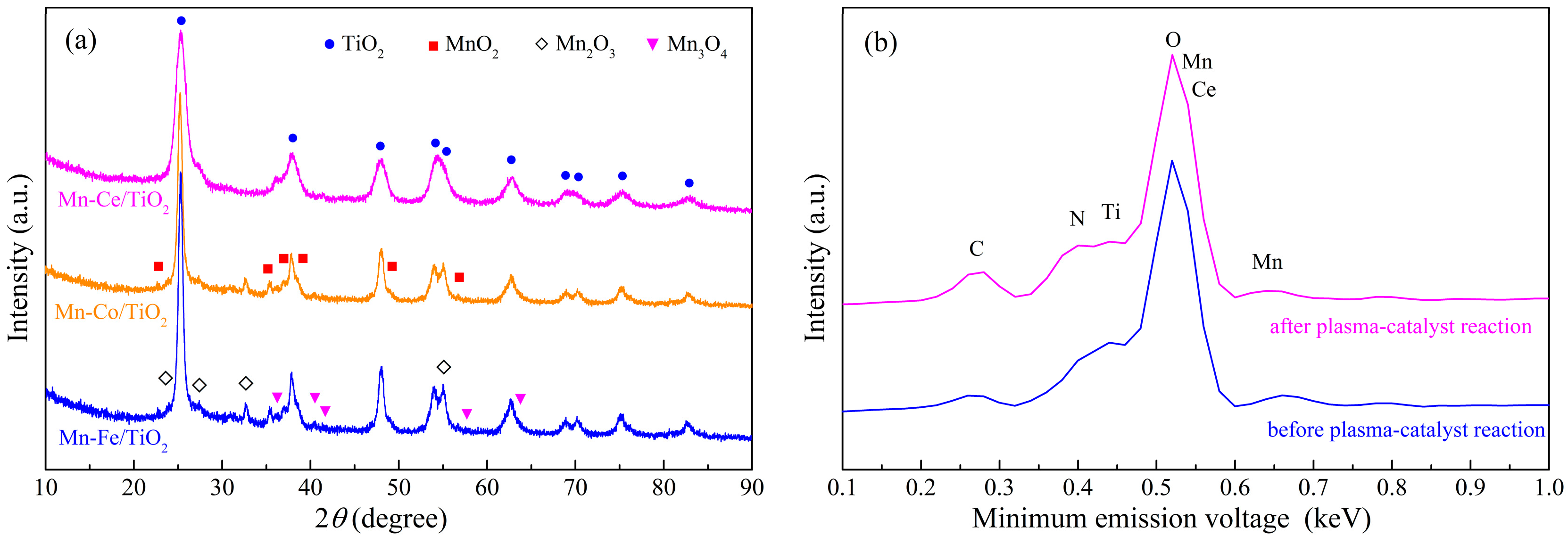
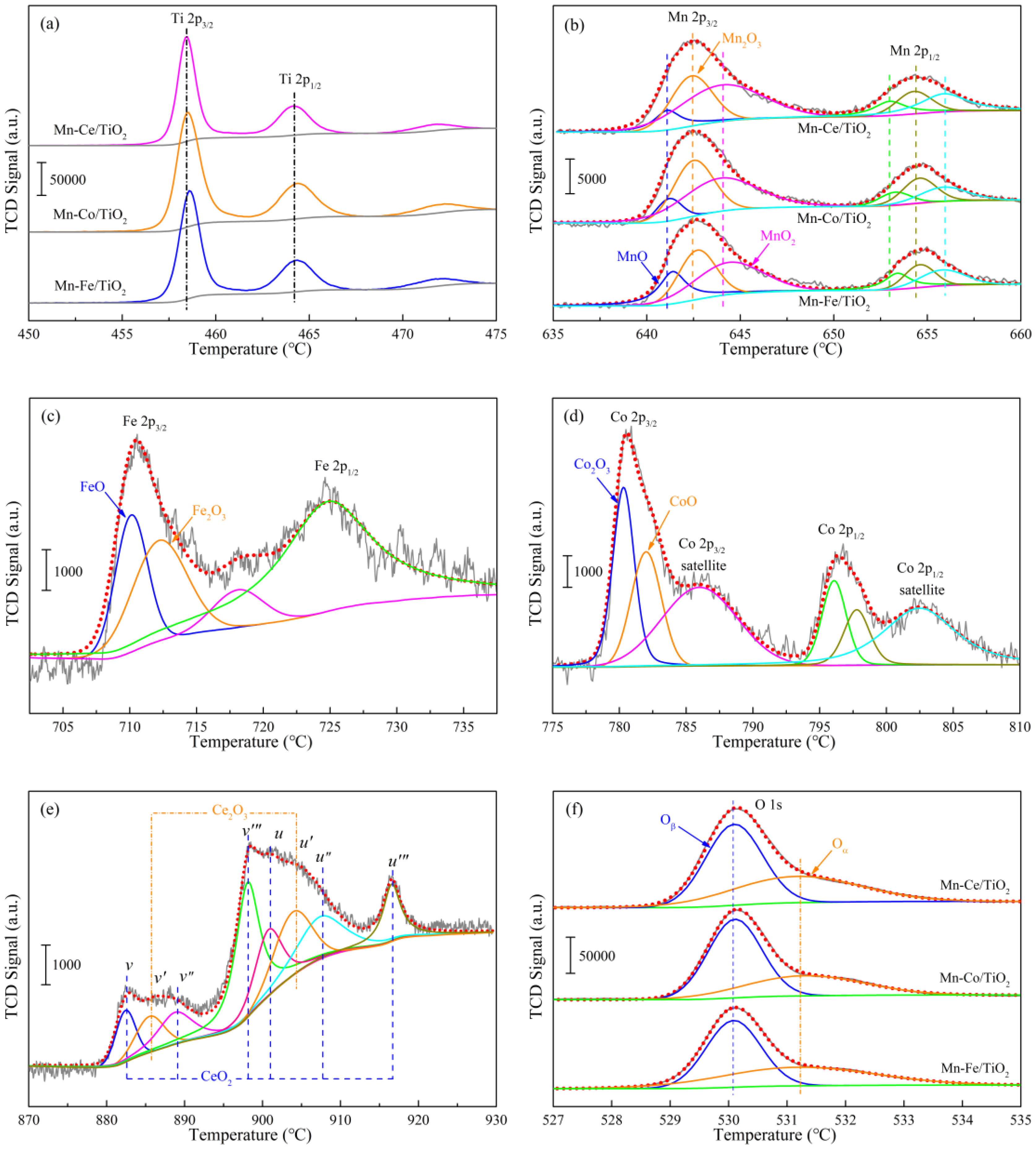
2.4.4. Oxidation States of Active Species
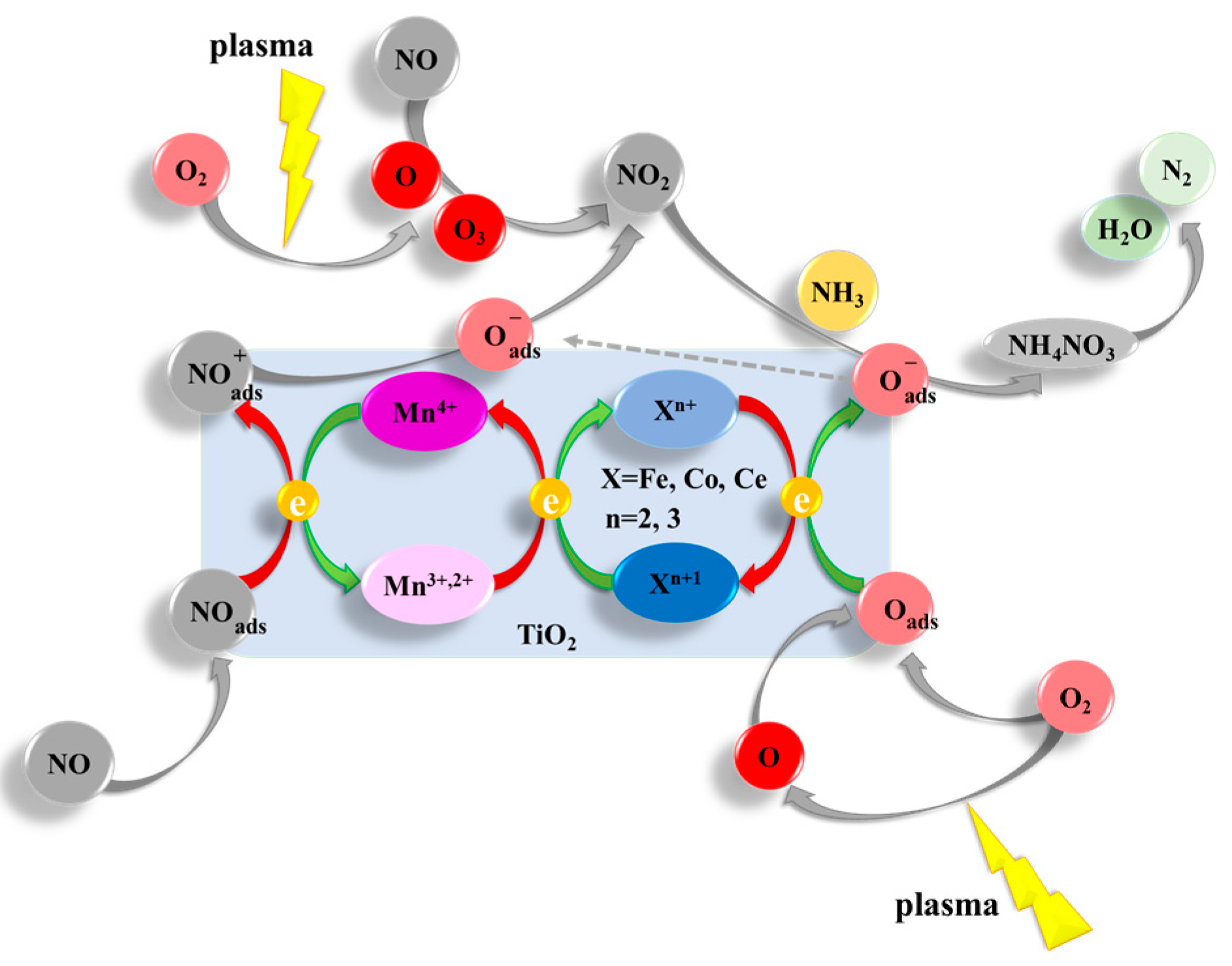
2.5. Reaction Mechanism Analysis
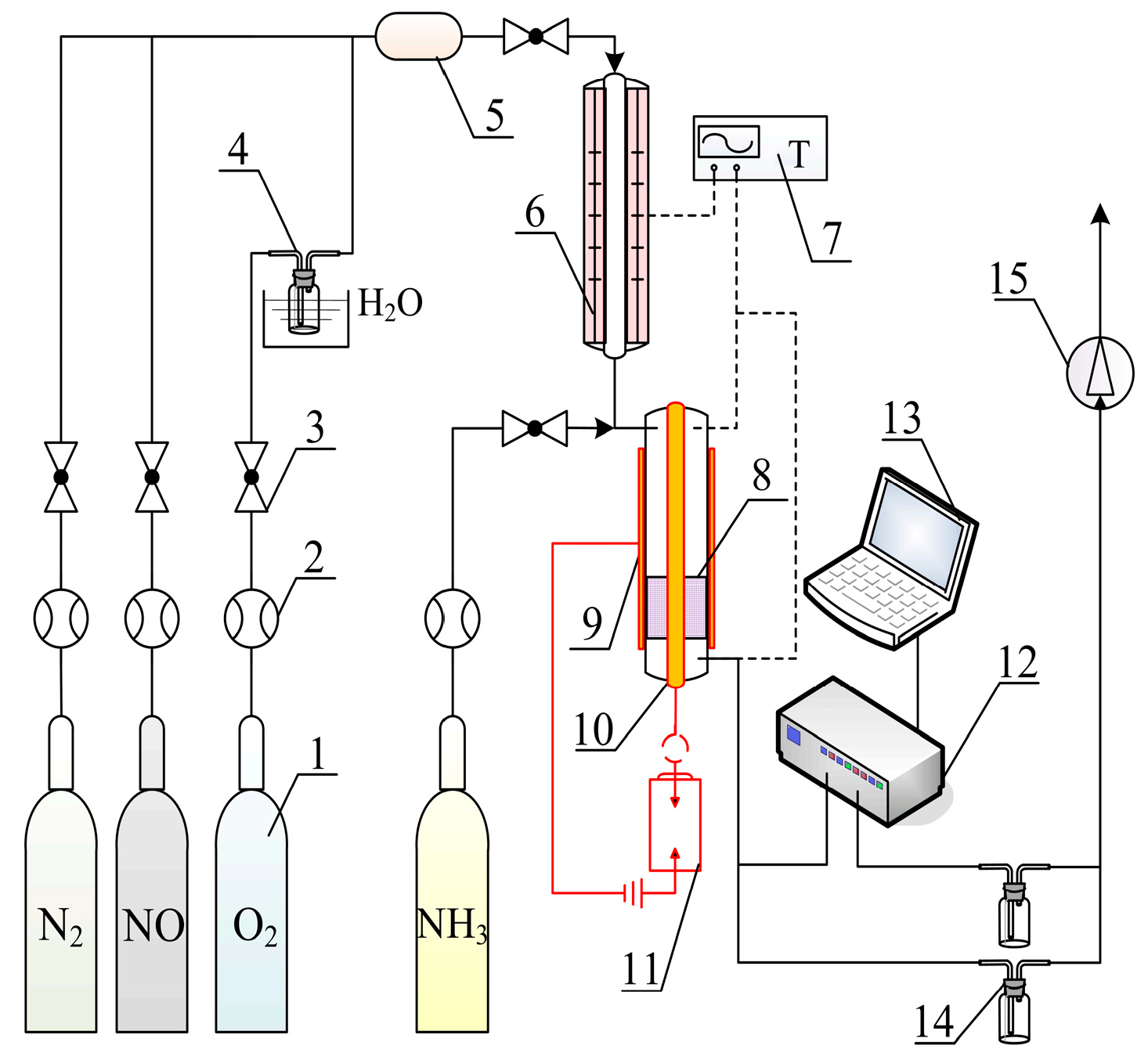
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Performance Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, B.; Chi, C.; Xu, M.; Wang, C.; Meng, D. Plasma-catalytic removal of toluene over CeO2-MnOx catalysts in an atmosphere dielectric barrier discharge. Chem. Eng. J. 2017, 322, 679–692. [Google Scholar] [CrossRef]
- France, L.J.; Yang, Q.; Li, W.; Chen, Z.; Guang, J.; Guo, D.; Wang, L.; Li, X. Ceria modified FeMnOx—Enhanced performance and sulphur resistance for low-temperature SCR of NOx. Appl. Catal. B Environ. 2017, 206, 203–215. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Lu, T.; Cheng, K.; Xu, H. Performance of V2O5-WO3-MoO3/TiO2 catalyst for selective catalytic reduction of NOx by NH3. Chin. J. Chem. Eng. 2013, 21, 1–7. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Zhang, X.; Liu, J.; Zhang, Y.; Guo, Y.; Sun, B. Catalytic conversion of NO assisted by plasma over Mn-Ce/ZSM5-multi-walled carbon nanotubes composites: Investigation of acidity, activity and stability of catalyst in the synergic system. Appl. Surf. Sci. 2018, 457, 187–199. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, W.; Luo, N.; Li, P.; Lei, Z.; Chen, B. Low-temperature NH3-SCR of NO by lanthanum manganite perovskites: Effect of A-/B-site substitution and TiO2/CeO2 support. Appl. Catal. B Environ. 2014, 146, 94–104. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, M.; Zhang, W.; Feng, W. Structure–Activity Relationship Study of Mn/Fe Ratio Effects on Mn−Fe−Ce−Ox/γ-Al2O3 Nanocatalyst for NO Oxidation and Fast SCR Reaction. Catalysts 2018, 8, 642. [Google Scholar] [CrossRef]
- Miessner, H.; Francke, K.P.; Rudolph, R.; Hammer, T. NOx removal in excess oxygen by plasma-enhanced selective catalytic reduction. Catal. Today 2002, 75, 325–330. [Google Scholar] [CrossRef]
- Kim, H.-H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Plasma Catalysis for Environmental Treatment and Energy Applications. Plasma Chem. Plasma Process. 2016, 36, 45–72. [Google Scholar] [CrossRef]
- Penetrante, B.M.; Brusasco, R.M.; Merritt, B.T.; Pitz, W.J.; Vogtlin, G.E.; Kung, M.C.; Kung, H.H.; Wan, C.Z.; Voss, K.E. Plasma-Assisted Catalytic Reduction of NOx. In Proceedings of the International Fall Fuels and Lubricants Meeting and Exposition, San Francisco, CA, USA, 19–22 October 1998. [Google Scholar] [CrossRef]
- Patil, B.S.; Cherkasov, N.; Lang, J.; Ibhadon, A.O.; Hessel, V.; Wang, Q. Low temperature plasma-catalytic NOx synthesis in a packed DBD reactor: Effect of support materials and supported active metal oxides. Appl. Catal. B Environ. 2016, 194, 123–133. [Google Scholar] [CrossRef]
- Hammer, T.; Kishimoto, T.; Miessner, H.; Rudolph, R. Plasma Enhanced Selective Catalytic Reduction: Kinetics of NOx-Removal and Byproduct Formation. In Proceedings of the International Fuels & Lubricants Meeting & Exposition, Dearborn, MI, USA, 25 October 1999; Volume 1, pp. 3632–3641. [Google Scholar]
- McAdams, R.; Beech, P.; Shawcross, J.T. Low Temperature Plasma Assisted Catalytic Reduction of NOx in Simulated Marine Diesel Exhaust. Plasma Chem. Plasma Process. 2008, 28, 159–171. [Google Scholar] [CrossRef]
- Oda, T.; Kato, T.; Takahashi, T.; Shimizu, K. Nitric oxide decomposition in air by using non-thermal plasma processing—With additives and catalyst. J. Electrost. 1997, 42, 151–157. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, T.; Liu, H.; Chen, D.; Xu, B.; Qing, C. Low temperature SCR reaction over Nano-Structured Fe-Mn Oxides: Characterization, performance, and kinetic study. Appl. Surf. Sci. 2018, 457, 1116–1125. [Google Scholar] [CrossRef]
- Qiu, L.; Pang, D.; Zhang, C.; Meng, J.; Zhu, R.; Ouyang, F. In situ IR studies of Co and Ce doped Mn/TiO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2015, 357, 189–196. [Google Scholar] [CrossRef]
- You, X.; Sheng, Z.; Yu, D.; Yang, L.; Xiao, X.; Wang, S. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 423, 845–854. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yan, Z.; Liu, L.; Zhang, Z.; Wang, X. Promoting effect of Nd on the reduction of NO with NH3 over CeO2 supported by activated semi-coke: An in situ DRIFTS study. Catal. Sci. Technol. 2015, 5, 2251–2259. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Li, J.; Ma, L.; Woo, S.I. Novel Mn–Ce–Ti Mixed-Oxide Catalyst for the Selective Catalytic Reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2014, 6, 14500–14508. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Liu, J.; Liu, H.; Wang, Y.; Sun, B. Effects of temperature on NOx removal with Mn-Cu/ZSM5 catalysts assisted by plasma. Appl. Therm. Eng. 2018, 130, 1224–1232. [Google Scholar] [CrossRef]
- Penetrante, B.M.; Bardsley, J.N.; Hsiao, M.C. Kinetic Analysis of Non-Thermal Plasmas Used for Pollution Control. Jpn. J. Appl. Phys. 1997, 36, 5007. [Google Scholar] [CrossRef]
- Heck, R.M. Catalytic abatement of nitrogen oxides–stationary applications. Catal. Today 1999, 53, 519–523. [Google Scholar] [CrossRef]
- Kim, H.H.; Takashima, K.; Katsura, S.; Mizuno, A. Low-temperature NOx reduction processes using combined systems of pulsed corona discharge and catalysts. J. Phys. D Appl. Phys. 2001, 34, 604. [Google Scholar] [CrossRef]
- Niu, J.; Yang, X.; Zhu, A.; Shi, L.; Sun, Q.; Xu, Y.; Shi, C. Plasma-assisted selective catalytic reduction of NOx by C2H2 over Co-HZSM-5 catalyst. Catal. Commun. 2006, 7, 297–301. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Jia, Y.; Liu, X.; Zhong, Q. Selective catalytic oxidation of NO with O2 over Ce-doped MnOx/TiO2 catalysts. J. Nat. Gas Chem. 2012, 21, 17–24. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, H.; Li, Q.; Zheng, J.; Zhang, X. Catalytic reduction of NO by NH3 over Fe–Cu–Ox/CNTs-TiO2 composites at low temperature. Appl. Catal. A Gen. 2012, 427–428, 43–48. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, S.; Sun, H.; Shu, Y.; Quan, X. Low-temperature selective catalytic reduction of NOx with NH3 over hierarchically macro-mesoporous Mn/TiO2. Catal. Commun. 2013, 42, 10–13. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Chu, C.; Li, N.; Li, J.; Zhao, S. In-situ DRIFTS for the mechanistic studies of NO oxidation over α-MnO2, β-MnO2 and γ-MnO2 catalysts. Chem. Eng. J. 2017, 322, 525–537. [Google Scholar] [CrossRef]
- Gao, Y.; Luan, T.; Zhang, W.; Li, H. The promotional effects of cerium on the catalytic properties of Al2O3-supported MnFeOx for NO oxidation and fast SCR reaction. Res. Chem. Intermed. 2018, in press. [Google Scholar] [CrossRef]
- Pérez Vélez, R.; Ellmers, I.; Huang, H.; Bentrup, U.; Schünemann, V.; Grünert, W.; Brückner, A. Identifying active sites for fast NH3-SCR of NO/NO2 mixtures over Fe-ZSM-5 by operando EPR and UV–vis spectroscopy. J. Catal. 2014, 316, 103–111. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; Han, D.; He, F.; Li, F.; Qi, K. Effects of surface physicochemical properties on NH3-SCR activity of MnO2 catalysts with different crystal structures. Chin. J. Catal. 2017, 38, 1925–1934. [Google Scholar] [CrossRef]
- Boningari, T.; Pappas, D.K.; Ettireddy, P.R.; Kotrba, A.; Smirniotis, P.G. Influence of SiO2 on M/TiO2 (M = Cu, Mn, and Ce) Formulations for Low-Temperature Selective Catalytic Reduction of NOx with NH3: Surface Properties and Key Components in Relation to the Activity of NOx Reduction. Ind. Eng. Chem. Res. 2015, 54, 2261–2273. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, W.; Xie, A.; Wu, F.; Yao, C.; Li, X.; Zuo, S.; Liu, T. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2–Palygorskite. Chem. Eng. J. 2016, 286, 291–299. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, C.; Wang, H.; Zhang, M. Synthesis of highly efficient MnOx catalyst for low-temperature NH3-SCR prepared from Mn-MOF-74 template. Mater. Lett. 2016, 168, 17–19. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, X.; Su, D.; Wang, Z.; Chang, J.; Ma, C. NO reduction by CO over copper catalyst supported on mixed CeO2 and Fe2O3: Catalyst design and activity test. Appl. Catal. B Environ. 2018, 239, 485–501. [Google Scholar] [CrossRef]
- Stanciulescu, M.; Caravaggio, G.; Dobri, A.; Moir, J.; Burich, R.; Charland, J.P.; Bulsink, P. Low-temperature selective catalytic reduction of NOx with NH3 over Mn-containing catalysts. Appl. Catal. B Environ. 2012, 123–124, 229–240. [Google Scholar] [CrossRef]
- Kong, F.; Qiu, j.; Liu, H.; Zhao, R.; Zeng, H. Effect of NO/SO2 on Elemental Mercury Adsorption by Nano-Fe2O3. Proc. CSEE (China) 2010, 30, 43–48. [Google Scholar]
- Cao, L.; Chen, L.; Wu, X.; Ran, R.; Xu, T.; Chen, Z.; Weng, D. TRA and DRIFTS studies of the fast SCR reaction over CeO2/TiO2 catalyst at low temperatures. Appl. Catal. A Gen. 2018, 557, 46–54. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Seo, C.Y.; Nahata, M.; Chen, X.; Li, J.; Schwank, J.W. Shape dependence and sulfate promotion of CeO2 for selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2018, 232, 246–259. [Google Scholar] [CrossRef]
- Xiong, Z.-B.; Liu, J.; Zhou, F.; Liu, D.-Y.; Lu, W.; Jin, J.; Ding, S.-F. Selective catalytic reduction of Nox with NH3 over iron-cerium-tungsten mixed oxide catalyst prepared by different methods. Appl. Surf. Sci. 2017, 406, 218–225. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Shan, W.; Shi, X. Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst. Catal. Today 2011, 175, 18–25. [Google Scholar] [CrossRef]
- Fan, X.; Qiu, F.; Yang, H.; Tian, W.; Hou, T.; Zhang, X. Selective catalytic reduction of NOX with ammonia over Mn–Ce–Ox/TiO2-carbon nanotube composites. Catal. Commun. 2011, 12, 1298–1301. [Google Scholar] [CrossRef]
- Guan, D.S.; Wang, Y. Synthesis and growth mechanism of multilayer TiO2 nanotube arrays. Nanoscale 2012, 4, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shen, C.; Zhang, Z.; Barrios, E.; Zhai, L. Core–Shell Composite Fibers for High-Performance Flexible Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 4041–4049. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, F.; Zhu, M.; Wang, X.; Dan, J.; Zhang, J.; Cao, P.; Dai, B. Microspherical MnO2-CeO2-Al2O3 mixed oxide for monolithic honeycomb catalyst and application in selective catalytic reduction of NOx with NH3 at 50–150 °C. Chem. Eng. J. 2018, 346, 182–192. [Google Scholar] [CrossRef]
- Kapteijn, F.; Singoredjo, L.; Andreini, A.; Moulijn, J.A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 1994, 3, 173–189. [Google Scholar] [CrossRef]
- Gao, G.; Shi, J.-W.; Liu, C.; Gao, C.; Fan, Z.; Niu, C. Mn/CeO2 catalysts for SCR of NOx with NH3: Comparative study on the effect of supports on low-temperature catalytic activity. Appl. Surf. Sci. 2017, 411, 338–346. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Li, X.; Liu, Y.; Liu, Y.; Ma, J. Fabrication of hollow carbon nanospheres introduced with Fe and N species immobilized palladium nanoparticles as catalysts for the semihydrogenation of phenylacetylene under mild reaction conditions. Appl. Surf. Sci. 2017, 404, 398–408. [Google Scholar] [CrossRef]
- Wang, T.; Wan, Z.; Yang, X.; Zhang, X.; Niu, X.; Sun, B. Promotional effect of iron modification on the catalytic properties of Mn-Fe/ZSM-5 catalysts in the Fast SCR reaction. Fuel Process. Technol. 2018, 169, 112–121. [Google Scholar] [CrossRef]
- Hu, H.; Cai, S.; Li, H.; Huang, L.; Shi, L.; Zhang, D. Mechanistic Aspects of deNOx Processing over TiO2 Supported Co–Mn Oxide Catalysts: Structure–Activity Relationships and In Situ DRIFTs Analysis. ACS Catal. 2015, 5, 6069–6077. [Google Scholar] [CrossRef]
- Li, K.; Tang, X.; Yi, H.; Ning, P.; Kang, D.; Wang, C. Low-temperature catalytic oxidation of NO over Mn–Co–Ce–Ox catalyst. Chem. Eng. J. 2012, 192, 99–104. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx–CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- Huang, B.; Yu, D.; Sheng, Z.; Yang, L. Novel CeO2@TiO2 core–shell nanostructure catalyst for selective catalytic reduction of NOx with NH3. J. Environ. Sci. 2017, 55, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Boningari, T.; Somogyvari, A.; Smirniotis, P.G. Ce-Based Catalysts for the Selective Catalytic Reduction of NOx in the Presence of Excess Oxygen and Simulated Diesel Engine Exhaust Conditions. Ind. Eng. Chem. Res. 2017, 56, 5483–5494. [Google Scholar] [CrossRef]
- Ruggeri, M.P.; Grossale, A.; Nova, I.; Tronconi, E.; Jirglova, H.; Sobalik, Z. FTIR in situ mechanistic study of the NH3NO/NO2 “Fast SCR” reaction over a commercial Fe-ZSM-5 catalyst. Catal. Today 2012, 184, 107–114. [Google Scholar] [CrossRef]
- Huang, T.-J.; Zhang, Y.-P.; Zhuang, K.; Lu, B.; Zhu, Y.-W.; Shen, K. Preparation of honeycombed holmium-modified Fe-Mn/TiO2 catalyst and its performance in the low temperature selective catalytic reduction of NOx. J. Fuel Chem. Technol. 2018, 46, 319–327. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T.; Fuertes, A.B. Mechanism of low-temperature selective catalytic reduction of NO with NH3 over carbon-supported Mn3O4: Role of surface NH3 species: SCR mechanism. J. Catal. 2004, 226, 138–155. [Google Scholar] [CrossRef]
- Wang, J.; Yi, H.; Tang, X.; Zhao, S.; Gao, F.; Yang, Z. Oxygen plasma-catalytic conversion of NO over MnOx: Formation and reactivity of adsorbed oxygen. Catal. Commun. 2017, 100, 227–231. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Liu, J.; Liu, H.; Guo, Y.; Sun, B. Plasma-assisted catalytic conversion of NO over Cu-Fe catalysts supported on ZSM-5 and carbon nanotubes at low temperature. Fuel Process. Technol. 2018, 178, 53–61. [Google Scholar] [CrossRef]
| Samples | Specific Input Energy (W·h/m3) | NOx Conversion (%) | Temperature (°C) | Reductant | GHSV (h−1) | Gas Flow Rate (m3/h) | Ref |
|---|---|---|---|---|---|---|---|
| V2O5-WO3/TiO2 | 4.7 a | ~76.5 | 170 | NH3 | -- | 31.8 | [11] |
| H-mordenite | 5 | 76 | 160 | NH3 | 20,000 | 31 | [7] |
| Ag/Al2O3 | 16.7 a | ~91 | 350 | C3H6 | 10,000 | 1.2 | [12] |
| BaTiO3-Al2O3 | ~40.3 a | ~61.5 | 150 | CH3OH | 11,000 | -- | [22] |
| Cu-ZSM-5 | 37.5 a | ~90 | 25 b | C2H4 | -- | 0.12 | [13] |
| Co-ZSM-5 | 8.3 a | ~70.6 | 150 | C2H4+NH3 | 1000 | 0.12 | [22] |
| Co-HZSM-5 | 38.3 | ~92 | 300 | C2H2 | 12,000 | 0.03 | [23] |
| Mn−Ce/ZSM5−MWCNTs | 16.7 a | ~85 | 25 | NH3 | 60,000 | 0.12 | [4] |
| Mn−Ce/TiO2 | 15 | 99.5 | 25 | NH3 | 20,000 | 0.1 | This study |
| Samples | SBET (m2·g−1) | Vtotal (cm3·g−1) | Dp (nm) |
|---|---|---|---|
| Mn−Ce/TiO2 | 239.7 | 0.527 | 17.57 |
| Mn−Co/TiO2 | 189.9 | 0.531 | 33.06 |
| Mn−Fe/TiO2 | 104.6 | 0.424 | 54.85 |
| Samples | Temperature (°C) | H2 Consumption (mmol·g−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R 1 | R 2 | R 3 | R 4 | R 5 | R 1 | R 2 | R 3 | R 4 | R 5 | Total | |
| Mn−Ce/TiO2 | 261 | 336 | 367 | 423 | 717 | 2.52 | 1.24 | 0.36 | 0.67 | 0.07 | 4.86 |
| Mn−Co/TiO2 | 218 | 327 | 418 | 517 | -- | 1.14 | 0.21 | 2.12 | 0.96 | -- | 4.43 |
| Mn−Fe/TiO2 | 276 | 387 | 436 | 501 | -- | 0.83 | 0.41 | 0.54 | 1.59 | -- | 3.37 |
| Samples | Temperature (°C) | NH3 Composition (mmol·g−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 1 | Peak 2 | Peak 3 | Peak 4 | Total | |
| Mn−Ce/TiO2 | 245 | 396 | 583 | 647 | 0.27 | 0.52 | 0.58 | 0.39 | 1.76 |
| Mn−Co/TiO2 | 276 | 402 | 587 | 653 | 0.11 | 0.49 | 0.52 | 0.32 | 1.44 |
| Mn−Fe/TiO2 | 209 | 398 | 585 | 649 | 0.14 | 0.24 | 0.39 | 0.20 | 0.97 |
| Samples | Binding Energy (eV)/Atomic Composition (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | Fe | Co | Ce | O | |||||||
| Mn2+ | Mn3+ | Mn4+ | Fe2+ | Fe3+ | Co2+ | Co3+ | Ce3+ | Ce4+ | Oα | Oβ | |
| Mn−Ce/TiO2 | 640.9/ 9.3 | 642.4/ 34.2 | 644.0/ 56.5 | -/- | -/- | -/- | -/- | 885.6/ 40.4 | 882.4/ 59.6 | 531.2/ 40.4 | 530.2/ 59.6 |
| Mn−Co/TiO2 | 641.2/ 13.8 | 642.6/ 39.4 | 644.1/ 46.8 | -/- | -/- | 779.6 39.3 | 782.1/ 60.7 | -/- | -/- | 531.4/ 33.7 | 530.3/ 66.3 |
| Mn−Fe/TiO2 | 641.4/ 21.3 | 642.8/ 38.5 | 644.6/ 40.2 | 709.6/ 43.4 | 711.7/ 56.6 | -/- | -/- | -/- | -/- | 531.6/ 28.2 | 530.3/ 71.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Jiang, W.; Luan, T.; Li, H.; Zhang, W.; Feng, W.; Jiang, H. High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species. Catalysts 2019, 9, 103. https://doi.org/10.3390/catal9010103
Gao Y, Jiang W, Luan T, Li H, Zhang W, Feng W, Jiang H. High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species. Catalysts. 2019; 9(1):103. https://doi.org/10.3390/catal9010103
Chicago/Turabian StyleGao, Yan, Wenchao Jiang, Tao Luan, Hui Li, Wenke Zhang, Wenchen Feng, and Haolin Jiang. 2019. "High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species" Catalysts 9, no. 1: 103. https://doi.org/10.3390/catal9010103
APA StyleGao, Y., Jiang, W., Luan, T., Li, H., Zhang, W., Feng, W., & Jiang, H. (2019). High-Efficiency Catalytic Conversion of NOx by the Synergy of Nanocatalyst and Plasma: Effect of Mn-Based Bimetallic Active Species. Catalysts, 9(1), 103. https://doi.org/10.3390/catal9010103