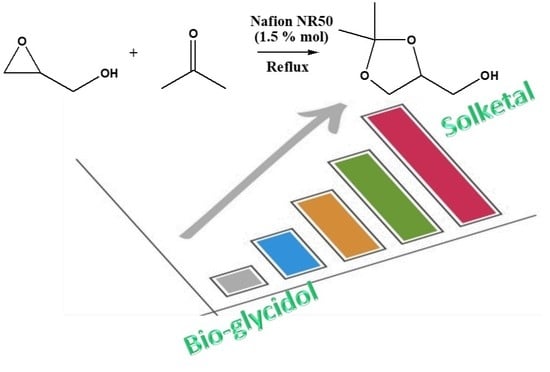
Bio-Glycidol Conversion to Solketal over Acid Heterogeneous Catalysts: Synthesis and Theoretical Approach
Abstract
:1. Introduction
2. Results and Discussion
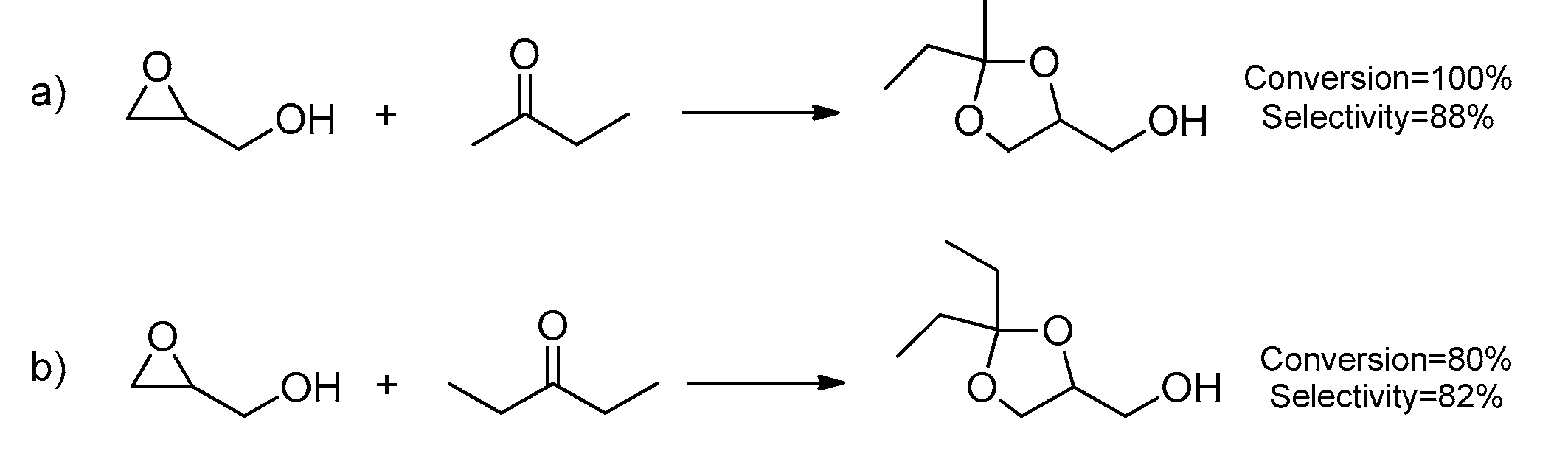
2.1. Glycidol Conversion to Solketal: Reaction Conditions Optimization
2.2. Theoretical Investigation of the Reaction Mechanism
3. Materials and Methods
3.1. Materials
3.2. Catalytic Conversion of Glycidol to Solketal: General Conditions
3.3. Gas-Chromatographic (GC-FID) Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cespi, D.; Passarini, F.; Vassura, I.; Cavani, F. Butadiene from biomass, a life cycle perspective to address sustainability in the chemical industry. Green Chem. 2016, 18, 1625–1638. [Google Scholar] [CrossRef]
- Tripodi, A.; Bahadori, E.; Cespi, D.; Passarini, F.; Cavani, F.; Tabanelli, T.; Rossetti, I. Acetonitrile from Bioethanol Ammoxidation: Process Design from the Grass-Roots and Life Cycle Analysis. ACS Sustain. Chem. Eng. 2018, 6, 5441–5451. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. In Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; pp. 29–56. ISBN 0-19-850234-6. [Google Scholar]
- Cespi, D.; Cucciniello, R.; Ricciardi, M.; Capacchione, C.; Vassura, I.; Passarini, F.; Proto, A. A simplified early stage assessment of process intensification: Glycidol as a value-added product from epichlorohydrin industry wastes. Green Chem. 2016, 18, 4559–4570. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. The Future of Glycerol; RSC Publishing: Cambridge, UK, 2010; pp. 1–25. ISBN 978-1-84973-046-4. [Google Scholar]
- Canale, V.; Tonucci, L.; Bressan, M.; d’Alessandro, N. Deoxydehydration of glycerol to allyl alcohol catalyzed by rhenium derivatives. Catal. Sci. Technol. 2014, 4, 3697–3704. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Cucciniello, R.; Pironti, C.; Capacchione, C.; Proto, A.; Di Serio, M. Efficient and selective conversion of glycidol to 1, 2-propanediol over Pd/C catalyst. Catal. Commun. 2016, 77, 98–102. [Google Scholar] [CrossRef]
- Cucciniello, R.; Ricciardi, M.; Vitiello, R.; Di Serio, M.; Proto, A.; Capacchione, C. Synthesis of Monoalkyl Glyceryl Ethers by Ring Opening of Glycidol with Alcohols in the Presence of Lewis Acids. ChemSusChem 2016, 9, 3272–3275. [Google Scholar] [CrossRef] [PubMed]
- Vicente, G.; Melero, J.A.; Morales, G.; Paniagua, M.; Martín, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12, 899–907. [Google Scholar] [CrossRef]
- Samoilov, V.O.; Onishchenko, M.O.; Ramazanov, D.N.; Maximov, A.L. Glycerol Isopropyl Ethers: Direct Synthesis from Alcohols and Synthesis by the Reduction of Solketal. ChemCatChem 2017, 9, 2839–2849. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Li, L.; Korányi, T.I.; Sels, B.F.; Pescarmona, P.P. Highly-efficient conversion of glycerol to solketal over heterogeneous Lewis acid catalysts. Green Chem. 2012, 14, 1611–1619. [Google Scholar] [CrossRef]
- Iranpoor, N.; Kazemi, F. Ru(III) catalyses the conversion of epoxides to 1,3-dioxolanes. Synth. Commun. 1998, 28, 3189–3193. [Google Scholar] [CrossRef]
- Iranpoor, N.; Adibi, H. Iron(III) Trifluoroacetate as an Efficient Catalyst for Solvolytic and Nonsolvolytic Nucleophilic Ring Opening of Epoxides. BCSJ 2000, 73, 675–680. [Google Scholar] [CrossRef]
- Procopio, A.; Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Nardi, M.; Russo, B. Synthesis of Acetonides from Epoxides Catalyzed by Erbium(III) Triflate. Adv. Synth. Catal. 2005, 347, 1447–1450. [Google Scholar] [CrossRef]
- Ricciardi, M.; Passarini, F.; Vassura, I.; Proto, A.; Capacchione, C.; Cucciniello, R.; Cespi, D. Glycidol, a Valuable Substrate for the Synthesis of Monoalkyl Glyceryl Ethers: A Simplified Life Cycle Approach. ChemSusChem 2017, 10, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.; Passarini, F.; Capacchione, C.; Proto, A.; Barrault, J.; Cucciniello, R.; Cespi, D. First Attempt of Glycidol-to-Monoalkyl Glyceryl Ethers Conversion by Acid Heterogeneous Catalysis: Synthesis and Simplified Sustainability Assessment. ChemSusChem 2018, 11, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, F.; Buonerba, A.; Grassi, A.; Capacchione, C.; Milione, S. Glycidol: An Hydroxyl-Containing Epoxide Playing the Double Role of Substrate and Catalyst for CO2 Cycloaddition Reactions. ChemSusChem 2016, 9, 3457–3464. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpoor-Baltork, I.; Khosropour, A.R.; Aliyan, H. Efficient Conversion of Epoxides to 1,3-Dioxolanes Catalyzed by Bismuth(III) Salts. Synth. Commun. 2001, 31, 3411–3416. [Google Scholar] [CrossRef]
- Lange, J.-P. Don’t Forget Product Recovery in Catalysis Research—Check the Distillation Resistance. ChemSusChem 2017, 10, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.; Cespi, D.; Celentano, M.; Genga, A.; Malitesta, C.; Proto, A.; Capacchione, C.; Cucciniello, R. Bio-propylene glycol as value-added product from Epicerol® process. Sustain. Chem. Pharm. 2017, 6, 10–13. [Google Scholar] [CrossRef]
- Tayade, K.N.; Mishra, M.; Munusamy, K.; Somani, R.S. Synthesis of aluminium triflate-grafted MCM-41 as a water-tolerant acid catalyst for the ketalization of glycerol with acetone. Catal. Sci. Technol. 2015, 5, 2427–2440. [Google Scholar] [CrossRef]
- Cucciniello, R.; Cespi, D. Recycling within the Chemical Industry: The Circular Economy Era. Recycling 2018, 3, 22. [Google Scholar] [CrossRef]


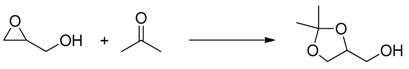
| Experiment | Catalyst | Conversion (%) | Selectivity to Solketal (%) | Yield (%) |
|---|---|---|---|---|
| 1 | Nafion NR50 | 90 | 88 | 79 |
| 2 | Amberlyst-15 | 100 | 8 | 8 |
| 3 | Bi(OTf)3 on MS | 100 | 86 | 86 |
| 4 | Al(OTf)3 on MS | 100 | 93 | 93 |
| 5 | Fe(OTf)3 on MS | 100 | 87 | 87 |
| 6 | No catalyst | 0 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, M.; Falivene, L.; Tabanelli, T.; Proto, A.; Cucciniello, R.; Cavani, F. Bio-Glycidol Conversion to Solketal over Acid Heterogeneous Catalysts: Synthesis and Theoretical Approach. Catalysts 2018, 8, 391. https://doi.org/10.3390/catal8090391
Ricciardi M, Falivene L, Tabanelli T, Proto A, Cucciniello R, Cavani F. Bio-Glycidol Conversion to Solketal over Acid Heterogeneous Catalysts: Synthesis and Theoretical Approach. Catalysts. 2018; 8(9):391. https://doi.org/10.3390/catal8090391
Chicago/Turabian StyleRicciardi, Maria, Laura Falivene, Tommaso Tabanelli, Antonio Proto, Raffaele Cucciniello, and Fabrizio Cavani. 2018. "Bio-Glycidol Conversion to Solketal over Acid Heterogeneous Catalysts: Synthesis and Theoretical Approach" Catalysts 8, no. 9: 391. https://doi.org/10.3390/catal8090391
APA StyleRicciardi, M., Falivene, L., Tabanelli, T., Proto, A., Cucciniello, R., & Cavani, F. (2018). Bio-Glycidol Conversion to Solketal over Acid Heterogeneous Catalysts: Synthesis and Theoretical Approach. Catalysts, 8(9), 391. https://doi.org/10.3390/catal8090391