Zeolite Supported Ionic Liquid Catalysts for the Hydrochlorination of Acetylene
Abstract
1. Introduction
2. Results and Discussion
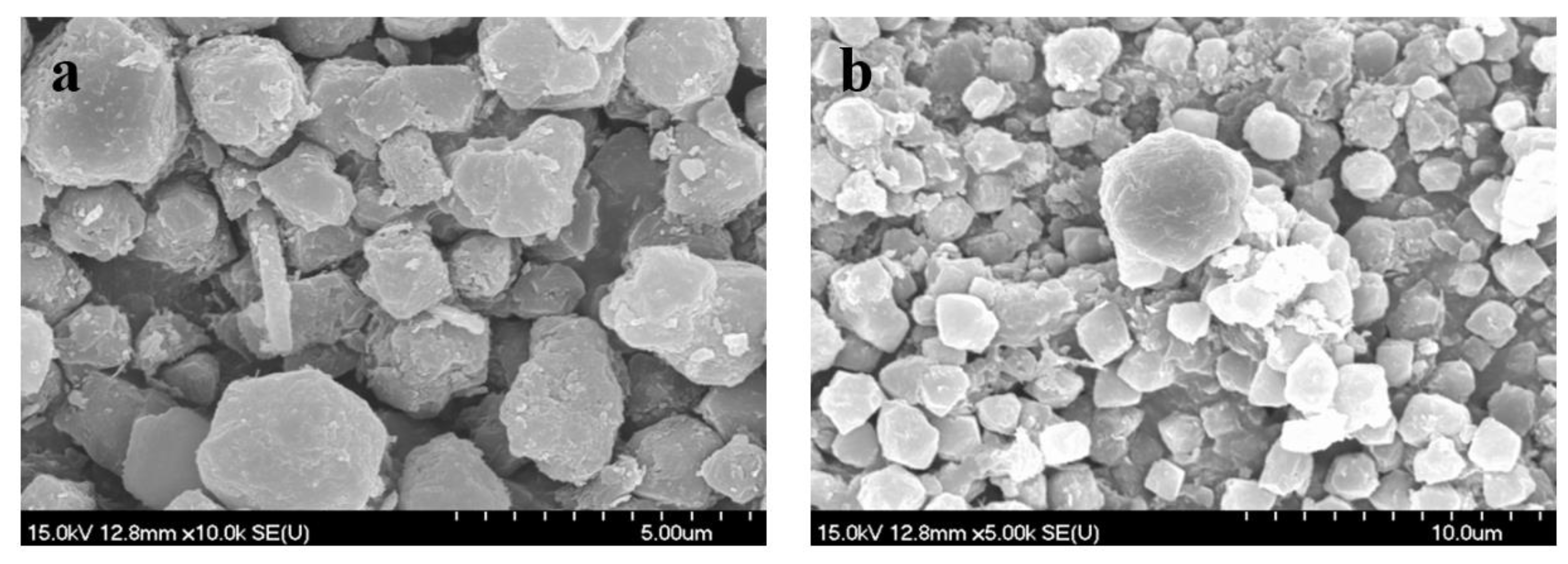
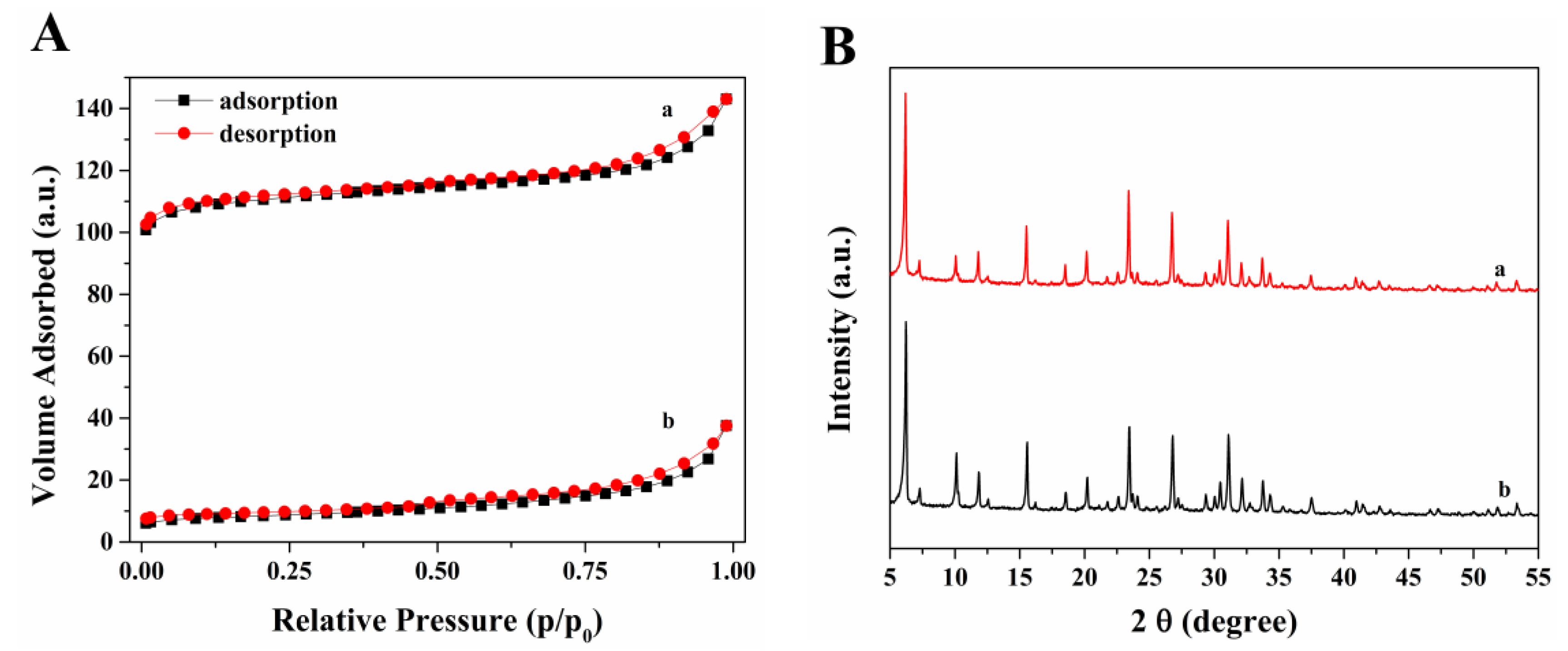
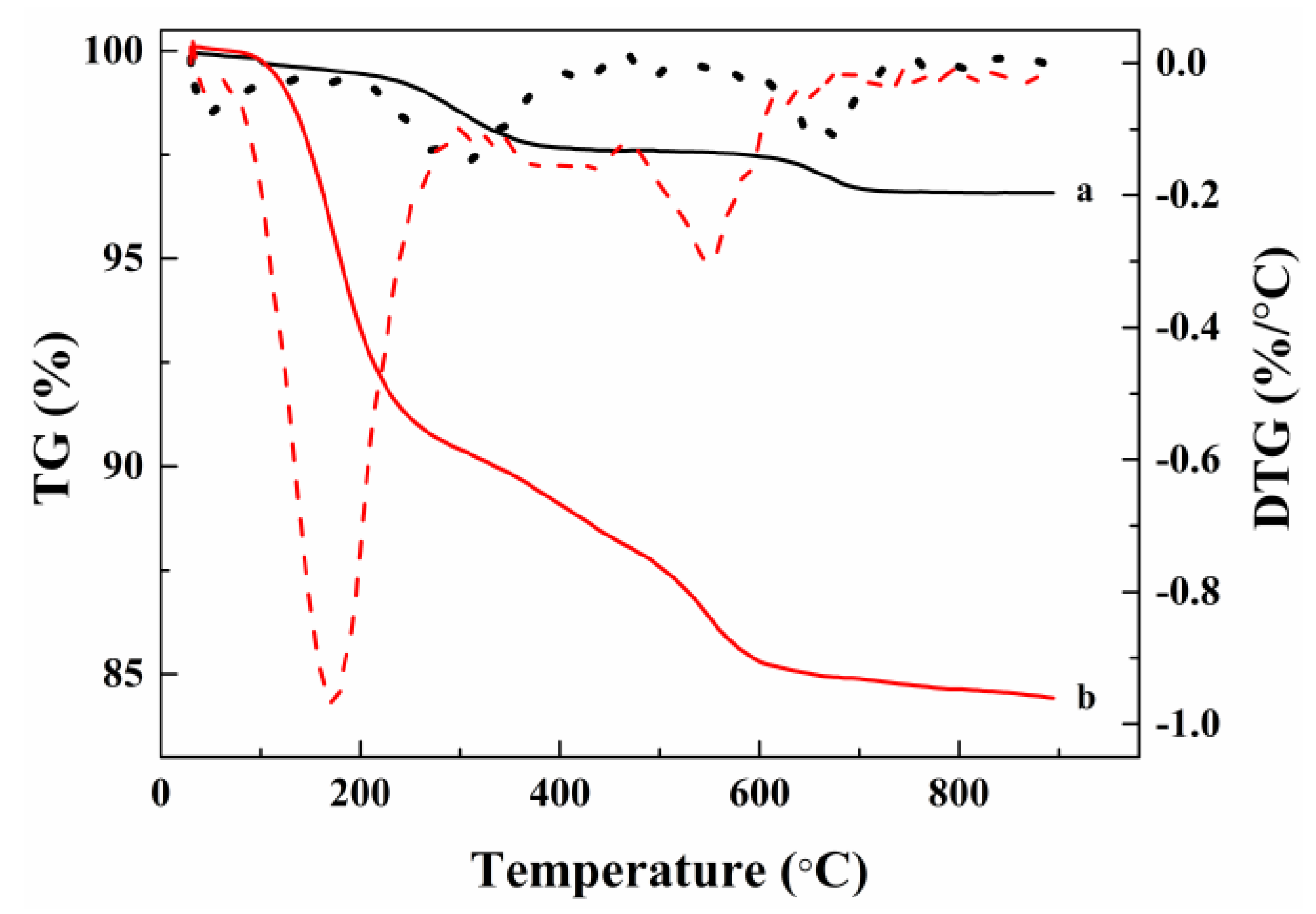
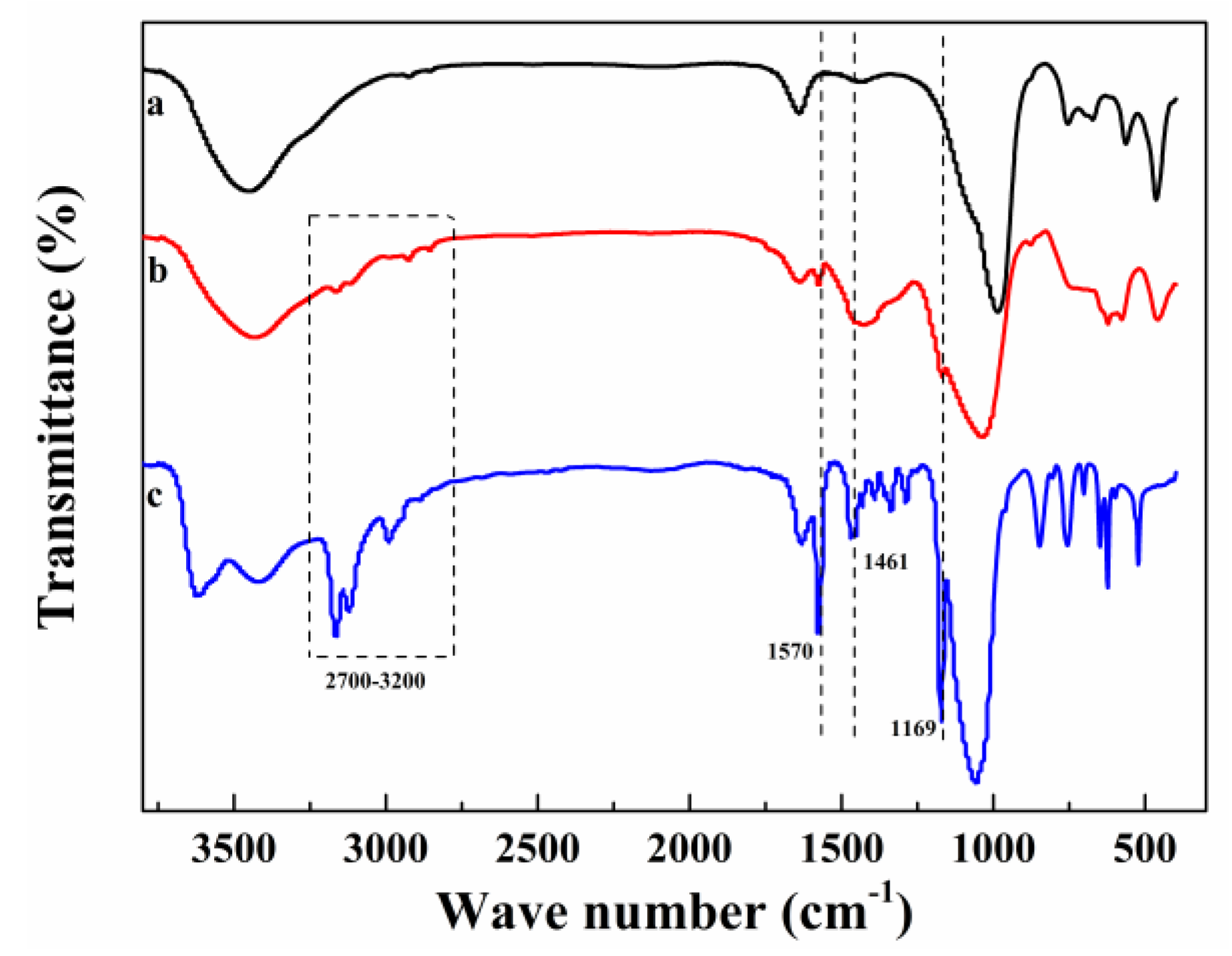
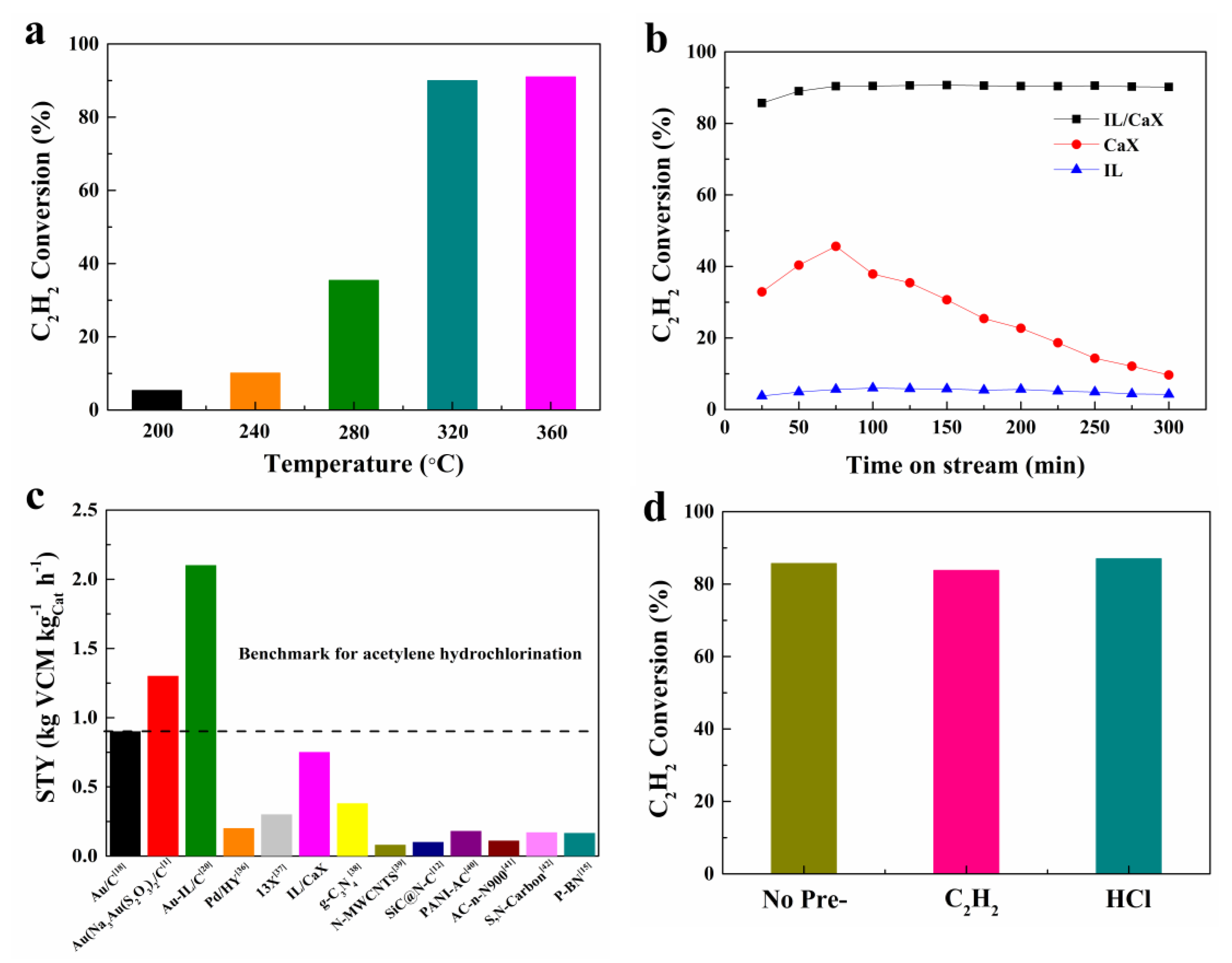
2.1. Catalytic Characterization
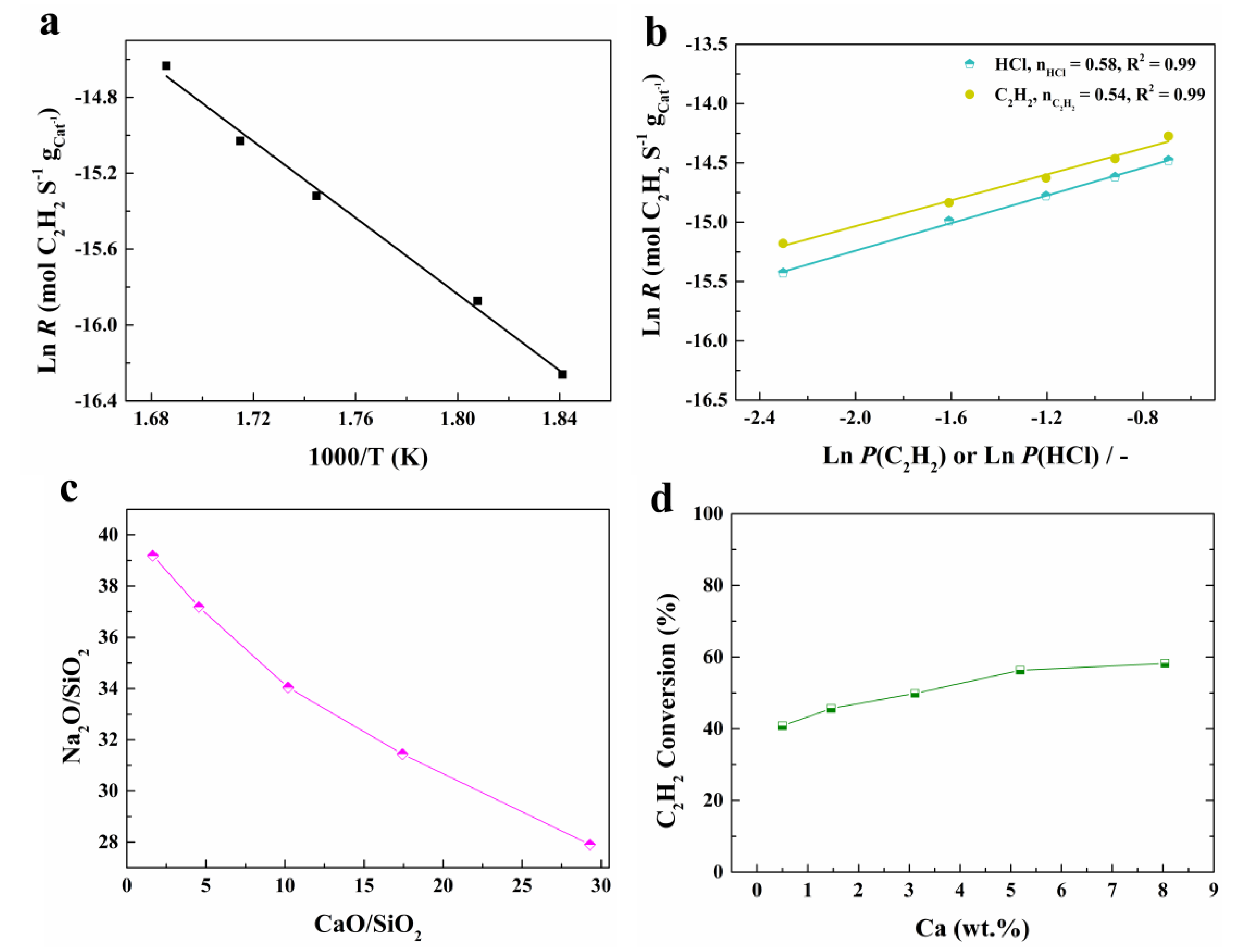
2.2. Catalytic Performance
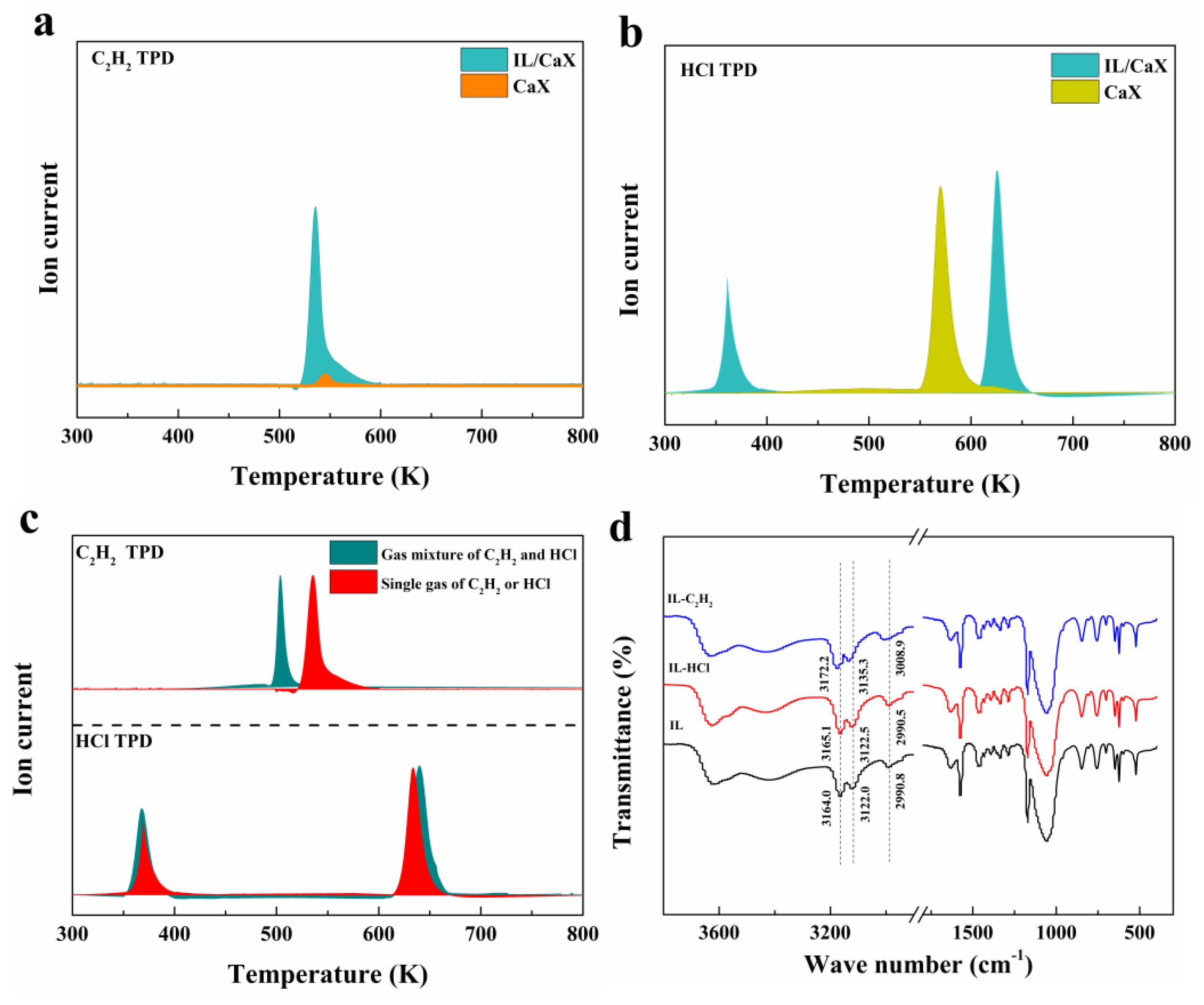
2.3. Catalytic Active Sites and Reaction Mechanism
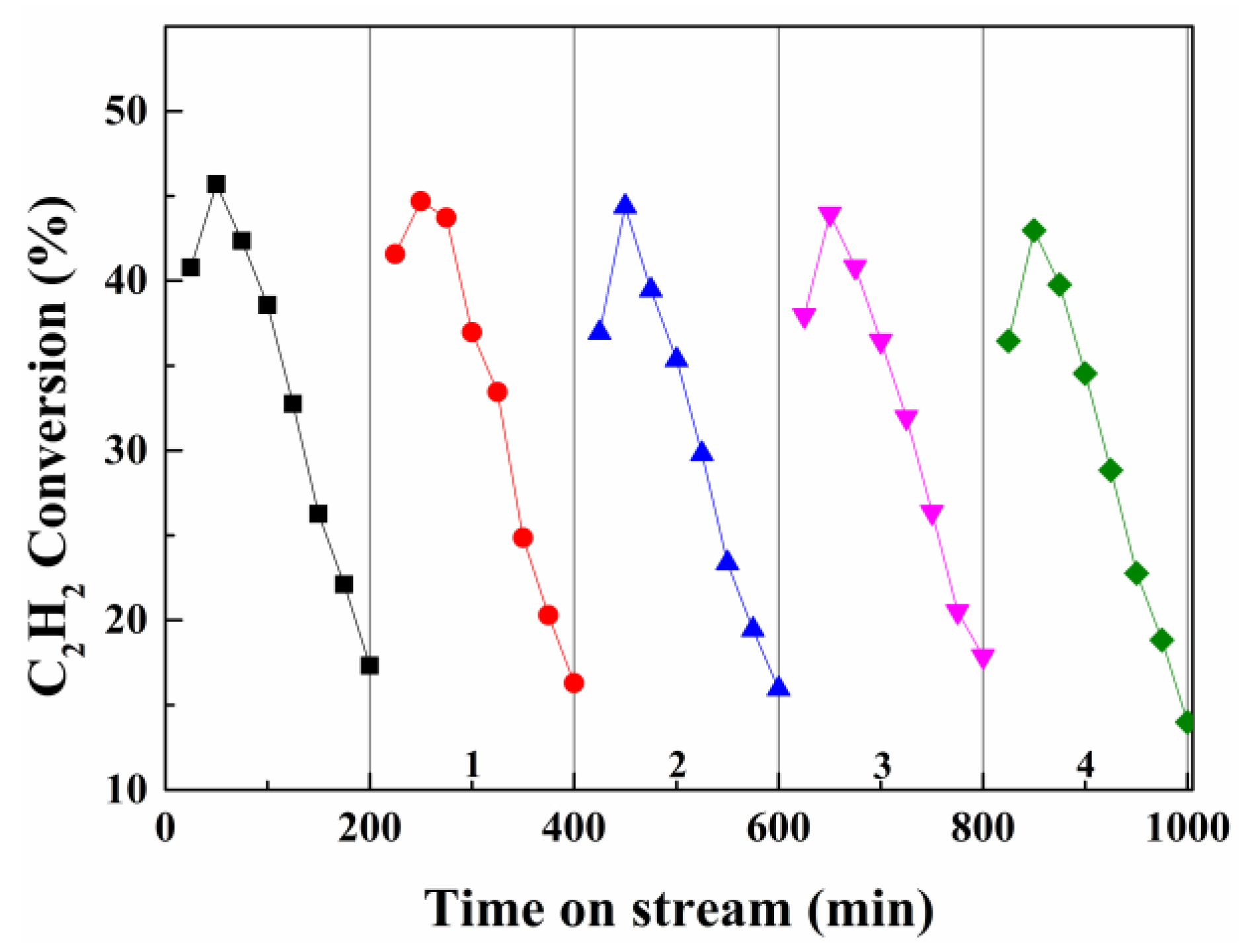
2.4. Deactivation and Regeneration Studies
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Reaction Evaluation
3.3. Characterization of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Johnston, P.; Carthey, N.; Hutchings, G.J. Discovery, development, and commercialization of gold catalysts for acetylene hydrochlorination. J. Am. Chem. Soc. 2015, 137, 14548–14557. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.W.; Xu, Y.P.; Liu, Z.M. Heterogeneous non-mercury catalysts for acetylene hydrochlorination: Progress, challenges, and opportunities. Green Chem. 2018, 20, 2412–2427. [Google Scholar] [CrossRef]
- Hutchings, G. Vapor phase hydrochlorination of acetylene: Correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 1985, 96, 292–295. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.L.; Xu, X.L.; Yu, Y.; Di, S.X.; Xu, H.; Zhai, Y.Y.; He, H.H.; Guo, L.L.; Pan, Z.Y.; et al. Alternative solvent to aqua regia to activate Au/AC catalysts for the hydrochlorination of acetylene. J. Catal. 2017, 350, 149–158. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.L.; Yue, Y.X.; Di, S.X.; Zhai, Y.Y.; He, H.H.; Sheng, G.F.; Lai, H.X.; Zhu, Y.H.; Guo, L.L.; et al. Towards a greener approach for the preparation of highly active gold/carbon catalyst for the hydrochlorination of ethyne. J. Catal. 2018, 365, 153–162. [Google Scholar] [CrossRef]
- Mitchenko, S.A.; Krasnyakova, T.V.; Zhikharev, I.V. Effect of mechanicochemical treatment on the activity of K2PdCl4 in the heterogeneous catalytic hydrochlorination of acetylene. Theor. Exp. Chem. 2010, 46, 32–38. [Google Scholar] [CrossRef]
- Krasnyakova, T.V.; Nikitenko, D.V.; Khomutova, E.V.; Mitchenko, S.A. Catalytic hydrochlorination of acetylene on PdCl2/C supported catalysts: Kinetic isotopic effect of HCl/DCl, stereoselectivity, and mechanism. Kinet. Catal. 2017, 58, 533–540. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, J.; Yu, L.; Jin, Y.; Li, W. Active ruthenium species in acetylene hydrochlorination. Appl. Catal. A Gen. 2014, 488, 28–36. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Zhang, T.; Di, X.; Gu, S.; Ni, J.; Li, X. Ultra-low Ru-promoted CuCl2 as highly active catalyst for the hydrochlorination of acetylene. RSC Adv. 2015, 5, 38159–38163. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Cai, W.; Zhang, J.; Zhang, X. Hydrochlorination of acetylene using supported phosphorus-doped Cu-based catalysts. Catal. Sci. Technol. 2015, 5, 5174–5184. [Google Scholar] [CrossRef]
- Zhai, Y.Y.; Zhao, J.; Di, X.X.; Di, S.X.; Wang, B.L.; Yue, Y.X.; Sheng, G.F.; Lai, H.X.; Guo, L.L.; Wang, H.; et al. Carbon-supported perovskite-like CsCuCl3 nanoparticles: A highly active and cost-effective heterogeneous catalyst for the hydrochlorination of acetylene to vinyl chloride. Catal. Sci. Technol. 2018, 8, 2901–2908. [Google Scholar] [CrossRef]
- Li, X.; Pan, X.; Yu, L.; Ren, P.; Wu, X.; Sun, L.; Jiao, F.; Bao, X. Silicon carbide-derived carbon nanocomposite as a substitute for mercury in the catalytic hydrochlorination of acetylene. Nat. Commun. 2014, 5, 3688. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zhang, J.L.; Han, Y.; Zhu, M.Y.; Shang, S.S.; Li, W. MOF-derived various morphologies of N-doped carbon composites for acetylene hydrochlorination. J. Mater. Sci. 2018, 53, 4913–4926. [Google Scholar] [CrossRef]
- Chao, S.L.; Zou, F.; Wan, F.F.; Dong, X.B.; Wang, Y.L.; Wang, Y.X.; Guan, Q.X.; Wang, G.C.; Li, W. Nitrogen-doped carbon derived from ZIF-8 as a high-performance metal-free catalyst for acetylene hydrochlorination. Sci. Rep. 2017, 7, 39789. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, H.; Pan, X.; Tie, K.; Cui, T.; Ding, M.; Bao, X. Catalytically active boron nitride in acetylene hydrochlorination. ACS Catal. 2017, 7, 8572–8577. [Google Scholar] [CrossRef]
- Nkosi, B.; Coville, N.J.; Hutchings, G.J.; Adams, M.D.; Friedl, J.; Wagner, F.E. Hydrochlorination of acetylene using gold catalysts: A study of catalyst deactivation. J. Catal. 1991, 128, 366–377. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, P.; Pan, X.L.; Ma, H.; Bao, X.H. Deactivation mechanism and regeneration of carbon nanocomposite catalyst for acetylene hydrochlorination. Appl. Catal. B Environ. 2017, 210, 116–120. [Google Scholar] [CrossRef]
- Conte, M.; Carley, A.F.; Hutchings, G.J. Reactivation of a carbon-supported gold catalyst for the hydrochlorination of acetylene. Catal. Lett. 2008, 124, 165–167. [Google Scholar] [CrossRef]
- Oliver-Meseguer, J.; Doménech-Carbó, A.; Boronat, M.; Leyva-Pérez, A.; Corma, A. Partial reduction and selective transfer of hydrogen chloride on catalytic gold nanoparticles. Angew. Chem. Int. Ed. 2017, 56, 6435–6439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gu, S.; Xu, X.; Zhang, T.; Yu, Y.; Di, X.; NI, J.; Pan, Z.; Li, X. Supported ionic-liquid-phase-stabilized Au(III) catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 3263–3270. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Y.; Xu, X.L.; Di, S.X.; Wang, B.L.; Xu, H.; Ni, J.; Guo, L.L.; Pan, Z.Y.; Li, X.N. Stabilizing Au(III) in supported-ionic-liquid-phase (SILP) catalyst using CuCl2 via a redox mechanism. Appl. Catal. B Environ. 2017, 206, 175–183. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Xu, J.; Zhang, T.; Di, X.; Ni, J.; Li, X. Enhancement of Au/AC acetylene hydrochlorination catalyst activity and stability via nitrogen-modified activated carbon support. Chem. Eng. J. 2015, 262, 1152–1160. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J. Non-mercury catalytic acetylene hydrochlorination over a NH4F–urea-modified Pd/HY catalyst for vinyl chloride monomer production. New J. Chem. 2016, 40, 3019–3023. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Wang, J. Enhanced stability of hydrochlorination of acetylene using polyaniline-modified Pd/HY catalysts. Catal. Commun. 2016, 74, 55–59. [Google Scholar] [CrossRef]
- Cai, J.; Li, L.; Lv, X.; Yang, C.; Zhao, X. Large surface area ordered porous carbons via nanocasting zeolite 10x and high performance for hydrogen storage application. ACS Appl. Mater. Interfaces 2014, 6, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic−inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Saxena, P.; Velaga, B.; Peela, N.R. Ionic liquid-encapsulated zeolite catalysts for the conversion of glucose to 5-hydroxymethylfurfural. Chemistryselect 2017, 2, 10379–10386. [Google Scholar] [CrossRef]
- Catrinck, M.N.; Ribeiro, E.S.; Monteiro, R.S.; Ribas, R.M.; Barbosa, M.H.P.; Teófilo, R.F. Direct conversion of glucose to 5-hydroxymethylfurfural using a mixture of niobic acid and niobium phosphate as a solid acid catalyst. Fuel 2017, 210, 67–74. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Gravel, O.; Larachi, F. Torrefaction of ionic-liquid impregnated lignocellulosic biomass and its comparison to dry torrefaction. Fuel 2013, 103, 814–826. [Google Scholar] [CrossRef]
- Berijani, K.; Hosseini-Monfared, H. Collaborative effect of Mn-porphyrin and mesoporous SBA-15 in the enantioselective epoxidation of olefins with oxygen. Inorg. Chim. Acta 2018, 471, 113–120. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, H.; Luo, J.; Wei, Y. A magnetic nanoparticle supported dual acidic ionic liquid: A “quasi-homogeneous” catalyst for the one-pot synthesis of benzoxanthenes. Green Chem. 2012, 14, 201–208. [Google Scholar] [CrossRef]
- Kotov, N.; Šturcová, A.; Zhigunov, A.; Raus, V.; Dybal, J. Structural transitions of 1-butyl-3-methylimidazolium chloride/water mixtures studied by Raman and FTIR spectroscopy and waxs. Cryst. Growth Des. 2016, 16, 1958–1967. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Gao, L.; Pi, K.; Zhang, J.; Zheng, C. Improvement of the CO2 absorption performance using ionic liquid [NH2emim][BF4] and [emim][BF4]/[bmim][BF4] mixtures. Energy Fuels 2013, 27, 461–466. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Incorporation of acetate-based ionic liquids into a zeolitic imidazolate framework (ZIF-8) as efficient sorbents for carbon dioxide capture. Chem. Eng. J. 2018, 334, 817–828. [Google Scholar] [CrossRef]
- Xu, H.; Han, Z.; Zhang, D.; Zhan, J. Interface behaviors of acetylene and ethylene molecules with 1-butyl-3-methylimidazolium acetate ionic liquid: A combined quantum chemistry calculation and molecular dynamics simulation study. ACS Appl. Mater. Interfaces 2012, 4, 6646–6653. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, F.; Wang, J. Catalytic properties of Pd/HY catalysts modified with NH4F for acetylene hydrochlorination. Catal. Commun. 2015, 65, 41–45. [Google Scholar] [CrossRef]
- Song, Z.; Liu, G.; He, D.; Pang, X.; Tong, Y.; Wu, Y.; Yuan, D.; Liu, Z.; Xu, Y. Acetylene hydrochlorination over 13X zeolite catalysts at high temperature. Green Chem. 2016, 18, 5994–5998. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Kang, L.; Zhu, M.; Dai, B. A novel, non-metallic graphitic carbon nitride catalyst for acetylene hydrochlorination. J. Catal. 2014, 311, 288–294. [Google Scholar] [CrossRef]
- Li, X.; Pan, X.; Bao, X. Nitrogen doped carbon catalyzing acetylene conversion to vinyl chloride. J. Energy Chem. 2014, 23, 131–135. [Google Scholar] [CrossRef]
- Zhang, C.; Kang, L.; Zhu, M.; Dai, B. Nitrogen-doped active carbon as a metal-free catalyst for acetylene hydrochlorination. RSC Adv. 2015, 5, 7461–7468. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhao, J.; Xu, J.T.; Xu, J.H.; Di, X.X.; Li, X.N. Oxygen and nitrogen-doped metal-free carbon catalysts for hydrochlorination of acetylene. Chin. J. Chem. Eng. 2016, 24, 484–490. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, F.; Zhang, C.; Kang, L.; Zhu, M. A novel S, N dual doped carbon catalyst for acetylene hydrochlorination. Appl. Catal. A Gen. 2018, 549, 68–75. [Google Scholar] [CrossRef]
- Michalak, W.D.; Krier, J.M.; Alayoglu, S.; Shin, J.-Y.; An, K.; Komvopoulos, K.; Liu, Z.; Somorjai, G.A. CO oxidation on PtSn nanoparticle catalysts occurs at the interface of Pt and Sn oxide domains formed under reaction conditions. J. Catal. 2014, 312, 17–25. [Google Scholar] [CrossRef]
- Lin, R.; Kaiser, S.K.; Hauert, R.; Pérez-Ramírez, J. Descriptors for high-performance nitrogen-doped carbon catalysts in acetylene hydrochlorination. ACS Catal. 2018, 8, 1114–1121. [Google Scholar] [CrossRef]
- Tuma, D.; Maurer, G. Gas solubility in ionic liquids: Mixed gases in pure ionic liquids and single gases in binary liquid mixtures. In Ionic Liquids: Science and Applications; American Chemical Society: Washington, DC, USA, 2012; pp. 217–238. [Google Scholar]
- Shinoda, K. The vapor-phase hidrochlorination of acetylene over metal chlorides supported on activated carbon. Chem. Lett. 1975, 4, 219–220. [Google Scholar] [CrossRef]
- He, R.-H.; Long, B.-W.; Lu, Y.-Z.; Meng, H.; Li, C.-X. Solubility of hydrogen chloride in three 1-alkyl-3-methylimidazolium chloride ionic liquids in the pressure range (0 to 100) kPa and temperature range (298.15 to 363.15) k. J. Chem. Eng. Data 2012, 57, 2936–2941. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Xu, J.; Zhang, T.; Li, X.; Di, X.; Ni, J.; Wang, J.; Cen, J. Influence of surface chemistry of activated carbon on the activity of gold/activated carbon catalyst in acetylene hydrochlorination. Ind. Eng. Chem. Res. 2014, 53, 14272–14281. [Google Scholar] [CrossRef]
- Mehnert, C.P.; Cook, R.A.; Dispenziere, N.C.; Afeworki, M. Supported ionic liquid catalysis—A new concept for homogeneous hydroformylation catalysis. J. Am. Chem. Soc. 2002, 124, 12932–12933. [Google Scholar] [CrossRef] [PubMed]
- Valkenberg, M.H.; deCastro, C.; Hölderich, W.F. Immobilisation of ionic liquids on solid supports. Green Chem. 2001, 4, 88–93. [Google Scholar] [CrossRef]
- Fehrmann, R.; Riisager, A.; Haumann, M. Supported Ionic Liquids; Wiley-VCH: Weinheim, Germany, 2014; pp. 128–129. [Google Scholar]
- Bovio, S.; Podestà, A.; Lenardi, C.; Milani, P. Evidence of extended solidlike layering in [Bmim][NTf2] ionic liquid thin films at room-temperature. J. Phys. Chem. B 2009, 113, 6600–6603. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Lai, H.; Yue, Y.; Sheng, G.; Deng, Y.; He, H.; Guo, L.; Zhao, J.; Li, X. Zeolite Supported Ionic Liquid Catalysts for the Hydrochlorination of Acetylene. Catalysts 2018, 8, 351. https://doi.org/10.3390/catal8090351
Wang B, Lai H, Yue Y, Sheng G, Deng Y, He H, Guo L, Zhao J, Li X. Zeolite Supported Ionic Liquid Catalysts for the Hydrochlorination of Acetylene. Catalysts. 2018; 8(9):351. https://doi.org/10.3390/catal8090351
Chicago/Turabian StyleWang, Bolin, Huixia Lai, Yuxue Yue, Gangfeng Sheng, Yaqin Deng, Haihua He, Lingling Guo, Jia Zhao, and Xiaonian Li. 2018. "Zeolite Supported Ionic Liquid Catalysts for the Hydrochlorination of Acetylene" Catalysts 8, no. 9: 351. https://doi.org/10.3390/catal8090351
APA StyleWang, B., Lai, H., Yue, Y., Sheng, G., Deng, Y., He, H., Guo, L., Zhao, J., & Li, X. (2018). Zeolite Supported Ionic Liquid Catalysts for the Hydrochlorination of Acetylene. Catalysts, 8(9), 351. https://doi.org/10.3390/catal8090351



