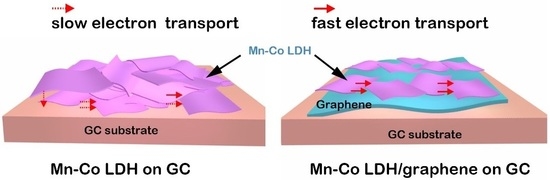
Two-Dimensional Mn-Co LDH/Graphene Composite towards High-Performance Water Splitting
Abstract
1. Introduction
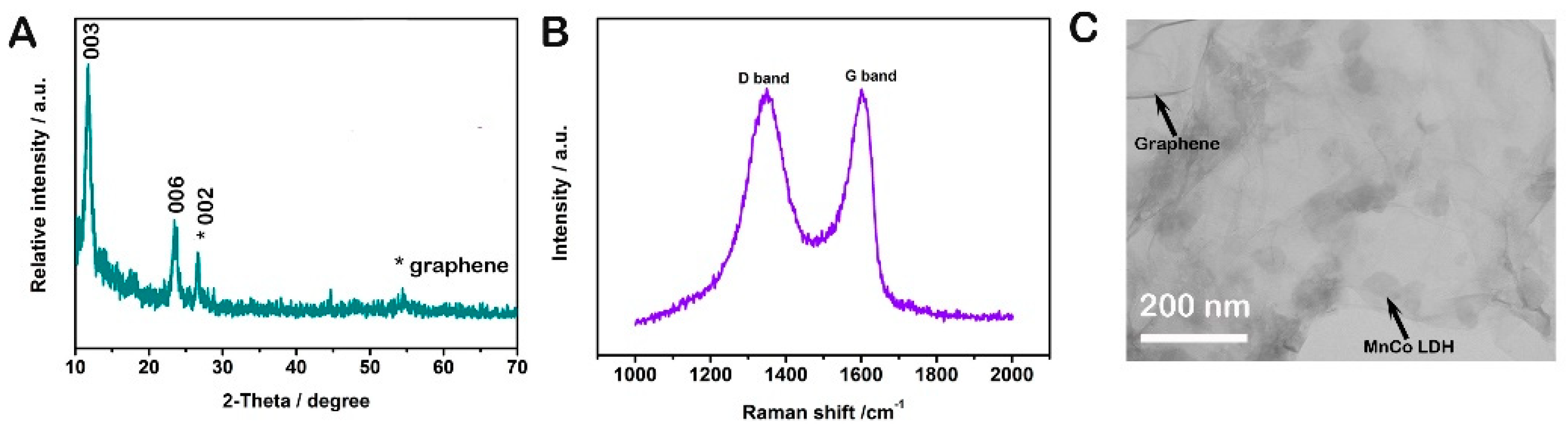
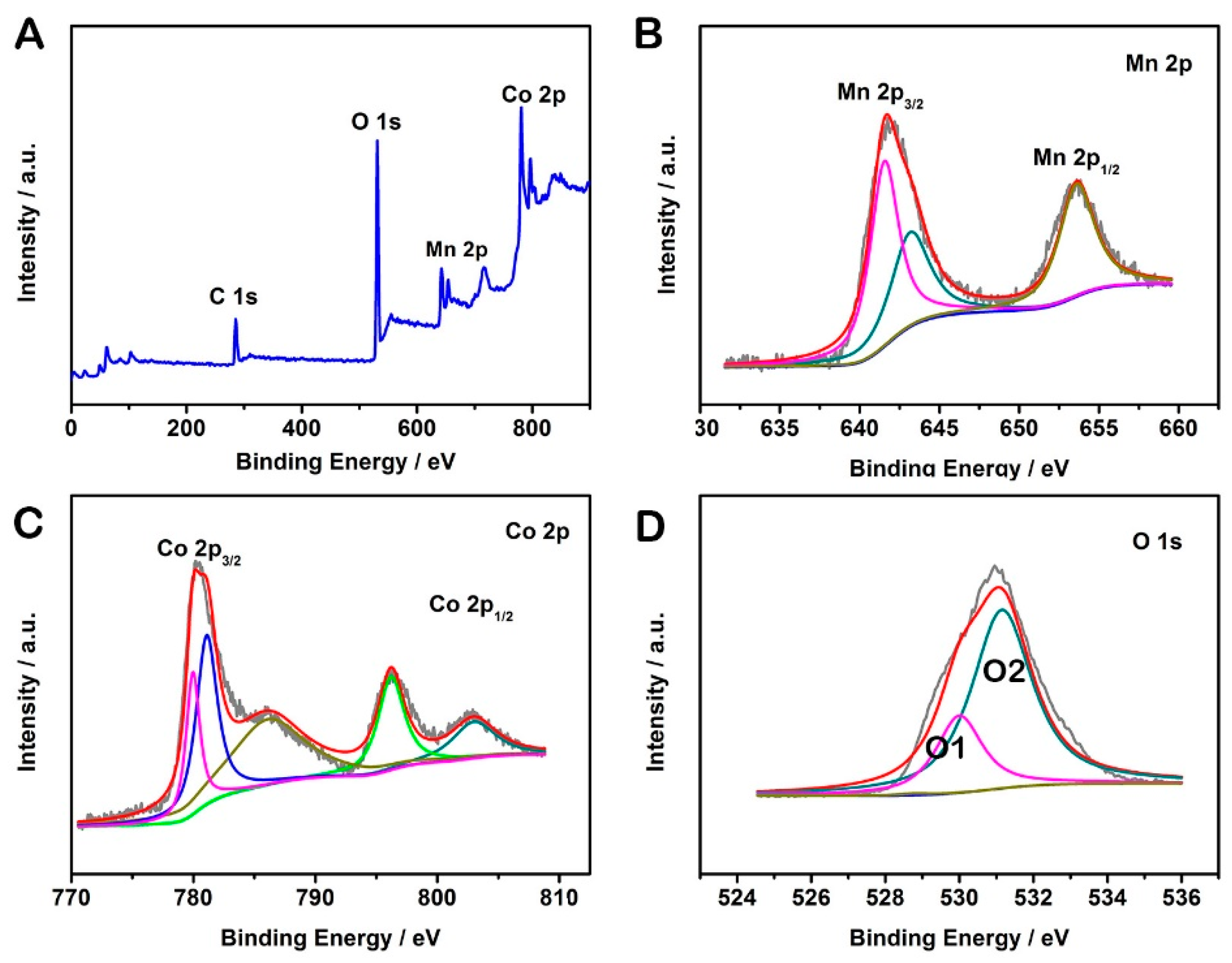
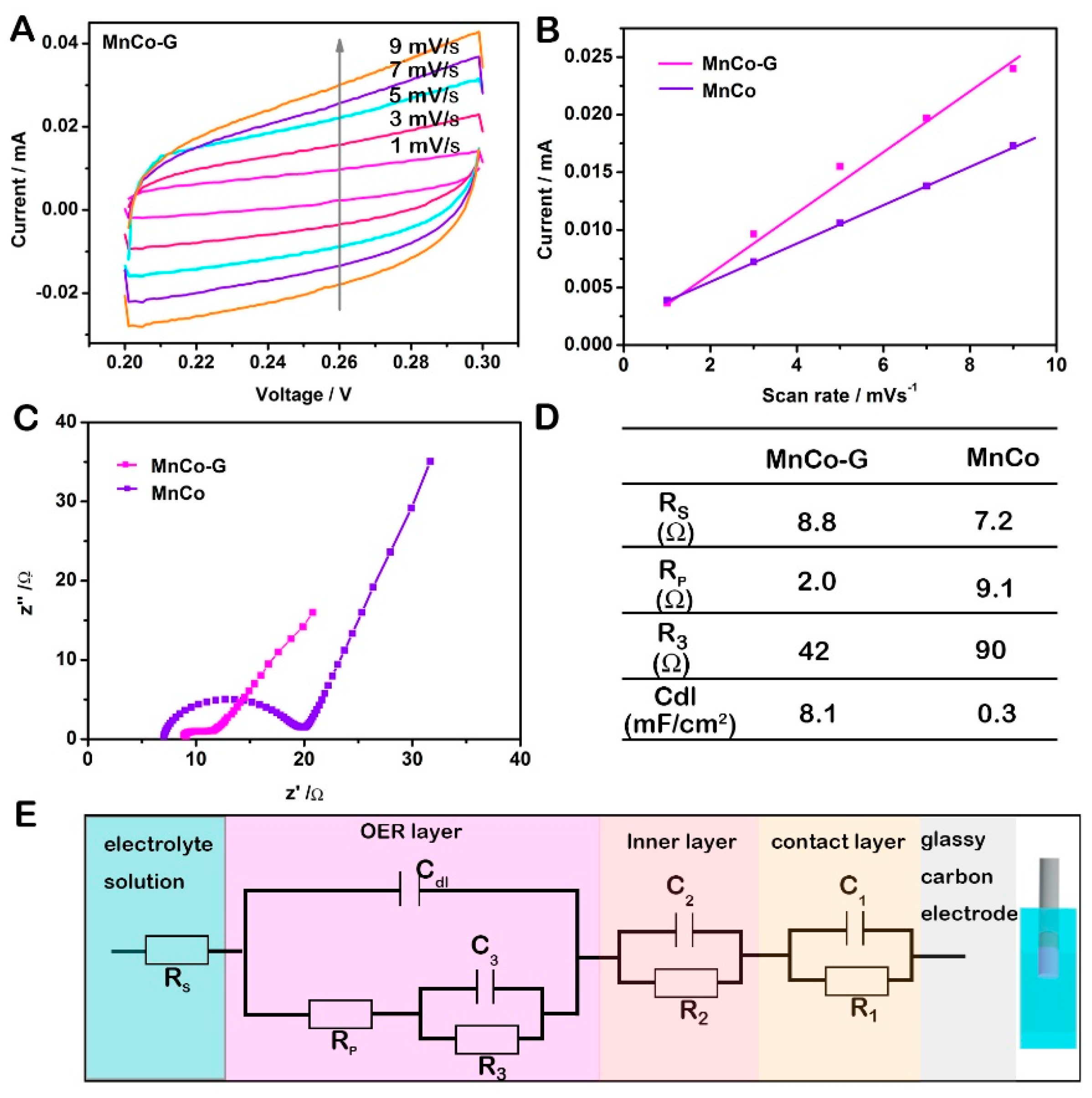
2. Results and Discussion
3. Experimental Section
3.1. Syntheses of the Mn-Co LDH/Graphene Ultrathin Composition Structure
3.2. Materials Characterizations
3.3. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xie, J.; Xie, Y. Structural Engineering of Electrocatalysts for the Hydrogen Evolution Reaction: Order or Disorder? ChemCatChem 2015, 7, 2568–2580. [Google Scholar] [CrossRef]
- Xie, J.; Xie, Y. Transition metal nitrides for electrocatalytic energy conversion: opportunities and challenges. Chem. Eur. J. 2015, 22, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Huang, S.; Yue, X.; Du, H.; Shen, P.K. Mo and Fe Modified Ni(OH)2/NiOOH Nanosheets as highly active and stable electrocatalysts for oxygen evolution reaction. ACS Catal. 2018, 8, 2359–2363. [Google Scholar] [CrossRef]
- Long, X.; Wang, Z.; Xiao, S.; An, Y.; Yang, S. Transition metal based layered double hydroxides tailored for energy conversion and storage. Mater. Today 2016, 19, 213–226. [Google Scholar] [CrossRef]
- Xie, J.; Liu, W.; Xin, J.; Lei, F.; Gao, L.; Qu, H.; Zhang, X.; Xie, Y. Dual effect in fluorine-doped hematite nanocrystals for efficient water oxidation. ChemSusChem 2017, 10, 4465–4471. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kang, Z.; Zhang, X.; Xie, J.; Wang, H.; Shao, W.; Zheng, X.S.; Yan, W.; Pan, B.; Xie, Y. Highly active Fe sites in ultrathin pyrrhotite Fe7S8 nanosheets realizing efficient electrocatalytic oxygen evolution. ACS Cent. Sci. 2017, 3, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, W.; Lei, F.; Zhang, X.; Qu, H.; Gao, L.; Hao, P.; Tang, B.; Xie, Y. Iron-Incorporated α-Ni(OH)2 Hierarchical Nanosheet Arrays for Electrocatalytic Urea Oxidation. Chem. Eur. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, R.; Bao, J.; Zhang, X.; Zhang, H.; Li, S.; Xie, Y. Zirconium trisulfide ultrathin nanosheets as efficient catalysts for water oxidation in both alkaline and neutral solutions. Inorg. Chem. Front. 2014, 1, 751–756. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Jia, X.; Waterhouse, G.I.N.; Shi, R.; Zhang, X.; Zhan, F.; Tao, Y.; Wu, L.; Tung, C.; et al. Sub-3 nm ultrafine monolayer layered double hydroxide nanosheets for electrochemical water oxidation. Adv. Energy Mater. 2018, 8, 1703585. [Google Scholar] [CrossRef]
- Wang, F.F.; Wang, T.; Sun, S.G.; Xu, Y.Q.; Yu, R.J.; Li, H.J. One-step synthesis of nickle iron-layered double hydroxide/reduced graphene oxide/carbon nanofibres composite as electrode materials for asymmetric supercapacitor. Sci. Rep. 2018, 8, 8908. [Google Scholar] [CrossRef] [PubMed]
- Liardet, L.; Hu, X. Amorphous cobalt vanadium oxide as a highly active electrocatalyst for oxygen evolution. ACS Catal. 2017, 8, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Qu, H.; Xin, J.; Zhang, X.; Cui, G.; Zhang, X.; Bao, J.; Tang, B.; Xie, Y. Defect-rich MoS2 nanowall catalyst for efficient hydrogen evolution reaction. Nano Res. 2017, 10, 1178–1188. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.D.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Masudy-Panah, S.; Siavash Moakhar, R.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal engineering of sputter-grown CuO photocathode for visible-light-driven electrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xin, J.; Cui, G.; Zhang, X.; Zhou, L.; Wang, Y.; Liu, W.; Wang, C.; Ning, M.; Xia, X.; et al. Vertically aligned oxygen-doped molybdenum disulfide nanosheets grown on carbon cloth realizing robust hydrogen evolution reaction. Inorg. Chem. Front. 2016, 3, 1160–1166. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.; Liu, W.; Xu, L.; Lei, F.; Xie, J.; Zhao, Y.; Huang, Y.; Guan, M.; Li, H. ZnCo2O4 Ultrathin nanosheets towards the high performance of flexible supercapacitors and bifunctional electrocatalysis. J. Alloys Compd. 2018, 764, 565–573. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Zhang, H.; Zhang, J.; Li, S.; Wang, R.; Pan, B.; Xie, Y. Intralayered ostwald ripening to ultrathin nanomesh catalyst with robust oxygen-evolving performance. Adv. Mater. 2017, 29, 1604765. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, A.; Masa, J.; Rösler, C.; Xia, W.; Weide, P.; Botz, A.J.R.; Fischer, R.A.; Schuhmann, W.; Muhler, M. Co@Co3O4 encapsulated in carbon nanotube-grafted nitrogen-doped carbon polyhedra as an advanced bifunctional oxygen electrode. Angew. Chem. Int. Ed. 2016, 55, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.S.; Bell, A.T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2011, 133, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hasin, P.; Wu, Y. NixCo3−xO4 nanowire arrays for electrocatalytic oxygen evolution. Adv. Mater. 2010, 22, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.; Bogdanoff, P.; Friedrich, D.; Fiechter, S. Synthesis of Ca2Mn3O8 films and their electrochemical studies for the oxygen evolution reaction (OER) of water. Nano Energy 2012, 1, 282–289. [Google Scholar] [CrossRef]
- Prabu, M.; Ketpang, K.; Shanmugam, S. Hierarchical nanostructured NiCo2O4 as an efficient bifunctional non-precious metal catalyst for rechargeable zinc–air batteries. Nanoscale 2014, 6, 3173. [Google Scholar] [CrossRef] [PubMed]
- Manivasakan, P.; Ramasamy, P.; Kim, J. Use of urchin-like NixCo3−xO4 hierarchical nanostructures based on non-precious metals as bifunctional electrocatalysts for anion-exchange membrane alkaline alcohol fuel cells. Nanoscale 2014, 6, 9665–9672. [Google Scholar] [CrossRef] [PubMed]
- Mondschein, J.S.; Callejas, J.F.; Read, C.G.; Chen, J.Y.C.; Holder, C.F.; Badding, C.K.; Schaak, R.E. Crystalline cobalt oxide films for sustained electrocatalytic oxygen evolution under strongly acidic conditions. Chem. Mater. 2017, 29, 950–957. [Google Scholar] [CrossRef]
- Lübke, M.; Sumboja, A.; McCafferty, L.; Armer, C.F.; Handoko, A.D.; Du, Y.; McColl, K.; Cora, F.; Brett, D.; Liu, Z.; et al. Transition-metal-doped α-MnO2 nanorods as bifunctional catalysts for efficient oxygen reduction and evolution reactions. Chem. Select. 2018, 9, 2613–2622. [Google Scholar] [CrossRef]
- Xie, J.; Xin, J.; Wang, R.; Zhang, X.; Lei, F.; Qu, H.; Hao, P.; Cui, G.; Tang, B.; Xie, Y. Sub-3 nm pores in two-dimensional nanomesh promoting the generation of electroactive phase for robust water oxidation. Nano Energy 2018, 53, 74–82. [Google Scholar] [CrossRef]
- Wang, L.; Lin, C.; Huang, D.; Zhang, F.; Wang, M.; Jin, J. A comparative study of composition and morphology effect of NixCo1−x(OH)2 on oxygen evolution/reduction reaction. ACS Appl. Mater. Interfaces 2014, 6, 10172–10180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, H.; He, H.; Xu, X.; Jin, Y. A high-performance binary Ni-CO hydroxide-based water oxidation electrode with three-dimensional coaxial nanotube array structure. Adv. Funct. Mater. 2014, 24, 4698–4705. [Google Scholar] [CrossRef]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient water oxidation using nanostructured α-nickel-hydroxide as an electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.W.; Yano, J.; Surendranath, Y.; Dincă, M.; Yachandra, V.K.; Nocera, D.G. Structure and valency of a cobalt–phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 2010, 132, 13692–13701. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Jung, N.; Jang, J.H.; Kim, H.-J.; Yoo, S.J. In Situ transformation of hydrogen-evolving CoP nanoparticles: Toward efficient oxygen evolution catalysts bearing dispersed morphologies with Co-oxo/hydroxo molecular units. ACS Catal. 2015, 5, 4066–4074. [Google Scholar] [CrossRef]
- Bediako, D.K.; Surendranath, Y.; Nocera, D.G. Mechanistic studies of the oxygen evolution reaction mediated by a nickel–borate thin film electrocatalyst. J. Am. Chem. Soc. 2013, 135, 3662–3674. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-R.; Cao, X.; Gao, Q.; Xu, Y.-F.; Zheng, Y.-R.; Jiang, J.; Yu, S.-H. Nitrogen-doped graphene supported CoSe2 nanobelt composite catalyst for efficient water oxidation. ACS Nano 2014, 8, 3970–3978. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tang, J.; Qian, H.; Wang, Z.; Yamauchi, Y. One-Pot synthesis of zeolitic imidazolate framework 67-derived hollow Co3S4@MoS2 heterostructures as efficient bifunctional catalysts. Chem. Mater. 2017, 29, 5566–5573. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, W.; Zhu, W.; Yang, Q.; Lei, X.; Liu, J.; Li, Y.; Sun, X.; Duan, X. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 2014, 50, 6479–6482. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Chen, H.; Ji, Y.; Huang, H.; Claesson, P.M.; Daniel, Q.; Philippe, B.; Rensmo, H.; Li, F.; Luo, Y.; et al. Nickel–vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 2016, 7, 11981. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Hu, X. Ultrathin cobalt–manganese layered double hydroxide is an efficient oxygen evolution catalyst. J. Am. Chem. Soc. 2014, 136, 16481–16484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Bandurin, D.A.; Pellegrino, F.M.D.; Cao, Y.; Principi, A.; Guo, H.; Auton, G.H.; Shalom, M.B.; Ponomarenko, L.A.; Falkovich, G.; et al. Superballistic flow of viscous electron fluid through graphene constrictions. Nat. Phys. 2017, 13, 1182–1185. [Google Scholar] [CrossRef]
- Sanchez-Yamagishi, J.D.; Luo, J.Y.; Young, A.F.; Hunt, B.M.; Watanabe, K.; Taniguchi, T.; Ashoori, R.C.; Jarillo-Herrero, P. Helical edge states and fractional quantum hall effect in a graphene electron-hole bilayer. Nat. Nanotechnol. 2017, 12, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Peng, X.; Liu, B.; Wu, C.; Xie, Y.; Yu, G. Ultrathin two-dimensional MnO2/graphene hybrid nanostructures for high-performance, flexible planar supercapacitors. Nano Lett. 2013, 13, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, J.F.; Guan, B.Y.; Lu, Y.; Lou, X.W.D. Hierarchical hollow nanoprisms based on ultrathin Ni-Fe layered double hydroxide nanosheets with enhanced electrocatalytic activity towards oxygen evolution. Angew. Chem. Int. Ed. 2018, 57, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, C.; Zhang, Z.; Liu, D.; Chen, R.; Wang, S. In Situ exfoliated, N-doped, and edge-rich ultrathin layered double hydroxides nanosheets for oxygen evolution reaction. Adv. Funct. Mater. 2018, 28, 1703363. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Sun, X.; Zhang, N.; Xu, K.; Zhou, M.; Xie, Y. Layer-by-layer β-Ni(OH)2/graphene nanohybrids for ultraflexible all-solid-state thin-film supercapacitors with high electrochemical performance. Nano Energy 2013, 2, 65–74. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states inXPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wexler, D.; Shi, D.; Liang, J.; Liu, H.; Xiong, S.; Qian, Y. Simple synthesis of yolk-shelled ZnCo2O4 microspheres towards enhancing the electrochemical performance of lithium-ion batteries in conjunction with a sodium carboxymethyl cellulose binder. J. Mater. Chem. A 2013, 1, 15292–15299. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation. Angew. Chem. Int. Ed. 2015, 127, 7507–7512. [Google Scholar] [CrossRef]
- Jia, G.; Hu, Y.; Qian, Q.; Yao, Y.; Zhang, S.; Li, Z.; Zou, Z. Formation of hierarchical structure composed of (Co/Ni)Mn-LDH nanosheets on MWCNT backbones for efficient electrocatalytic water oxidation. ACS Appl. Mater. Interfaces 2016, 8, 14527–14534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, J.; Zhou, L.; Li, Z.; Shao, M.; Wei, M. Layer-by-layer assembly of exfoliated layered double hydroxide nanosheets for enhanced electrochemical oxidation of water. J. Mater. Chem. A 2016, 4, 11516–11523. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; Papakonstantinou, P. CuCo2O4 nanoparticles on nitrogenated graphene as highly efficient oxygen evolution catalyst. J. Power Sources 2015, 281, 243–251. [Google Scholar] [CrossRef]
- Yu, M.Q.; Jiang, L.X.; Yang, H.G. Ultrathin nanosheets constructed CoMoO4 porous flowers with high activity for electrocatalytic oxygen evolution. Chem. Commun. 2015, 51, 14361–14364. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sliozberg, K.; Sinev, I.; Antoni, H.; Bähr, A.; Ollegott, K.; Xia, W.; Masa, J.; Grünert, W.; Cuenya, B.R.; et al. Synergistic effect of cobalt and iron in layered double hydroxide catalysts for the oxygen evolution reaction. ChemSusChem 2016, 10, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Qu, H.; Lei, F.; Peng, X.; Liu, W.; Gao, L.; Hao, P.; Cui, G.; Tang, B. Partially amorphous nickel-iron layered double hydroxide nanosheet arrays for robust bifunctional electrocatalysis. J. Mater. Chem. A 2018, 6, 16121–16129. [Google Scholar] [CrossRef]
- Xie, J.; Li, S.; Zhang, X.; Zhang, J.; Wang, R.; Zhang, H.; Pan, B.; Xie, Y. Atomically-thin molybdenum nitride nanosheets with exposed active surface sites for efficient hydrogen evolution. Chem. Sci. 2014, 5, 4615–4620. [Google Scholar] [CrossRef]



| Materials | Electrolyte | Overpotential for 10 mA cm−2/V | Tafel Slope/mV decade−1 | Ref. |
|---|---|---|---|---|
| MnCo-G | KOH | 0.33 | 48 | This work |
| RuO2 | 1 M KOH | 0.3 | 42 | This work |
| Ni5Mn-LDH-MWCNT | 1 M KOH | 0.35 (iR-corrected) | 83 | [52] |
| Co5Mn-LDH-MWCNT | 1 M KOH | 0.3 (iR-corrected) | 74 | [52] |
| CoNi-LDH/Fe-PP-M | 1 M KOH | 0.32 | 53 | [53] |
| CuCo2O4/N-rGO | 1 M KOH | 0.36 | 64 | [54] |
| Co3S4@MoS2 | 1 M KOH | 0.33 | 59 | [36] |
| CoMoO4 | 1 M KOH | 0.31 | 56 | [55] |
| CoP | 1 M KOH | 0.36 | 66 | [33] |
| CoFe LDH | 0.1 M KOH | 0.36 | 49 | [56] |
| NiFe LDH | 1 M KOH | 0.33 | 41 | [38] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, J.; Xie, J.; Lei, F.; Wang, Z.; Liu, W.; Xu, L.; Guan, M.; Zhao, Y.; Li, H. Two-Dimensional Mn-Co LDH/Graphene Composite towards High-Performance Water Splitting. Catalysts 2018, 8, 350. https://doi.org/10.3390/catal8090350
Bao J, Xie J, Lei F, Wang Z, Liu W, Xu L, Guan M, Zhao Y, Li H. Two-Dimensional Mn-Co LDH/Graphene Composite towards High-Performance Water Splitting. Catalysts. 2018; 8(9):350. https://doi.org/10.3390/catal8090350
Chicago/Turabian StyleBao, Jian, Junfeng Xie, Fengcai Lei, Zhaolong Wang, Wenjun Liu, Li Xu, Meili Guan, Yan Zhao, and Huaming Li. 2018. "Two-Dimensional Mn-Co LDH/Graphene Composite towards High-Performance Water Splitting" Catalysts 8, no. 9: 350. https://doi.org/10.3390/catal8090350
APA StyleBao, J., Xie, J., Lei, F., Wang, Z., Liu, W., Xu, L., Guan, M., Zhao, Y., & Li, H. (2018). Two-Dimensional Mn-Co LDH/Graphene Composite towards High-Performance Water Splitting. Catalysts, 8(9), 350. https://doi.org/10.3390/catal8090350