Modification to L-H Kinetics Model and Its Application in the Investigation on Photodegradation of Gaseous Benzene by Nitrogen-Doped TiO2
Abstract
:1. Introduction
2. Results and Discussion
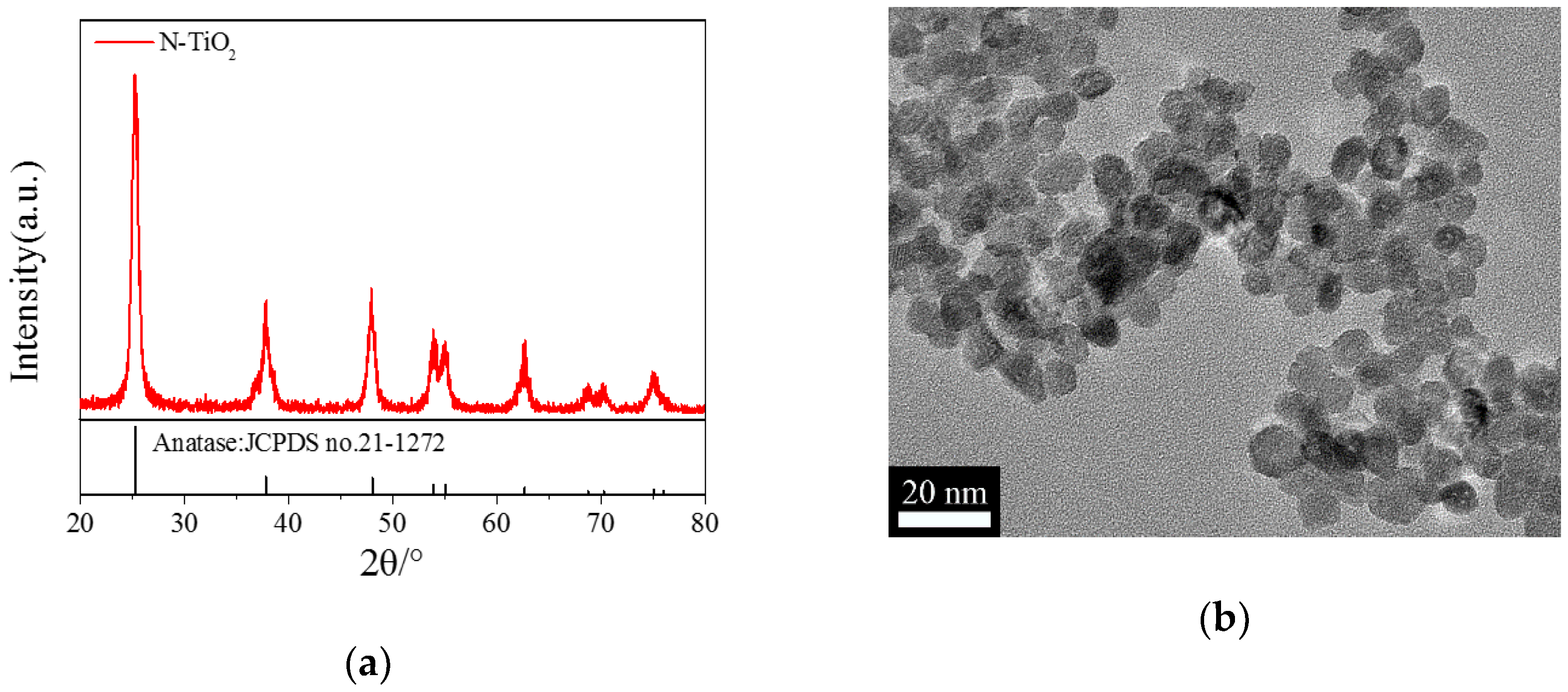
2.1. Characterization of the N-TiO2 Photocatalysts
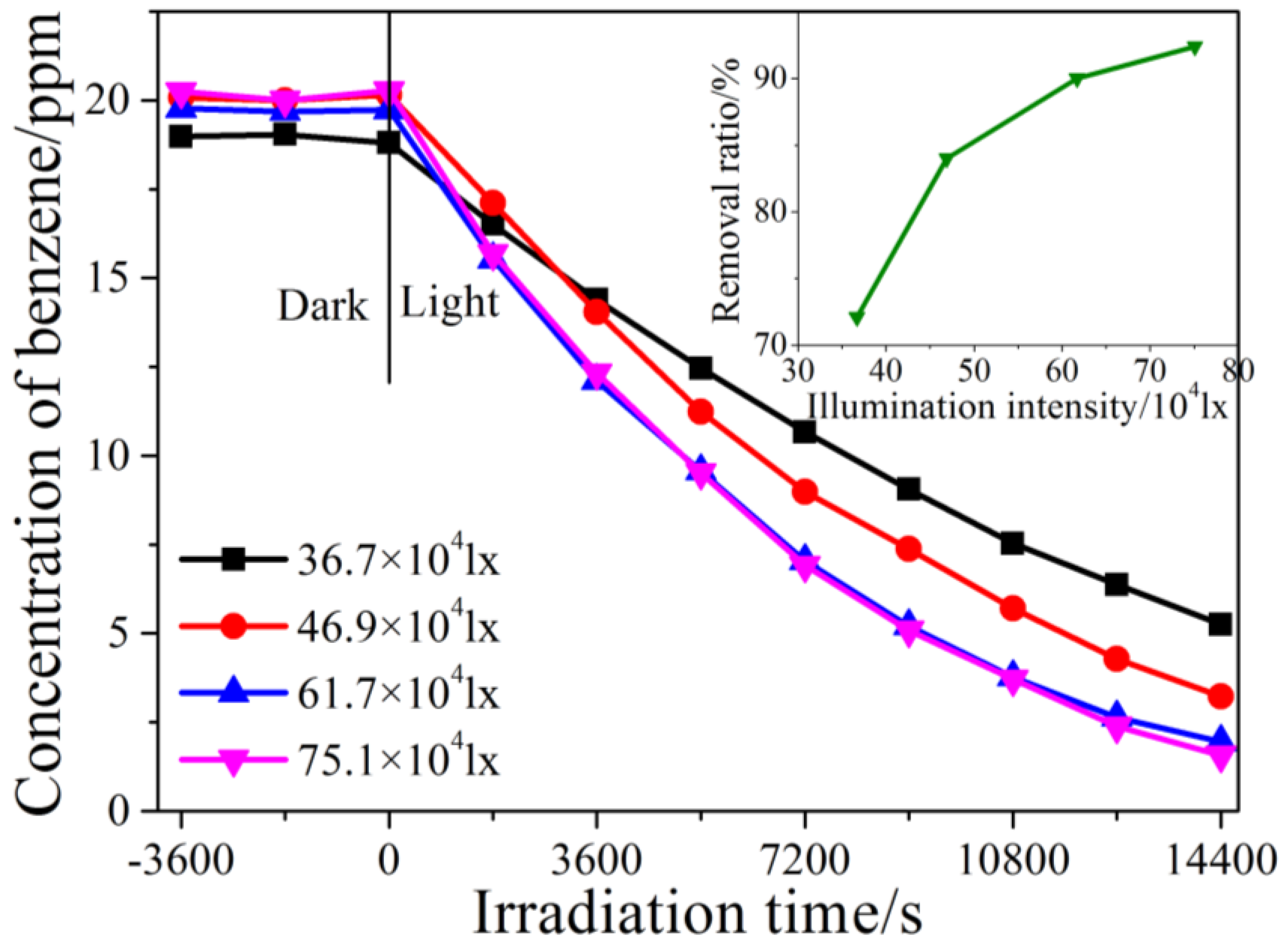
2.2. Kinetic Study of Photocatalytic Degradation of Benzene under Different Illumination Intensity
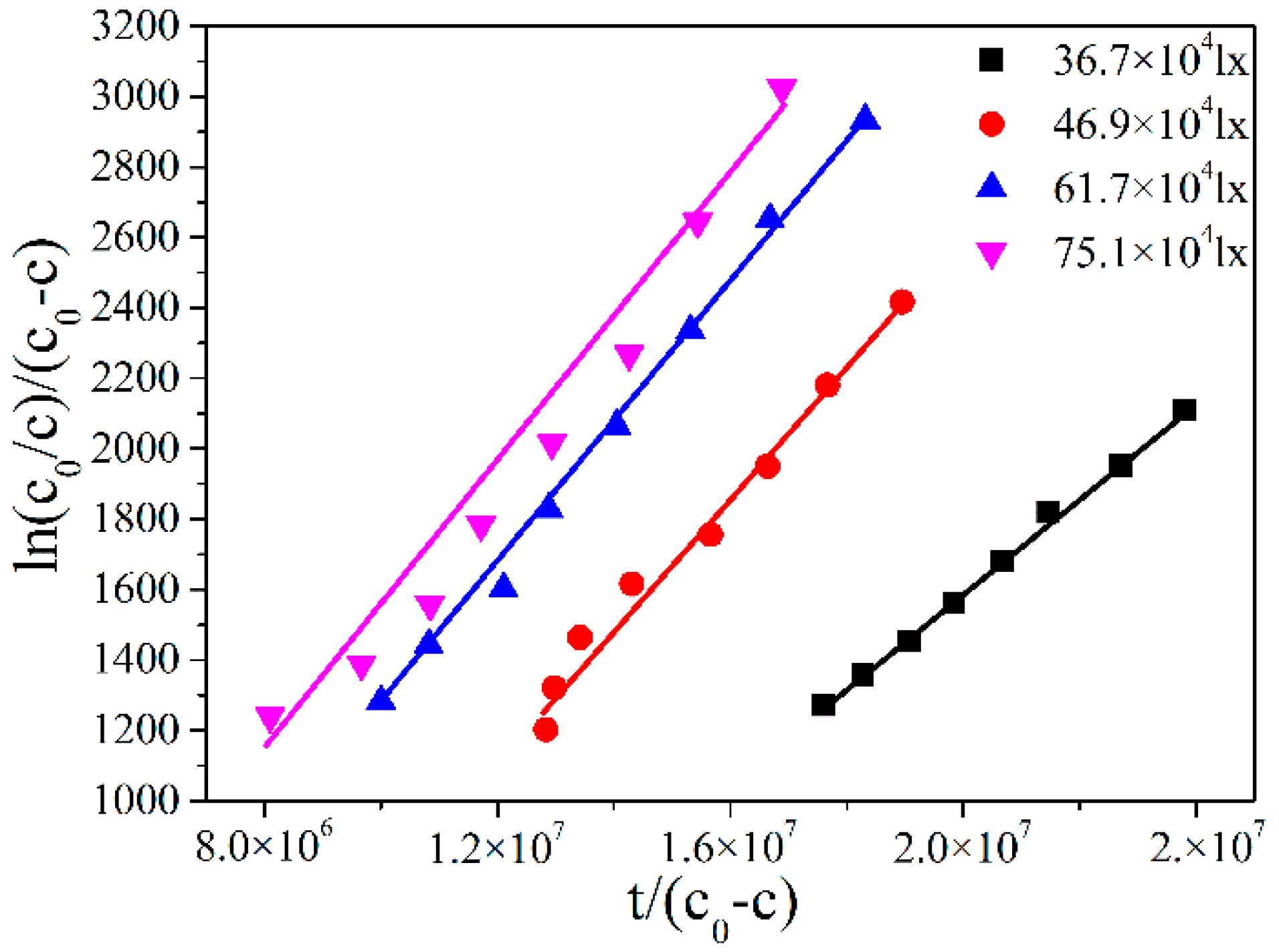
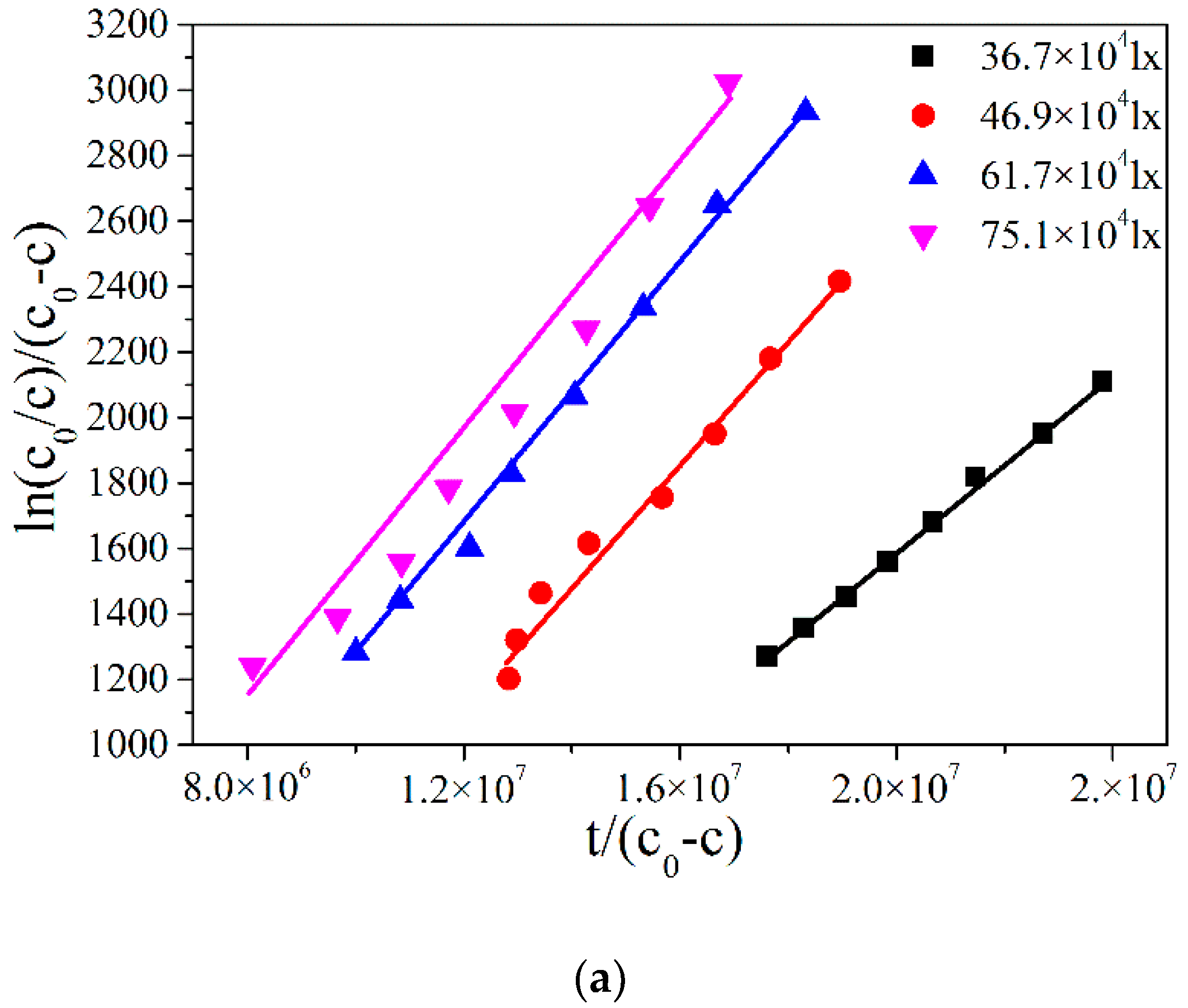
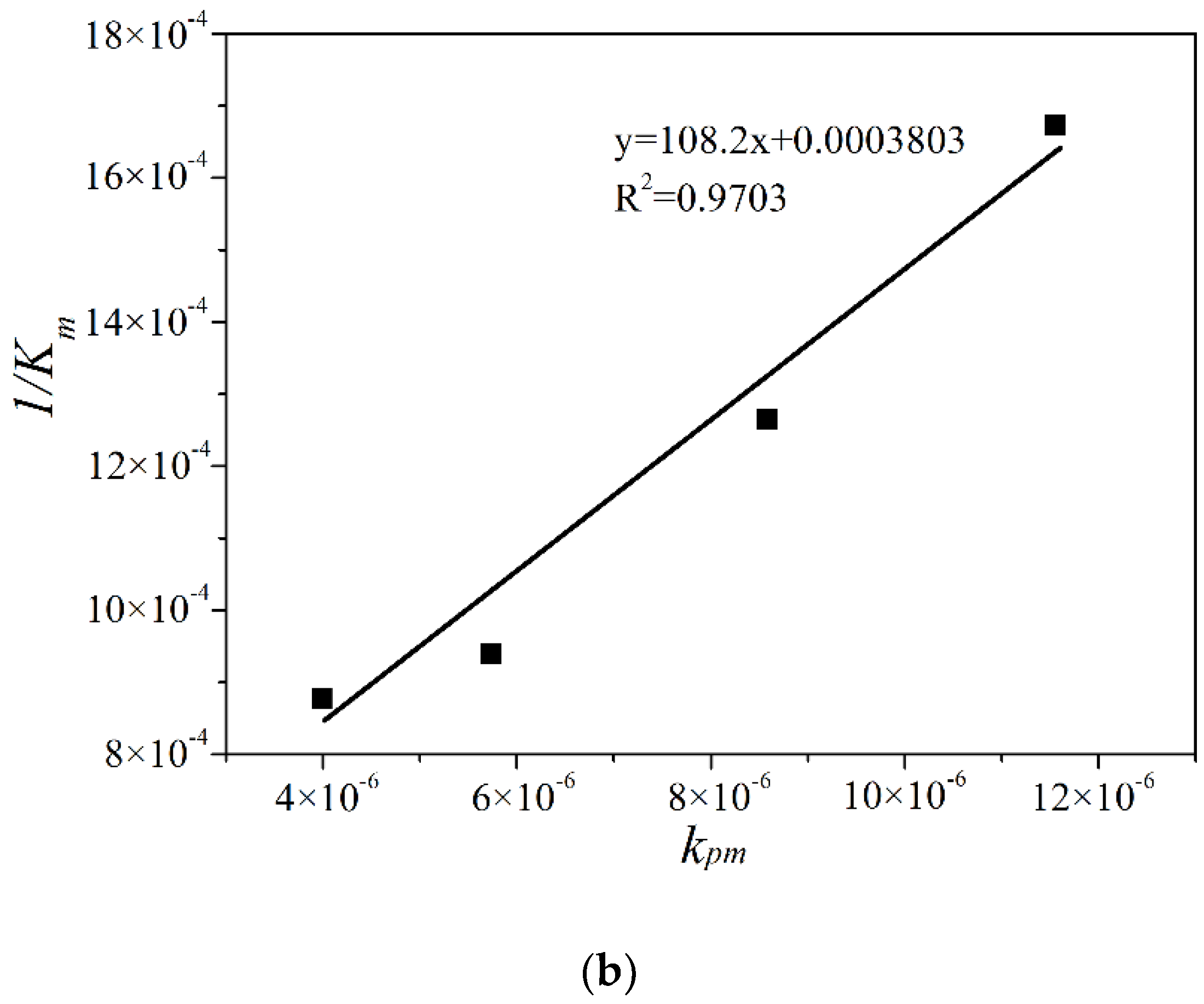
2.3. Modication to the L-H Model and Kinetic Results under Different Illumination Intensity
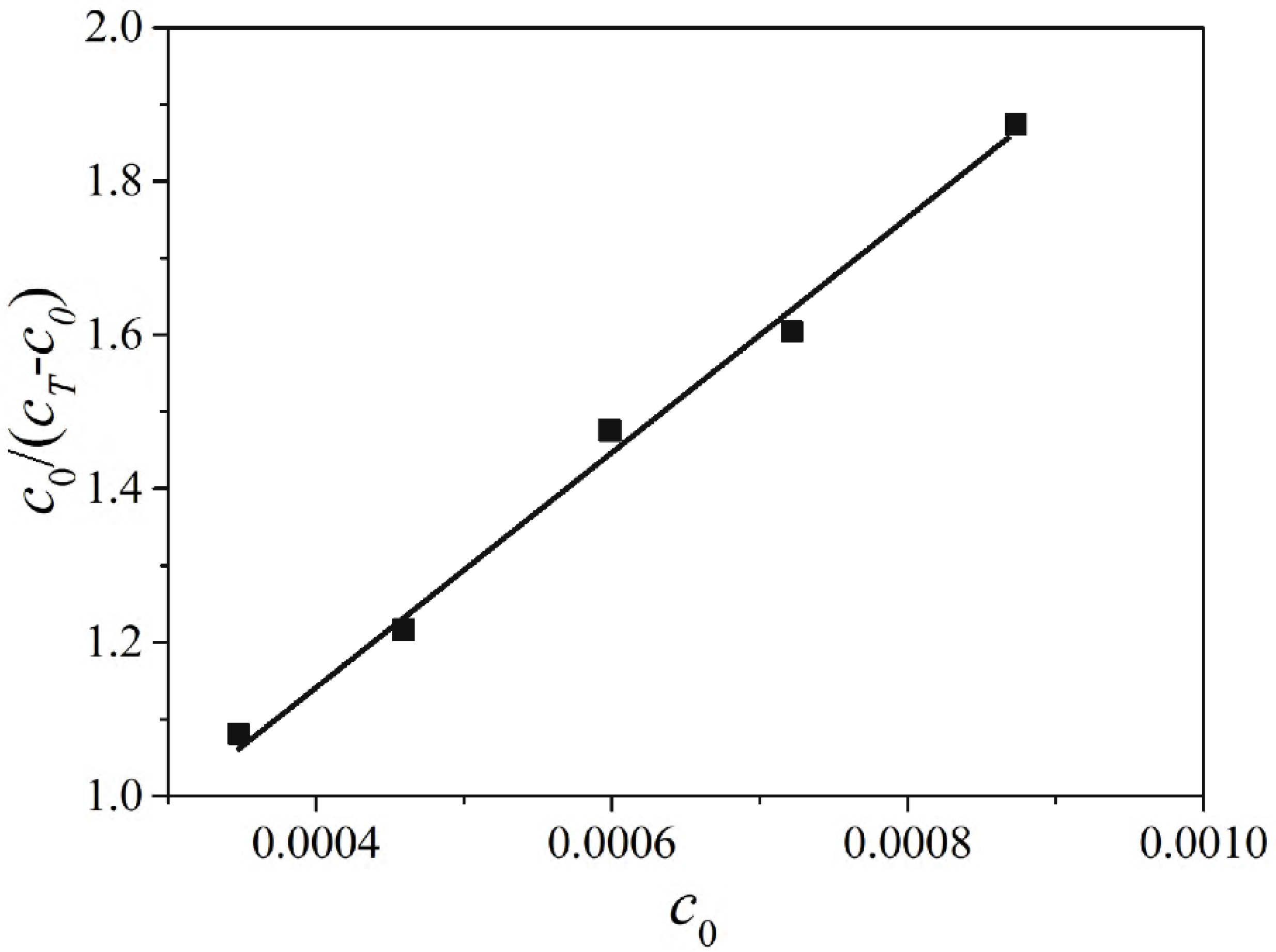
2.4. The Adsorption Equilibrium Constant KL Obtained by Using Adsorption Theory
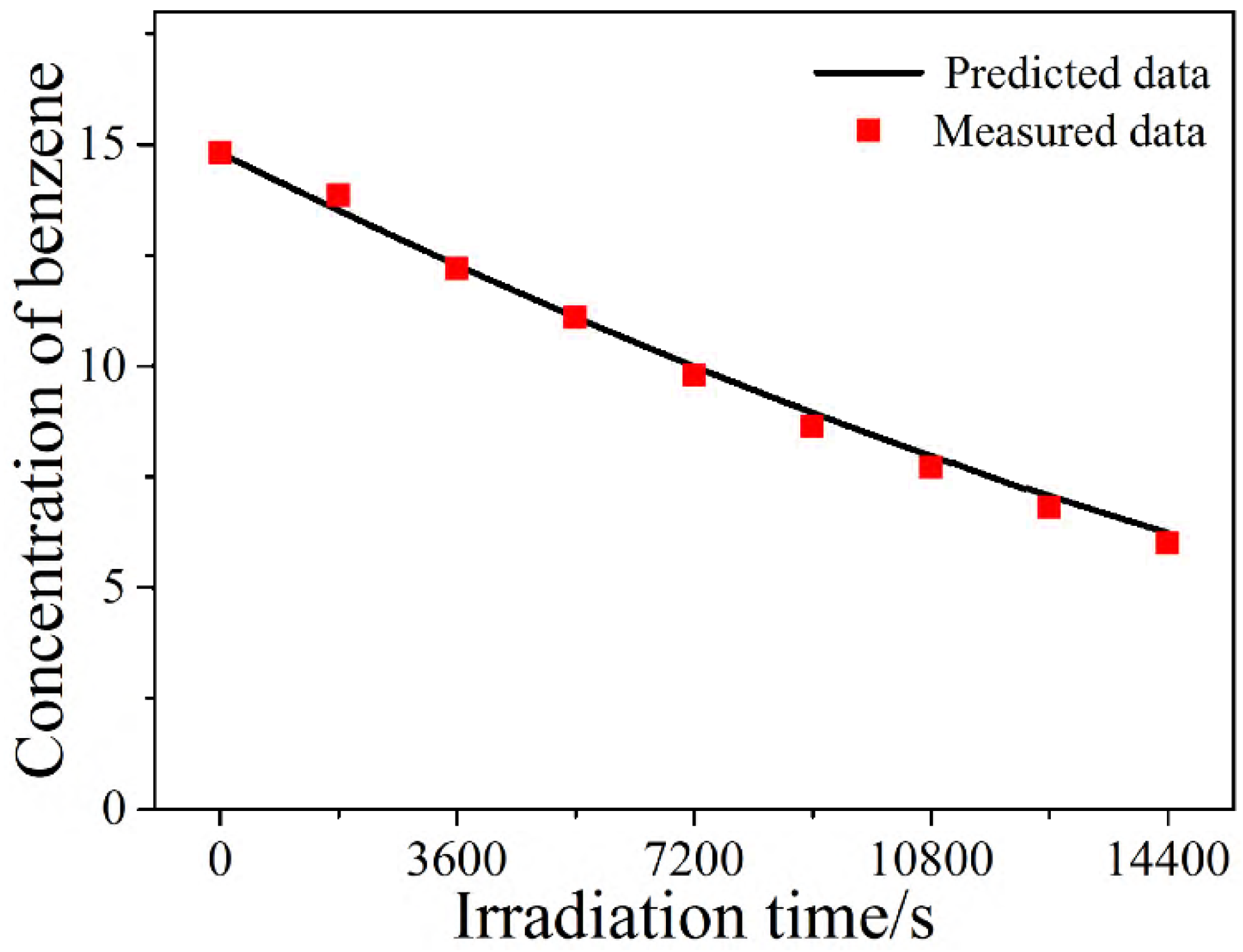
2.5. Verification of the Modified L-H Model
3. Materials and Methods
3.1. Preparation and Characterization of Samples
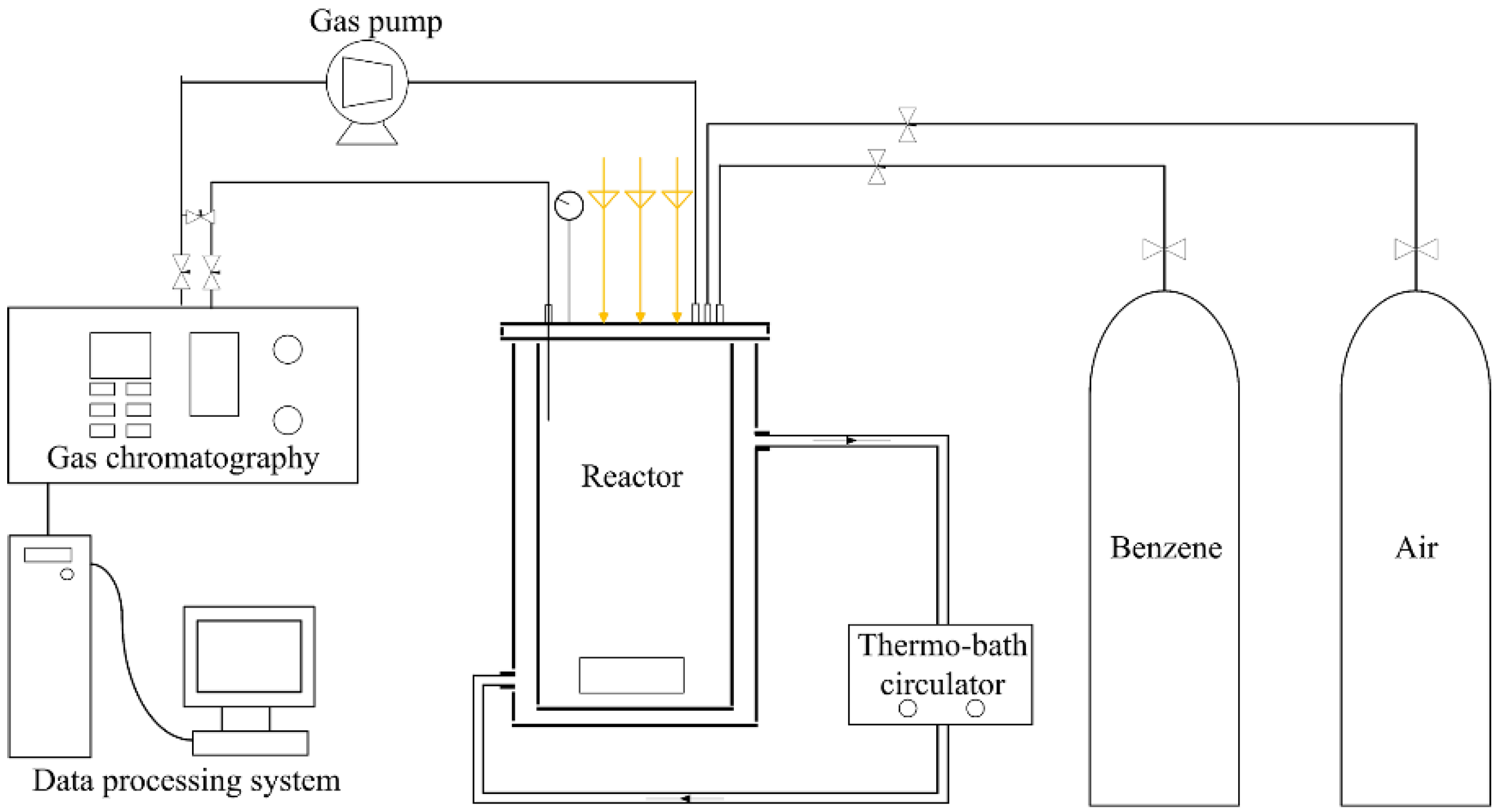
3.2. Photocatalytic Reaction System
3.3. Photocatalytic Reaction Procedures
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sui, H.; Zhang, T.; Cui, J.; Li, X.; Crittenden, J.; Li, X.; He, L. Novel off-gas treatment technology to remove volatile organic compounds with high concentration. Ind. Eng. Chem. Res. 2016, 55, 2594–2603. [Google Scholar] [CrossRef]
- Jiang, N.; Hui, C.-X.; Li, J.; Lu, N.; Shang, K.-F.; Wu, Y.; Mizuno, A. Improved performance of parallel surface/packed-bed discharge reactor for indoor VOCs decomposition: Optimization of the reactor structure. J. Phys. D Appl. Phys. 2015, 48, 40. [Google Scholar] [CrossRef]
- Ye, C.Z.; Ariya, P.A. Co-adsorption of gaseous benzene, toluene, ethylbenzene, m-xylene (btex) and SO2 on recyclable Fe3O4 nanoparticles at 0–101% relative humidities. J. Environ. Sci. 2015, 31, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lu, Z.; Li, M.; Yang, J.; Song, W.; Zeng, D.; Xie, C. A modular calcination method to prepare modified N-doped TiO2 nanoparticle with high photocatalytic activity. Appl. Catal. B Environ. 2016, 183, 308–316. [Google Scholar] [CrossRef]
- Ren, L.; Mao, M.; Li, Y.; Lan, L.; Zhang, Z.; Zhao, X. Novel photothermocatalytic synergetic effect leads to high catalytic activity and excellent durability of anatase TiO2 nanosheets with dominant {001} facets for benzene abatement. Appl. Catal. B Environ. 2016, 198, 303–310. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S. Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J. Alloys Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Fujimoto, T.M.; Ponczek, M.; Rochetto, U.L.; Landers, R.; Tomaz, E. Photocatalytic oxidation of selected gas-phase VOCs using UV light, TiO2, and TiO2/Pd. Environ. Sci. Pollut. Res. 2017, 24, 6390–6396. [Google Scholar] [CrossRef] [PubMed]
- Wongaree, M.; Chiarakorn, S.; Chuangchote, S.; Sagawa, T. Photocatalytic performance of electrospun CNT/TiO2 nanofibers in a simulated air purifier under visible light irradiation. Environ. Sci. Pollut. Res. 2016, 23, 21395–21406. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghi, S.; Mohammadi, M.; Ebadi, H. Photocatalytic degradation of benzene wastewater using PANI-TiO2 nanocomposite under UV and solar light radiation. J. Environ. Eng. 2016, 142, 05015003. [Google Scholar] [CrossRef]
- Fang, J.; Chen, Z.; Zheng, Q.; Li, D. Photocatalytic decomposition of benzene enhanced by the heating effect of light: Improving solar energy utilization with photothermocatalytic synergy. Catal. Sci. Technol. 2017, 7, 3303–3311. [Google Scholar] [CrossRef]
- Lan, L.; Li, Y.; Zeng, M.; Mao, M.; Ren, L.; Yang, Y.; Liu, H.; Yun, L.; Zhao, X. Efficient UV-Vis-Infrared light-driven catalytic abatement of benzene on amorphous manganese oxide supported on anatase TiO2 nanosheet with dominant {001} facets promoted by a photothermocatalytic synergetic effect. Appl. Catal. B Environ. 2017, 203, 494–504. [Google Scholar] [CrossRef]
- Melián, E.P.; Díaz, O.G.; Araña, J.; Rodríguez, J.M.D.; Rendón, E.T.; Melián, J.A.H. Kinetics and adsorption comparative study on the photocatalytic degradation of o-, m- and p-cresol. Catal. Today 2007, 129, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Bao, C.; Cun, T.; Huang, Q. One-pot synthesis of ZnO/oligoaniline nanocomposites with improved removal of organic dyes in water: Effect of adsorption on photocatalytic degradation. Mater. Res. Bull. 2017, 95, 459–467. [Google Scholar] [CrossRef]
- Zhi, Y.; Li, Y.; Zhang, Q.; Wang, H. Zno nanoparticles immobilized on flaky layered double hydroxides as photocatalysts with enhanced adsorptivity for removal of acid red g. Langmuir 2010, 26, 15546–15553. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Lee, C.W.; Lu, X.; Sun, Y.; Hua, W.; Zhuang, G.; Zhang, S.; Chen, J.; Hou, H.; Zhao, D. Synchronous role of coupled adsorption and photocatalytic oxidation on ordered mesoporous anatase TiO2-SiO2 nanocomposites generating excellent degradation activity of rhb dye. Appl. Catal. B Environ. 2010, 95, 197–207. [Google Scholar] [CrossRef]
- Kim, S.B.; Hong, S.C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst. Appl. Catal. B Environ. 2002, 35, 305–315. [Google Scholar] [CrossRef]
- Golshan, M.; Zare, M.; Goudarzi, G.; Abtahi, M.; Babaei, A.A. Fe3O4@hap-enhanced photocatalytic degradation of Acid Red73 in aqueous suspension: Optimization, kinetic, and mechanism studies. Mater. Res. Bull. 2017, 91, 59–67. [Google Scholar] [CrossRef]
- Deng, X.-Q.; Liu, J.-L.; Li, X.-S.; Zhu, B.; Zhu, X.; Zhu, A.-M. Kinetic study on visible-light photocatalytic removal of formaldehyde from air over plasmonic Au/TiO2. Catal. Today 2017, 281, 630–635. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Liu, P.; Yu, X.; Wang, F.; Zhang, W.; Yang, L.; Liu, Y. Degradation of formaldehyde and benzene by TiO2 photocatalytic cement based materials. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2017, 32, 391–396. [Google Scholar] [CrossRef]
- Yuzawa, H.; Aoki, M.; Otake, K.; Hattori, T.; Itoh, H.; Yoshida, H. Reaction mechanism of aromatic ring hydroxylation by water over platinum-loaded titanium oxide photocatalyst. J. Phys. Chem. C 2012, 116, 25376–25387. [Google Scholar] [CrossRef]
- Einaga, H.; Mochiduki, K.; Teraoka, Y. Photocatalytic oxidation processes for toluene oxidation over TiO2 catalysts. Catalysts 2013, 3, 219. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Hsueh, H.-T.; Chang, C.-W.; Chu, H. The visible light-driven photodegradation of dimethyl sulfide on S-doped TiO2: Characterization, kinetics, and reaction pathways. Appl. Catal. B Environ. 2016, 199, 1–10. [Google Scholar] [CrossRef]
- Dhada, I.; Nagar, P.K.; Sharma, M. Photo-catalytic oxidation of individual and mixture of benzene, toluene and p-xylene. Int. J. Environ. Sci. Technol. 2016, 13, 39–46. [Google Scholar] [CrossRef]
- Cheng, L.; Kang, Y.; Li, G. Effect factors of benzene adsorption and degradation by nano-TiO2 immobilized on diatomite. J. Nanomaterials 2012, 2012, 6. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of liquid phase photocatalyzed reactions: An illuminating approach. J. Phys. Chem. B 2005, 109, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Langford, C.H. Variation of langmuir adsorption constant determined for TiO2-photocatalyzed degradation of acetophenone under different light intensity. J. Photochem. Photobiol. A Chem. 2000, 133, 67–71. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; D’Amato, C.; Zannotti, M. Kinetic model for simultaneous adsorption/photodegradation process of alizarin red s in water solution by nano-TiO2 under visible light. Catalysts 2016, 6, 84. [Google Scholar] [CrossRef]
- Silva, C.G.; Faria, J.L. Effect of key operational parameters on the photocatalytic oxidation of phenol by nanocrystalline sol-gel TiO2 under uv irradiation. J. Mol. Catal. A Chem. 2009, 305, 147–154. [Google Scholar] [CrossRef]
- Du, E.; Zhang, Y.X.; Zheng, L. Photocatalytic degradation of dimethyl phthalate in aqueous TiO2 suspension: A modified langmuir–hinshelwood model. React. Kinet. Catal. Lett. 2009, 97, 83–90. [Google Scholar] [CrossRef]
- Brosillon, S.; Lhomme, L.; Vallet, C.; Bouzaza, A.; Wolbert, D. Gas phase photocatalysis and liquid phase photocatalysis: Interdependence and influence of substrate concentration and photon flow on degradation reaction kinetics. Appl. Catal. B Environ. 2008, 78, 232–241. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Li, T.; Li, G. Solvothermal synthesis of mesoporous TiO2: The effect of morphology, size and calcination progress on photocatalytic activity in the degradation of gaseous benzene. Chem. Eng. J. 2014, 237, 312–321. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.-K. Novel and facile synthesis of Ba-doped BiFeO3 nanoparticles and enhancement of their magnetic and photocatalytic activities for complete degradation of benzene in aqueous solution. J. Hazard. Mater. 2016, 316, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Hsiao, Y.-C.; Chiu, Y.-J.; Tseng, Y.-H. A simple route in fabricating carbon-modified titania films with glucose and their visible-light-responsive photocatalytic activity. Catalysts 2018, 8, 178. [Google Scholar] [CrossRef]
- Li, C.X.; Jin, H.Z.; Yang, Z.Z.; Yang, X.; Dong, Q.Z.; Li, T.T. Preparation and photocatalytic properties of mesporous RGO/TiO2 composites. J. Inorg. Mater. 2017, 32, 357–364. [Google Scholar]
- Xu, J.; Liu, Q.; Lin, S.; Cao, W. One-step synthesis of nanocrystalline N-doped TiO2 powders and their photocatalytic activity under visible light irradiation. Res. Chem. Intermed. 2013, 39, 1655–1664. [Google Scholar] [CrossRef]
- Wang, J.; Ruan, H.; Li, W.; Li, D.; Hu, Y.; Chen, J.; Shao, Y.; Zheng, Y. Highly efficient oxidation of gaseous benzene on novel Ag3VO4/TiO2 nanocomposite photocatalysts under visible and simulated solar light irradiation. J. Phys. Chem. C 2012, 116, 13935–13943. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M.; He, D. Preparation, photocatalytic activity, and mechanism of nano- TiO2 co-doped with nitrogen and iron (iii). J. Phys. Chem. C 2007, 111, 10618–10623. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wei, D.; Li, S.; Fang, L.; Zhang, Y. Effect of environmental factors on enhanced adsorption and photocatalytic regeneration of molecular imprinted TiO2 polymers for fluoroquinolones. Environ. Sci. Pollut. Res. 2018, 25, 6729–6738. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kim, J.K.; Chaudhari, V.; Mayadevi, S.; Campos, L.C. Degradation of metaldehyde in water by nanoparticle catalysts and powdered activated carbon. Environ. Sci. Pollut. Res. 2017, 24, 17861–17873. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, P.; Zhang, X.; Jiang, P.; Cao, W.; Chen, P.; Jin, H. Synthesis of N-doped TiO2 with different nitrogen concentrations by mild hydrothermal method. Mater. Manuf. Processes 2014, 29, 1162–1167. [Google Scholar] [CrossRef]

| Illumination Intensity/104 lx | kpm/ 10−6 mol·kg−1·s−1 | KL/ m3·mol−1 | R2 |
|---|---|---|---|
| 36.7 | 3.992 | 1139 | 0.9981 |
| 46.9 | 5.731 | 1064 | 0.9847 |
| 61.7 | 8.589 | 791 | 0.9961 |
| 75.1 | 11.55 | 597 | 0.9674 |
| Illumination Intensity/104 lx | Photoreaction Coefficient kpm/10−6 mol·kg−1·s−1 | Coverage Coefficient Km/m3·mol−1 | Adsorption Constant ka/m3·kg−1·−1 | Desorption Constant kd/mol·kg−1·s−1 | Adsorption Equilibrium Constant KL/m3·mol−1 |
|---|---|---|---|---|---|
| 36.7 | 3.992 | 1139 | 9.242 × 10−3 | 3.514 × 10−6 | 2629 |
| 46.9 | 5.731 | 1064 | |||
| 61.7 | 8.589 | 791 | |||
| 75.1 | 11.55 | 597 |
| Total Concentration Filled into the Reactor ct/ppm | Initial Concentration after Adsorption Equilibrium c0/ppm |
|---|---|
| 15 | 7.79 |
| 18.75 | 10.29 |
| 22.5 | 13.41 |
| 26.25 | 16.17 |
| 30 | 19.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Zhang, J.; Liu, W.; Wang, Q.; Cao, W. Modification to L-H Kinetics Model and Its Application in the Investigation on Photodegradation of Gaseous Benzene by Nitrogen-Doped TiO2. Catalysts 2018, 8, 326. https://doi.org/10.3390/catal8080326
Sun P, Zhang J, Liu W, Wang Q, Cao W. Modification to L-H Kinetics Model and Its Application in the Investigation on Photodegradation of Gaseous Benzene by Nitrogen-Doped TiO2. Catalysts. 2018; 8(8):326. https://doi.org/10.3390/catal8080326
Chicago/Turabian StyleSun, Peng, Jun Zhang, Wenxiu Liu, Qi Wang, and Wenbin Cao. 2018. "Modification to L-H Kinetics Model and Its Application in the Investigation on Photodegradation of Gaseous Benzene by Nitrogen-Doped TiO2" Catalysts 8, no. 8: 326. https://doi.org/10.3390/catal8080326






