Synthesis of Stable Hierarchical MIL-101(Cr) with Enhanced Catalytic Activity in the Oxidation of Indene
Abstract
:1. Introduction
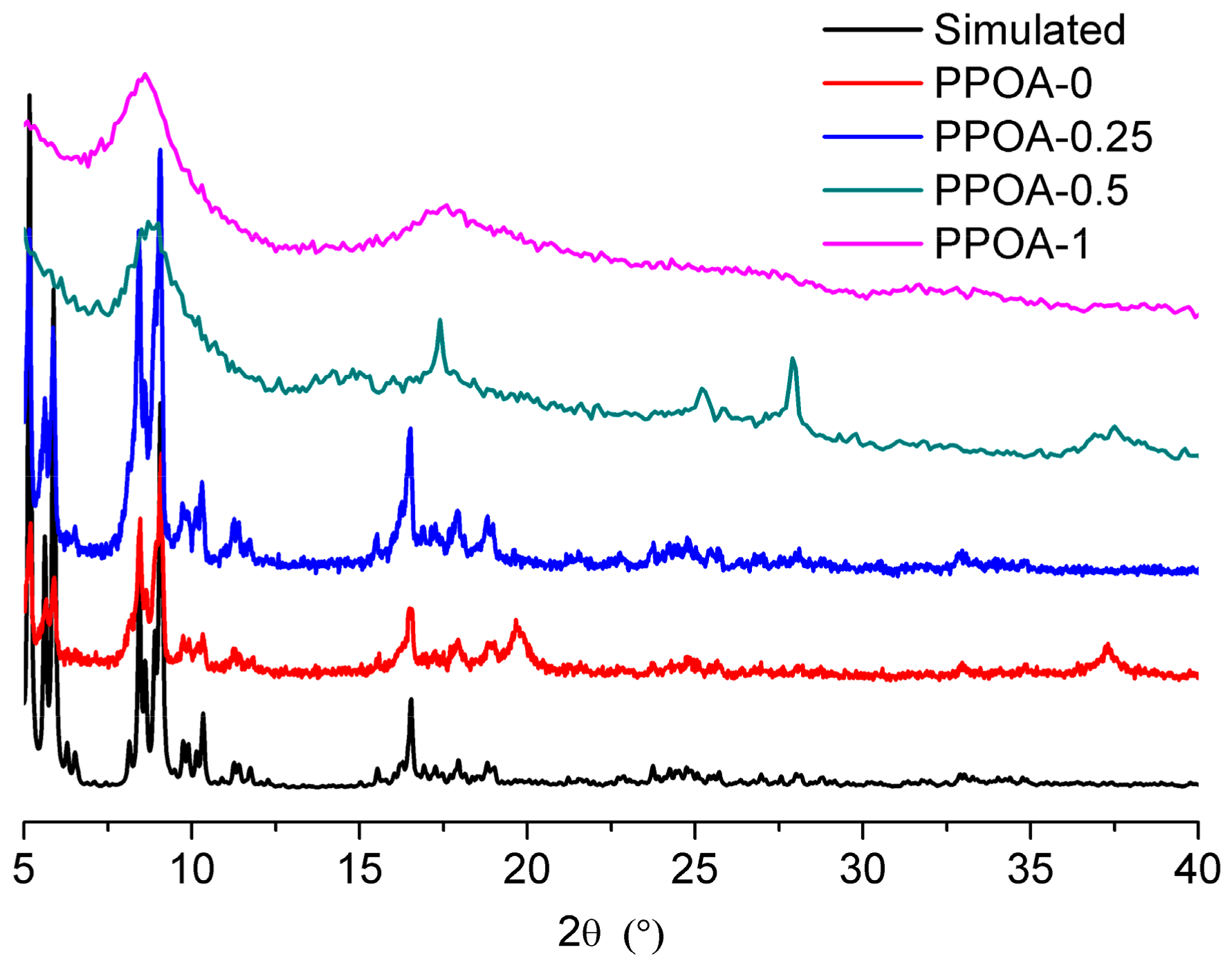
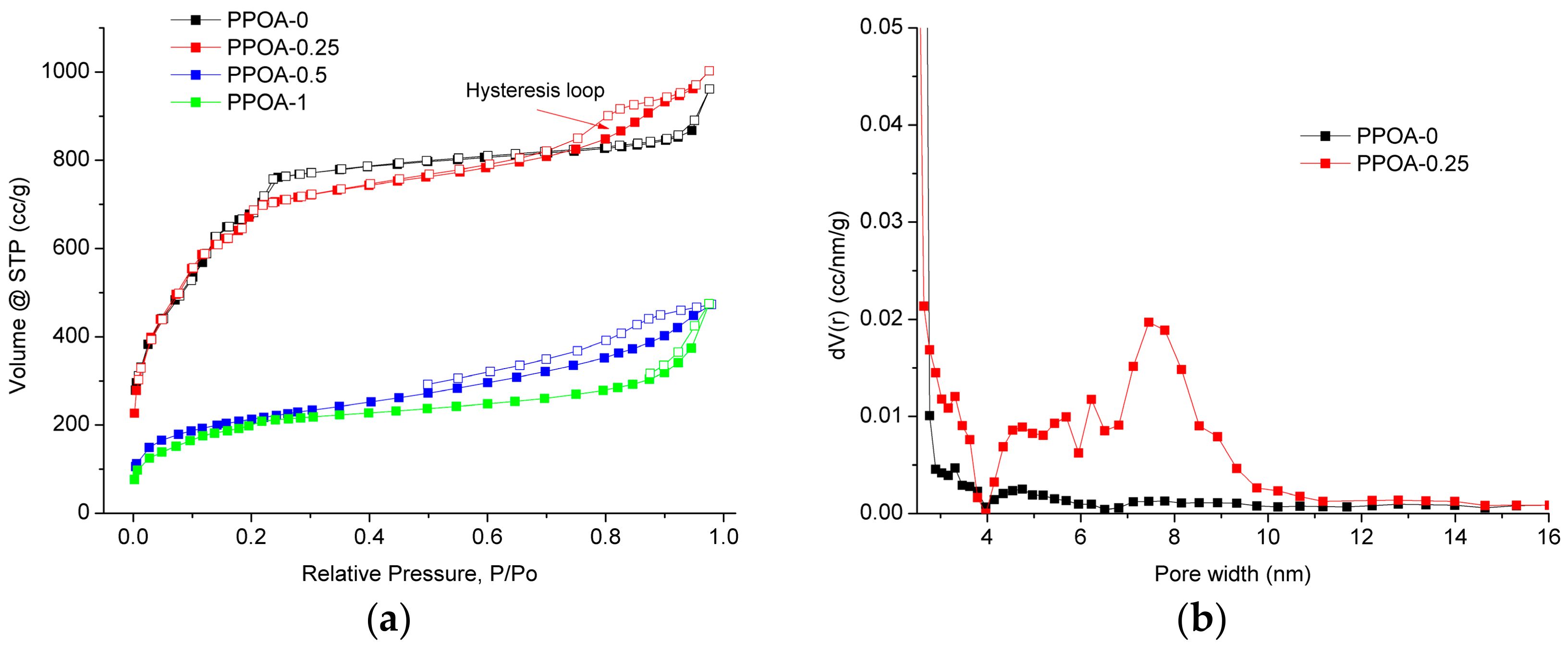
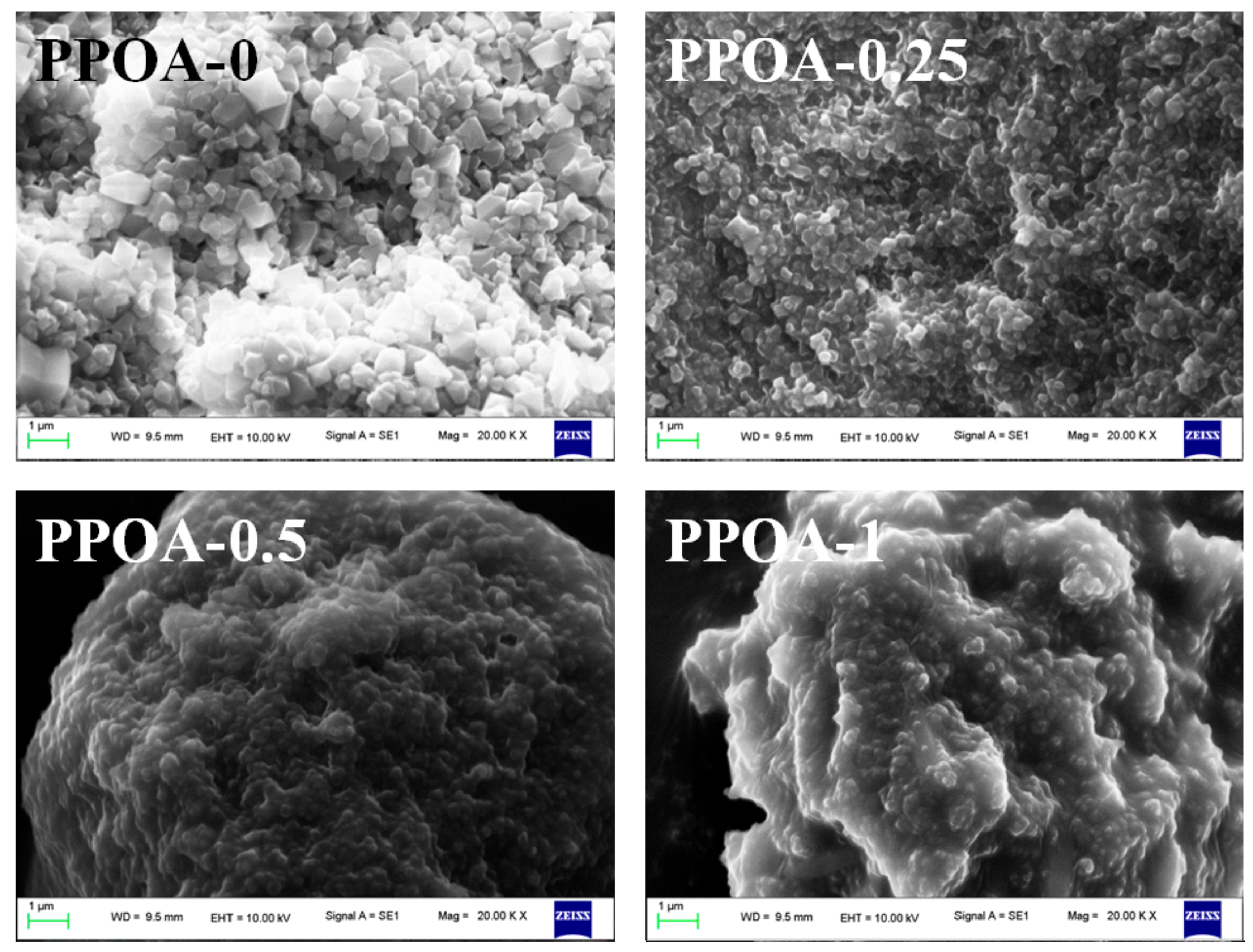
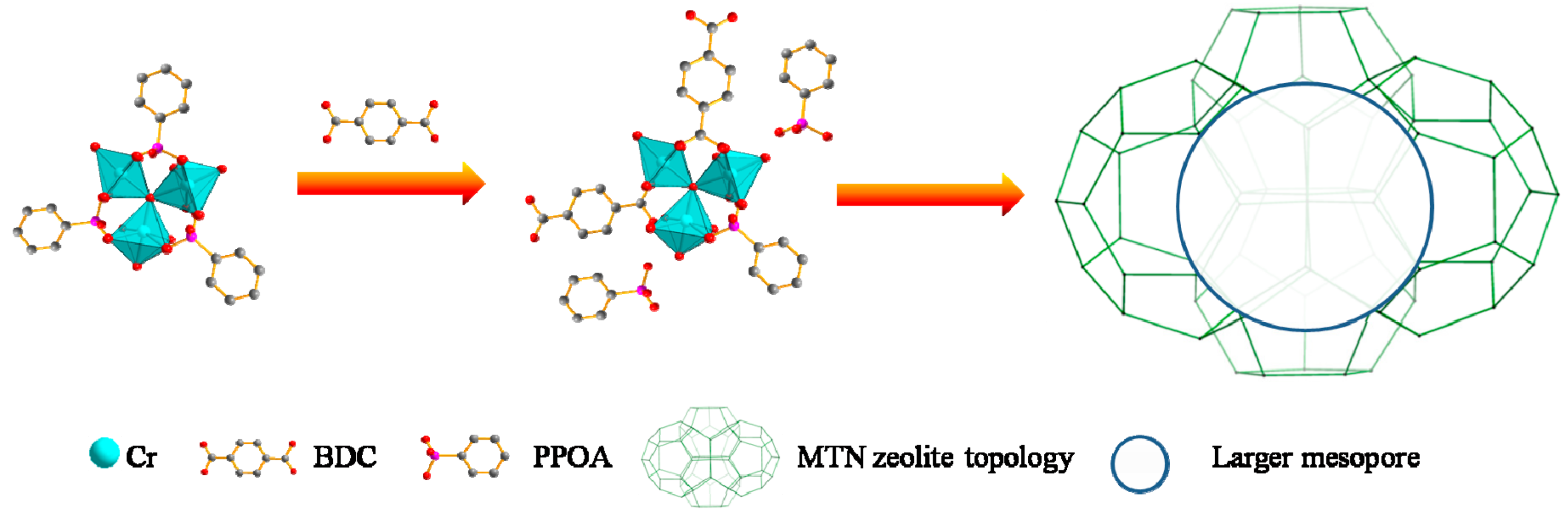
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of Hierarchical MIL-101(Cr)
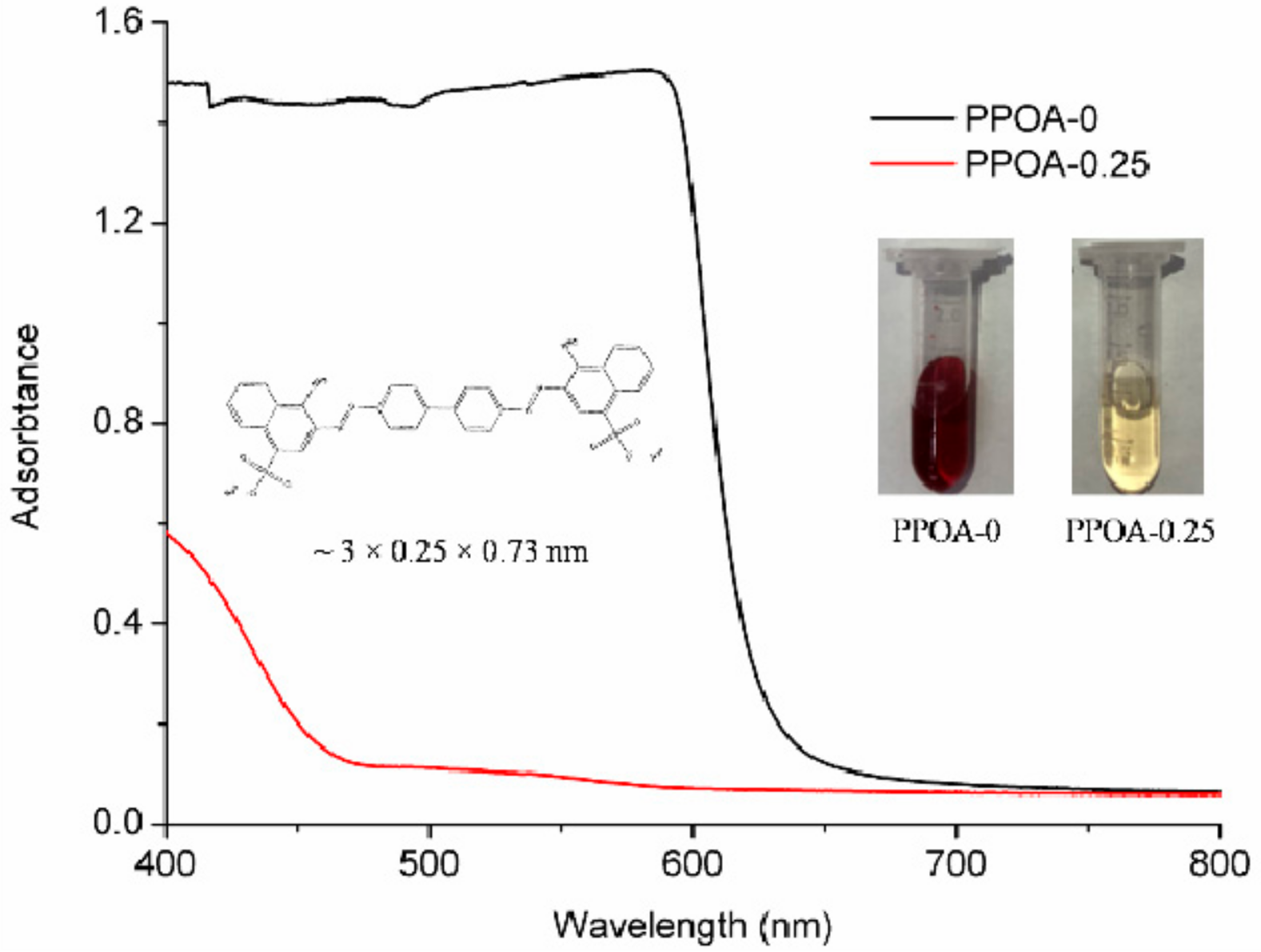
3.4. The Uptake of Large Molecules
3.5. Catalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal-organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001–3004. [Google Scholar] [CrossRef]
- Zhao, T.; Heering, C.; Boldog, I.; Domasevitchc, K.V.; Janiak, C. A view on systematic truncation of tetrahedralligands for coordination polymers. CrystEngComm 2017, 19, 776–780. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Zhao, T.; Dong, M.; Zhao, Y.; Liu, Y. Preparation and application of nano-sized metal-organic frameworks. Prog. Chem. 2017, 29, 1252–1259. [Google Scholar]
- Paik Suh, M.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar]
- Wang, Y.; Rui, M.; Lu, G. Recent applications of metal-organic frameworks in sample pretreatment. J. Sep. Sci. 2018, 41, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Liu, H.; Wang, Z.; Yu, X.; Yi, P.; Bai, J. Porous NbO-type metal-organic framework with inserted acylamide groups exhibiting highly selective CO2 capture. CrystEngComm 2013, 15, 3517–3520. [Google Scholar] [CrossRef]
- Xin, C.; Zhan, H.; Huang, X.; Li, H.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. Effect of various alkaline agents on the size and morphology of nano-sized HKUST-1 for CO2 adsorption. RSC Adv. 2015, 5, 27901–27911. [Google Scholar] [CrossRef]
- Herbst, A.; Khutia, A.; Janiak, C. Bronsted instead of Lewis Acidity in functionalized MIL-101Cr MOFs for eficient heterogeneous (nano-MOF) catalysis in the condensation reaction of aldehydes with alcohols. Inorg. Chem. 2014, 53, 7319–7333. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Huang, S.; Chen, L.; Li, Y.; Yang, X.; Yuan, Z.; Su, B. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Advancement of sorption-based heat transformation by a metal coating of highly-stable, hydrophilic aluminium fumarate MOF. RSC Adv. 2014, 4, 24073–24082. [Google Scholar] [CrossRef] [Green Version]
- Orellana-Tavra, C.; Mercado, S.A.; Fairen-Jimenez, D. Endocytosis mechanism of nano metal-organic Frameworks for drug delivery. Adv. Healthc. Mater. 2016, 5, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, L.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, G.; Chen, L.; Li, Y.; Rooke, J.C.; Wei, Y.; Liu, Z.; Deng, Z.; Tendeloo, G.V.; Su, B. Well-organized zeolite nanocrystal aggregates with interconnected hierarchically Micro-Meso-Macropore systems showing enhanced catalytic performance. Chem. Eur. J. 2011, 17, 14987–14995. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Binder, A.; Song, R.; Cui, Y.; Chen, J.; Hensleyd, D.; Dai, S. Encapsulation of large dye molecules in hierarchically superstructured metal-organic frameworks. Dalton Trans. 2014, 43, 17893–17898. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Qiao, Z.; Fulvio, P.; Binder, A.; Tian, C.; Chen, J.; Nelson, K.; Zhu, X.; Dai, S. Template-free synthesis of hierarchical porous metal-organic frameworks. J. Am. Chem. Soc. 2013, 135, 9572–9575. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Jiang, H. A modulator-induced defect-formation strategy to hierarchically porous Metal-Organic Frameworks with high stability. Angew. Chem. Int. Edit. 2017, 56, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar]
- Zhao, T.; Jeremias, F.; Boldog, I.; Nguyen, B.; Henninger, S.K.; Janiak, C. High-yield, fluoride-free and large-scale synthesis of MIL-101(Cr). Dalton Trans. 2015, 44, 16791–16801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Yang, L.; Feng, P.; Gruber, I.; Janiak, C.; Liu, Y. Facile synthesis of nano-sized MIL-101(Cr) with the addition of acetic acid. Inorg. Chim. Acta 2018, 471, 440–445. [Google Scholar] [CrossRef]
- Crystal Impact. Diamond–Crystal and Molecular Structure Visualization, Version 3.2; K. Brandenburg & H. Putz Gbr; Crystal Impact: Bonn, Germany, 2007–2012. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Ying, J.; Herbst, A.; Xiao, Y.; Wei, H.; Tian, G.; Li, Z.; Yang, X.; Su, B.; Janiak, C. Nanocoating of hydrophobic mesoporous silica around MIL-101Cr for enhanced catalytic activity and stability. Inorg. Chem. 2018, 57, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metal-organic framework UiO-66 and their important effects on gas asorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef] [PubMed]
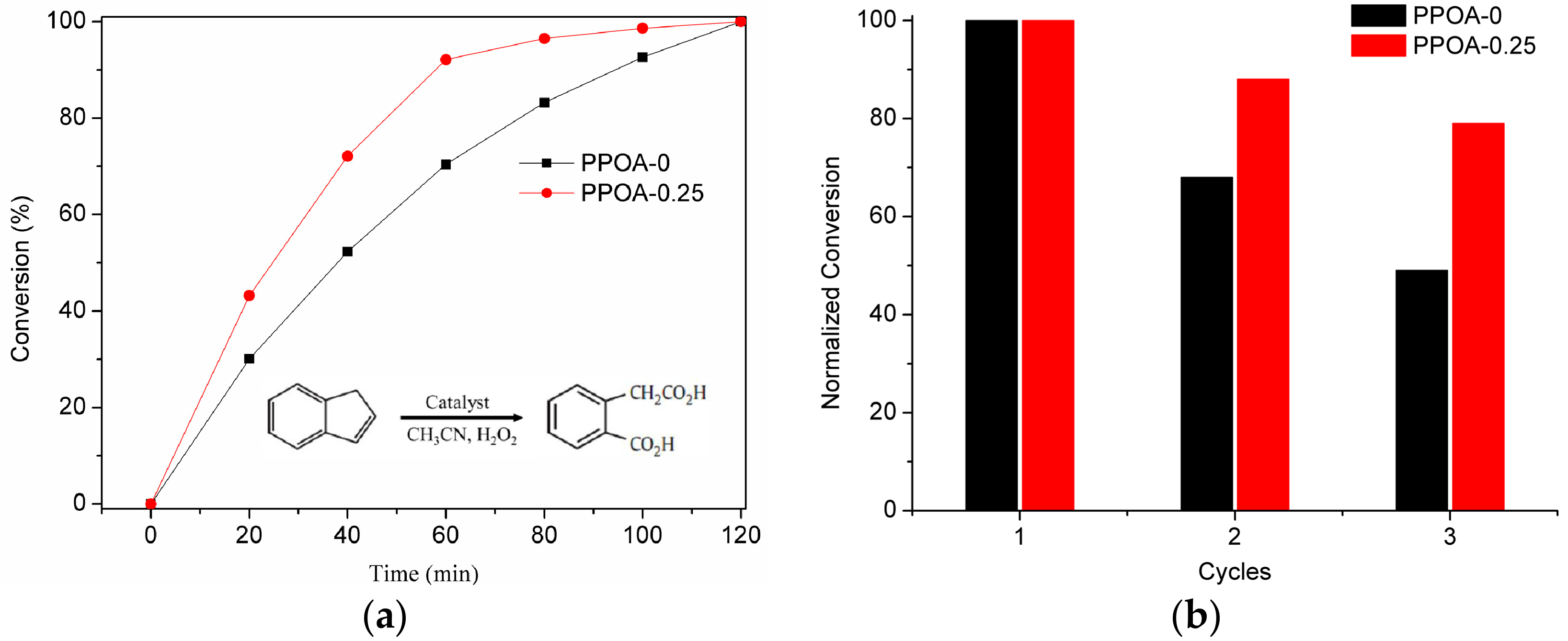
| Phenylphosphonic Acid | MOFtype | SBET | SLangmuir | Vpore | N2 Uptake |
|---|---|---|---|---|---|
| (PPOA)-(Equivalents) a | (m²/g) b | (m²/g) | (cm3/g) c | (cm3/g) c | |
| PPOA-0 | MIL-101 | 2487 | 3621 | 1.34 | 890 |
| PPOA-0.25 | MIL-101 | 2329 | 3350 | 1.49 | 970 |
| PPOA-0.5 | Unknown | 729 | 1039 | 0.69 | 448 |
| PPOA-1 | Unknown | 693 | 1020 | 0.58 | 442 |
| Catalysts/Weight | 0 mg (Conversion) | 5 mg (Conversion) | 10 mg (Conversion) | 15 mg (Conversion) |
|---|---|---|---|---|
| PPOA-0 | 0% | 69.2% | 70.4% | 71.9% |
| PPOA-0.25 | 0% | 82.1% | 92.3% | 92.7% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Dong, M.; Yang, L.; Liu, Y. Synthesis of Stable Hierarchical MIL-101(Cr) with Enhanced Catalytic Activity in the Oxidation of Indene. Catalysts 2018, 8, 394. https://doi.org/10.3390/catal8090394
Zhao T, Dong M, Yang L, Liu Y. Synthesis of Stable Hierarchical MIL-101(Cr) with Enhanced Catalytic Activity in the Oxidation of Indene. Catalysts. 2018; 8(9):394. https://doi.org/10.3390/catal8090394
Chicago/Turabian StyleZhao, Tian, Ming Dong, Ling Yang, and Yuejun Liu. 2018. "Synthesis of Stable Hierarchical MIL-101(Cr) with Enhanced Catalytic Activity in the Oxidation of Indene" Catalysts 8, no. 9: 394. https://doi.org/10.3390/catal8090394
APA StyleZhao, T., Dong, M., Yang, L., & Liu, Y. (2018). Synthesis of Stable Hierarchical MIL-101(Cr) with Enhanced Catalytic Activity in the Oxidation of Indene. Catalysts, 8(9), 394. https://doi.org/10.3390/catal8090394





