Ionic Liquids as Bifunctional Cosolvents Enhanced CO2 Conversion Catalysed by NADH-Dependent Formate Dehydrogenase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison between CO2 Conversions with and without IL
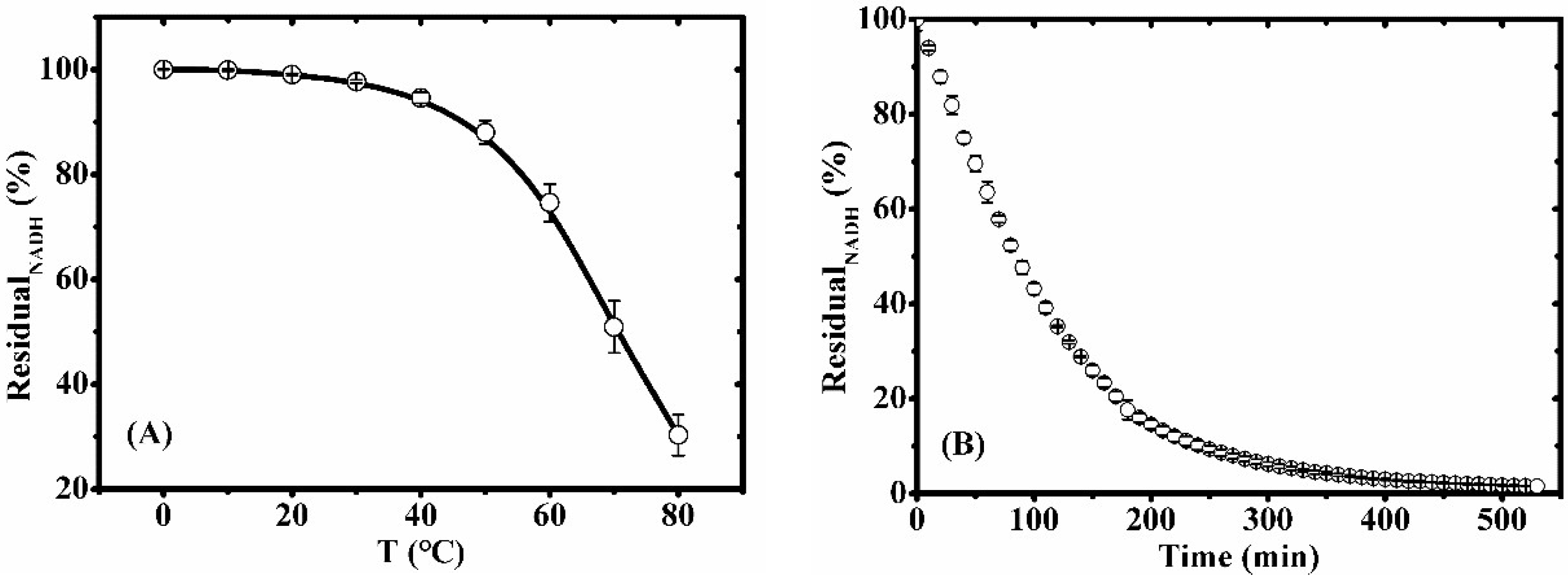
2.2. Stability of NADH in ILs
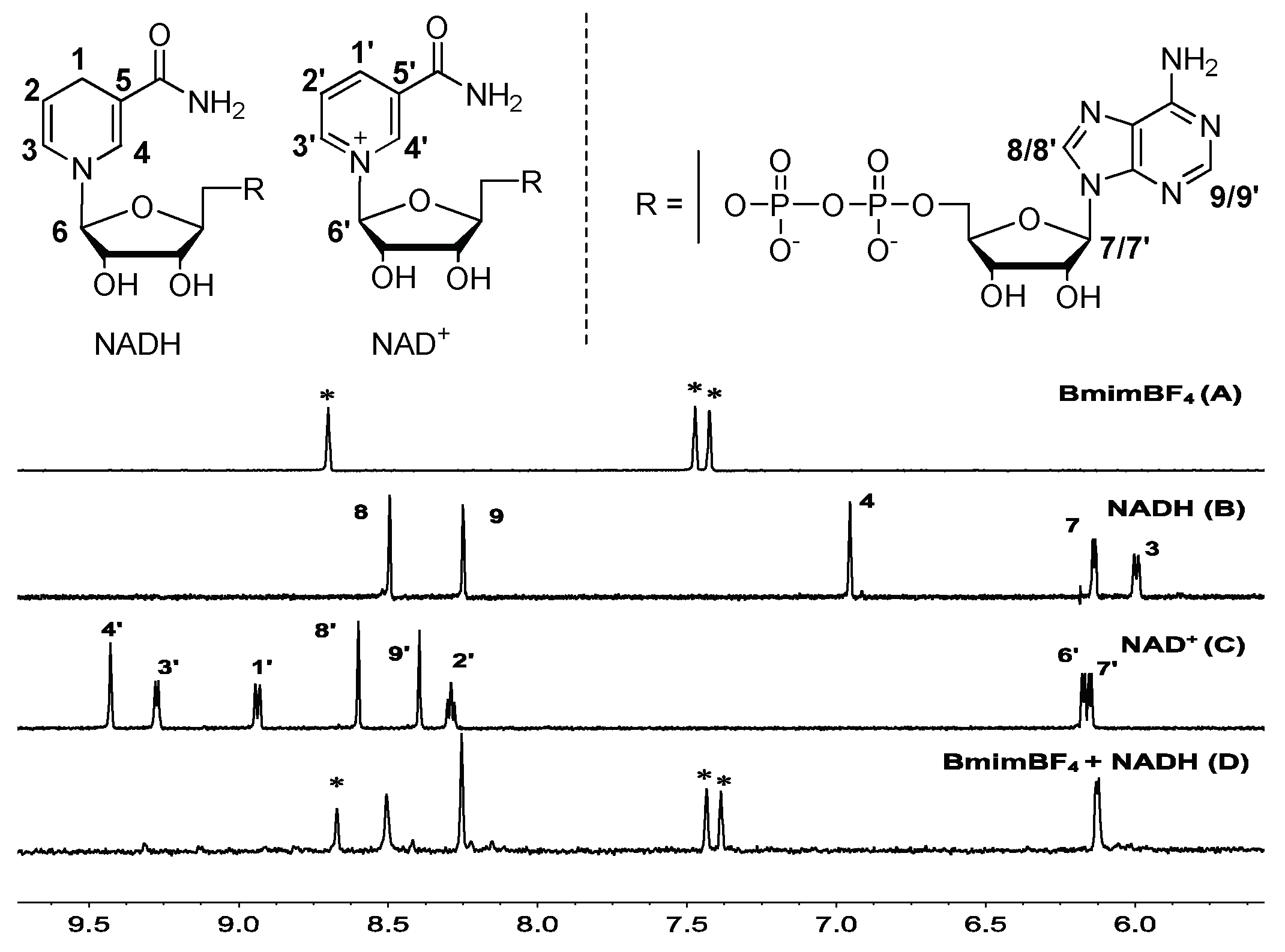
2.3. Mechanism Discussion for NADH Degradation
2.3.1. Effect of Acidity on the Degradation of NADH
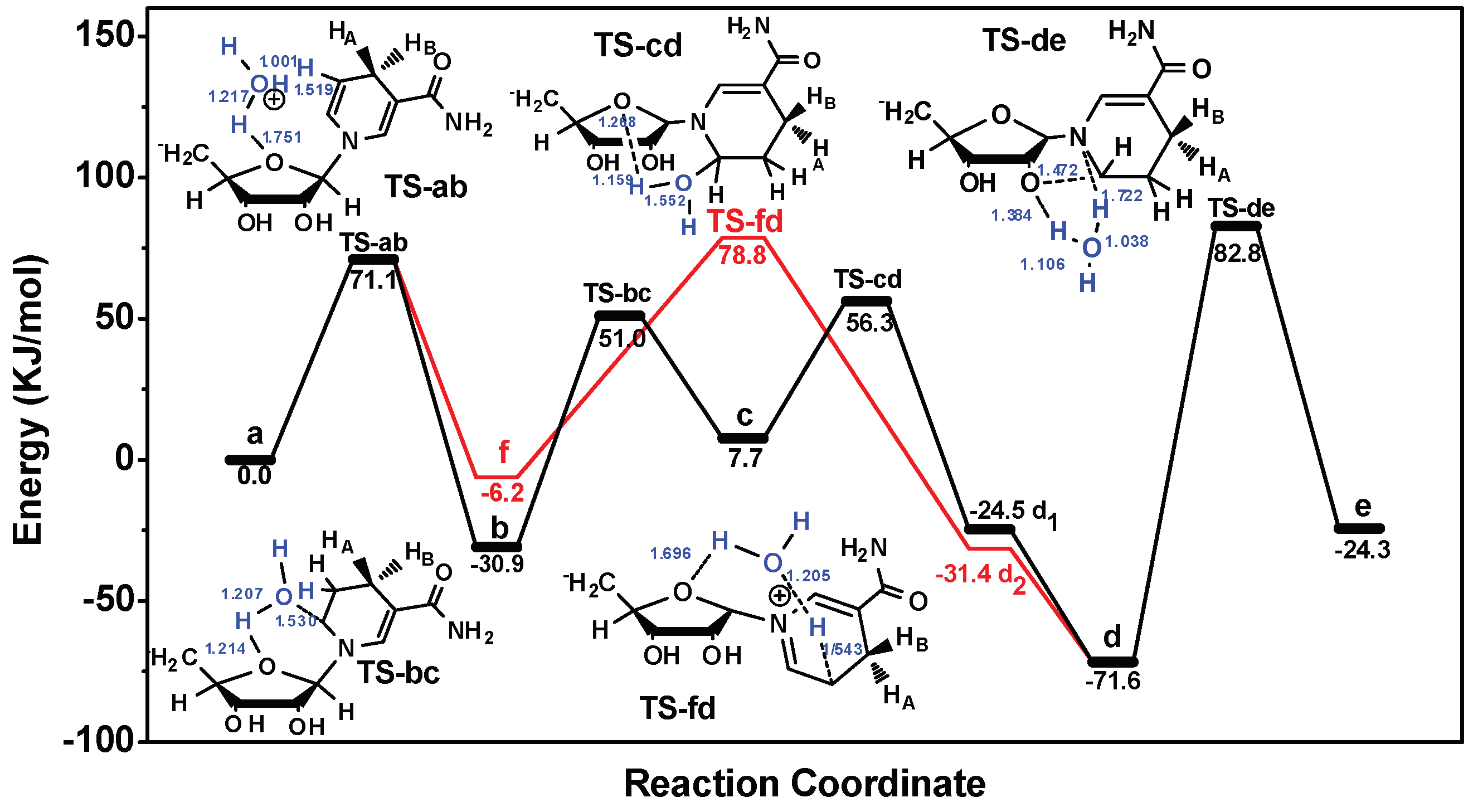
2.3.2. Proposed Mechanism of NADH Degradation
2.4. Enzymatic Reaction in BmimBF4 and BmimDCA
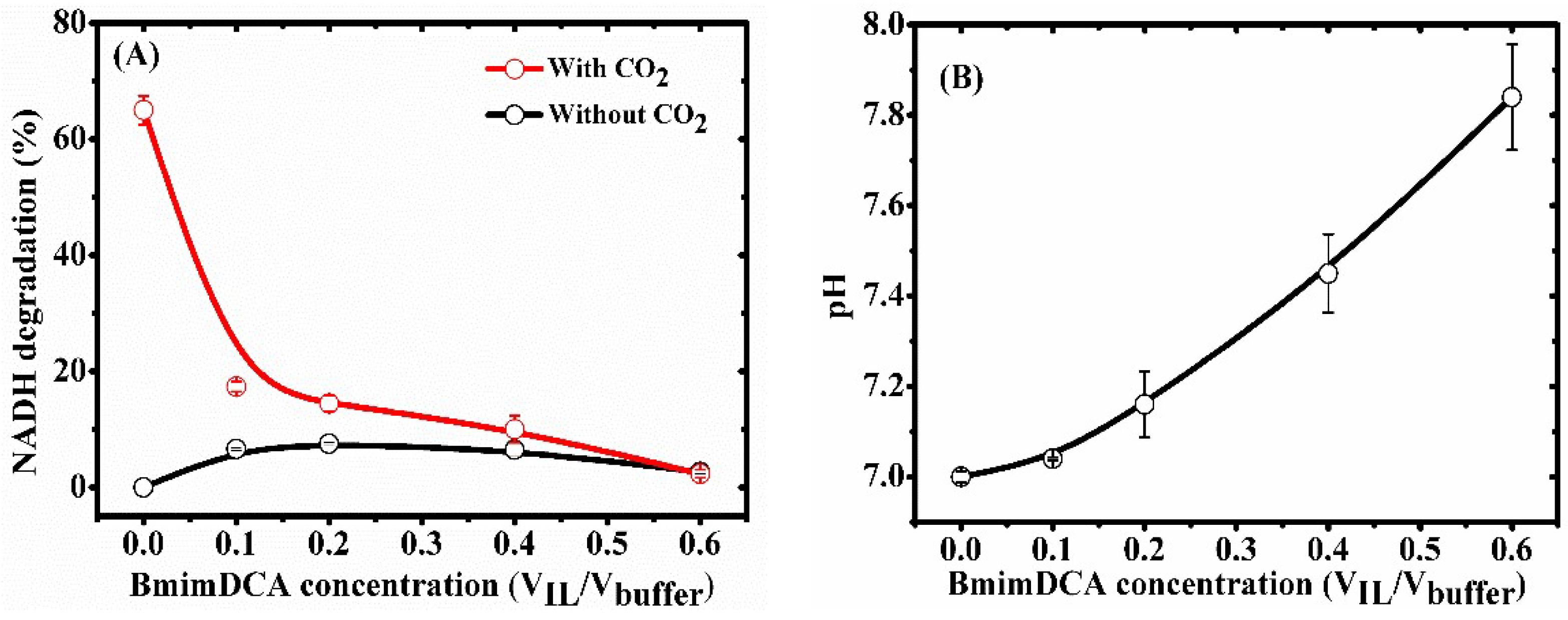
2.4.1. Effect of NADH Degradation in BmimBF4 and BmimDCA
2.4.2. Enzymatic Reaction in BmimBF4 and BmimDCA
2.4.3. Multi-Enzymatic Reaction of Converting CO2 to Methanol in BmimBF4
3. Materials and Methods
3.1. Materials
3.2. General Procedure for Enzymatic Conversion of CO2 to Formate
3.3. Kinetic Degradation of NADH in BmimBF4
3.4. Stability of NADH in Aqueous ILs
3.5. Effect of Acidity on the Degradation of NADH
3.6. NMR for NADH Degradation in BmimBF4
3.7. Effect of NADH Degradation in BmimBF4 and BmimDCA
3.8. Enzymatic Reaction in BmimBF4 and BmimDCA
3.9. Analytical Method
3.10. Method N for Determination Concentration of NADH
3.11. Method C for Determination Concentration of Formate
3.12. GC for Determination Concentration of Methanol
3.13. 1H Nuclear Magnetic Resonance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shi, J.F.; Jiang, Y.J.; Jiang, Z.Y.; Wang, X.Y.; Wang, X.L.; Zhang, S.H.; Han, P.P.; Yang, C. Enzymatic conversion of carbon dioxide. Chem. Soc. Rev. 2015, 44, 5981–6000. [Google Scholar] [CrossRef] [PubMed]
- He, M.Y.; Sun, Y.H.; Han, B.X. Green carbon science: Scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.H.; Wang, J.Q.; Sun, J.; Huang, Y.; Zhang, J.P.; Zhang, X.P.; Zhang, S.J. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach. Green Chem. 2015, 17, 108–122. [Google Scholar] [CrossRef]
- Cui, G.K.; Wang, J.J.; Zhang, S.J. Active chemisorption sites in functionalized ionic liquids for carbon capture. Chem. Soc. Rev. 2016, 45, 4307–4339. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.D.; Zhao, Y.F.; Yu, B.; Yang, Z.Z.; Zhang, H.Y.; Han, B.X.; Gao, X.; Liu, Z.M. Imidazolium-based ionic liquids catalyzed formylation of amines using carbon dioxide and phenylsilane at room temperature. ACS Catal. 2015, 5, 4989–4993. [Google Scholar] [CrossRef]
- Dominguez-Benetton, X.S.S.; Satyawali, Y.; Vanbroekhoven, K.; Pant, D. Enzymatic electrosynthesis: An overview on the progress in enzyme-electrodes for the production of electricity, fuels and chemicals. J. Microb. Biochem. Technol. 2013, S6, 007. [Google Scholar]
- Srikanth, S.G.Y.A.; Vanbroekhoven, K.; Pant, D. Enzymatic electrosynthesis of formic acid through carbon dioxide reduction in a bioelectrochemical system: Effect of immobilization and carbonic anhydrase addition. ChemPhysChem 2017, 18, 3174–3181. [Google Scholar] [CrossRef] [PubMed]
- Obert, R.; Dave, B.C. Enzymatic conversion of carbon dioxide to methanol: Enhanced methanol production in silica sol-gel matrices. J. Am. Chem. Soc. 1999, 121, 12192–12193. [Google Scholar] [CrossRef]
- Luo, J.Q.; Meyer, A.S.; Mateiu, R.V.; Pinelo, M. Cascade catalysis in membranes with enzyme immobilization for multi-enzymatic conversion of CO2 to methanol. New Biotechnol. 2015, 32, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Zhang, X.C.; Dong, H.F.; Zhao, Z.J.; Zhang, S.J.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Kenarsari, S.D.; Yang, D.L.; Jiang, G.D.; Zhang, S.J.; Wang, J.J.; Russell, A.G.; Wei, Q.; Fan, M.H. Review of recent advances in carbon dioxide separation and capture. RSC Adv. 2013, 3, 22739–22773. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Dong, H.F.; Zhang, X.P. The research progress of CO2 capture with ionic liquids. Chin. J. Chem. Eng. 2012, 20, 120–129. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Gu, Z.Y.; Brennecke, J.F. High-pressure phase behavior of ionic liquid/CO2 systems. J. Phys. Chem. B 2001, 105, 2437–2444. [Google Scholar] [CrossRef]
- Lau, R.M.; van Rantwijk, F.; Seddon, K.R.; Sheldon, R.A. Lipase-catalyzed reactions in ionic liquids. Org. Lett. 2000, 2, 4189–4191. [Google Scholar]
- Datta, S.; Holmes, B.; Park, J.I.; Chen, Z.W.; Dibble, D.C.; Hadi, M.; Blanch, H.W.; Simmons, B.A.; Sapra, R. Ionic liquid tolerant hyperthermophilic cellulases for biomass pretreatment and hydrolysis. Green Chem. 2010, 12, 338–345. [Google Scholar] [CrossRef]
- Eckstein, M.; Villela, M.; Liese, A.; Kragl, U. Use of an ionic liquid in a two-phase system to improve an alcohol dehydrogenase catalysed reduction. Chem. Commun. 2004, 1084–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.H.; Zhang, X.W.; Zheng, L.; Xu, H.; Li, M. Enantioselective esterification of (R,S)-flurbiprofen catalyzed by lipase in ionic liquid. Green Chem. Lett. Rev. 2017, 10, 23–28. [Google Scholar] [CrossRef]
- Zhu, C.H.; Guo, F.; Guo, X.Q.; Li, X.K. In situ saccharification of cellulose using a cellulase mixture and supplemental beta-glucosidase in aqueous-ionic liquid media. Bioresources 2016, 11, 9068–9078. [Google Scholar] [CrossRef]
- Amado, V.C.F. “One-Pot” Enzymatic Conversion of CO2 to Methanol; Faculdade de Ciências e Tecnologia: Caparica, Portugal, 2013. [Google Scholar]
- Kaftzik, N.; Wasserscheid, P.; Kragl, U. Use of ionic liquids to increase the yield and enzyme stability in the beta-galactosidase catalysed synthesis of N-acetyllactosamine. Org. Process Res. Dev. 2002, 6, 553–557. [Google Scholar] [CrossRef]
- Dabirmanesh, B.; Khajeh, K.; Ranjbar, B.; Ghazi, F.; Heydari, A. Inhibition mediated stabilization effect of imidazolium based ionic liquids on alcohol dehydrogenase. J. Mol. Liq. 2012, 170, 66–71. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Z.Y.; Xu, S.W.; Wu, H. Efficient conversion of CO2 to formic acid by formate dehydrogenase immobilized in a novel alginate-silica hybrid gel. Catal. Today 2006, 115, 263–268. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, J.; Wu, H.; Jiang, Z.; Zhang, W.; Song, X.; Ai, Q. Bioinspired approach to multienzyme cascade system construction for efficient carbon dioxide reduction. ACS Catal. 2014, 4, 962–972. [Google Scholar] [CrossRef]
- Liu, A.H.; Feng, R.R.; Liang, B. Microbial surface displaying formate dehydrogenase and its application in optical detection of formate. Enzyme Microb. Technol. 2016, 91, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.G.; Berkelhammer, G. A study of the primary acid reaction on model compounds of reduced diphosphopyridine nucleotide. J. Am. Chem. Soc. 1958, 80, 992–999. [Google Scholar] [CrossRef]
- Alivisatos, S.G.; Ungar, F.; Abraham, G.J. Spontaneous reactions of 1,3-substituted 1,4-dihydropyridines with acids in water at neutrality.I. Kinetic analysis and mechanism of reactions of dihydronicotinamide—adenine dinucleotide with orthophosphates. Biochemistry 1965, 4, 2616–2630. [Google Scholar]
- Oppenheimer, N.J.; Kaplan, N.O. Structure of the primary acid rearrangement product of reduced nicotinamide adenine dinucleotide (NADH). Biochemistry 1974, 13, 4675–4685. [Google Scholar] [CrossRef] [PubMed]
- Ruschig, U.; Muller, U.; Willnow, P.; Hopner, T. CO2 reduction to formate by nadh catalyzed by formate dehydrogenase from pseudomonas-oxalaticus. Eur. J. Biochem. 1976, 70, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Li, M.F.; Zhao, Z.P.; Liu, W.F. Effect of carbonic anhydrase on enzymatic conversion of CO2 to formic acid and optimization of reaction conditions. J. Mol. Catal. B-Enzym. 2015, 116, 89–94. [Google Scholar] [CrossRef]
- Rover, L.; Fernandes, J.C.B.; Neto, G.D.; Kubota, L.T.; Katekawa, E.; Serrano, S.H.P. Study of nadh stability using ultraviolet-visible spectrophotometric analysis and factorial design. Anal. Biochem. 1998, 260, 50–55. [Google Scholar] [CrossRef]
- Frisch, M.J.T.G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision d. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Baltrusaitis, J.; Grassian, V.H. Carbonic acid formation from reaction of carbon dioxide and water coordinated to al(oh)(3): A quantum chemical study. J. Phys. Chem. A 2010, 114, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Coutinho, J.A.P.; Fernandes, A.M. Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Carrete, J.; Mendez-Morales, T.; Cabeza, O.; Lynden-Bell, R.M.; Gallego, L.J.; Varela, M.L. Investigation of the local structure of mixtures of an ionic liquid with polar molecular species through molecular dynamics: Cluster formation and angular distributions. J. Phys. Chem. B 2012, 116, 5941–5950. [Google Scholar] [CrossRef] [PubMed]
- Yuki Kohno, H.O. Ionic liquid/water mixtures: From hostility to conciliation. Chem. Commun. 2012, 48, 7119–7130. [Google Scholar] [CrossRef] [PubMed]
- Sleat, R.; Mah, R.A. Quantitative method for colorimetric determination of formate in fermentation media. Appl. Environ. Microb. 1984, 47, 884–885. [Google Scholar]
- Denning, D.M.; Thum, M.D.; Falvey, D.E. Photochemical reduction of CO2 using 1,3-dimethylimidazolylidene. Org. Lett. 2015, 17, 4152–4155. [Google Scholar] [CrossRef] [PubMed]



| Entry | IL (VIL/Vbuffer) | NADH Conv. (%) (SD) | Yield of Formate (%) (SD) |
|---|---|---|---|
| Method N | Method C | ||
| 1 | Phosphate buffer (Blank) | 64.6 (±3.6) | 1.1 (±0.5) |
| 2 | BmimBF4 (20%) | 25.4 (±3.2) | 2.9 (±0.4) |
| Entry | IL | Residue(NADH) (%) (SD) | pH (SD) |
|---|---|---|---|
| 1 | BmimDCA | 100.0 (±0.1) | 7.8 (±0.20) |
| 2 | EmimOAc | 12.8 (±3.1) | 5.3 (±0.12) |
| 3 | EmimBF4 | 0 | 2.8 (±0.18) |
| 4 | BmimBF4 | 0 | 2.6 (±0.18) |
| 5 | BmimDMP | 0 | 2.3 (±0.16) |
| 6 | DBULat | 99.7 (±0.6) | 9.3 (±0.14) |
| Entry | Solvent (v/v) | NADH Conv. (%) (SD) | Yield of Formate (%) (SD) | pH (SD) | |
|---|---|---|---|---|---|
| Method N | Method C | Before | After | ||
| 1 | Phosphate buffer | 64.6 (±3.6) | 1.1 (±0.5) | 7.0 (±0.04) | 5.6 (±0.08) |
| 2 | 10% BmimBF4 | 46.8 (±2.3) | 0.2 (±0.3) | 6.7 (±0.08) | 6.2 (±0.12) |
| 3 | 20% BmimBF4 | 25.4 (±3.2) | 2.9 (±0.4) | 6.5 (±0.06) | 6.0 (±0.16) |
| 4 | 40% BmimBF4 | 36.3 (±2.0) | 2.3 (±0.4) | 6.0 (±0.05) | 5.8 (±0.18) |
| 5 | 60% BmimBF4 | 24.6 (±3.0) | 0.6 (±0.3) | 5.9 (±0.05) | 5.6 (±0.19) |
| 6 | 10% BmimDCA | 34.0 (±3.9) | 0.4 (±0.2) | 7.0 (±0.04) | 6.6 (±0.12) |
| 7 | 20% BmimDCA | 16.2 (±1.3) | 1.5 (±0.5) | 7.2 (±0.06) | 6.5 (±0.14) |
| 8 | 40% BmimDCA | 4.9 (±2.8) | 1.8 (±0.5) | 7.5 (±0.08) | 6.5 (±0.19) |
| 9 | 60% BmimDCA | 1.6 (±1.4) | 0.2 (±0.2) | 7.8 (±0.09) | 6.8 (±0.20) |

| Entry | Solvent (vIL/vbuffer) | Yield of Methanol (%) (SD) |
|---|---|---|
| 1 | Phosphate buffer | 24.3 (±1.2) |
| 2 | BmimBF4 (10%) | 20.2 (±1.9) |
| 3 | BmimBF4 (20%) | 67.1 (±2.4) |
| 4 | BmimBF4 (40%) | 48.3 (±2.1) |
| 5 | BmimBF4 (60%) | 15.2 (±2.6) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xu, B.-h.; Luo, J.; Solms, N.V.; He, H.; Zhang, Y.; Pinelo, M.; Zhang, S. Ionic Liquids as Bifunctional Cosolvents Enhanced CO2 Conversion Catalysed by NADH-Dependent Formate Dehydrogenase. Catalysts 2018, 8, 304. https://doi.org/10.3390/catal8080304
Zhang Z, Xu B-h, Luo J, Solms NV, He H, Zhang Y, Pinelo M, Zhang S. Ionic Liquids as Bifunctional Cosolvents Enhanced CO2 Conversion Catalysed by NADH-Dependent Formate Dehydrogenase. Catalysts. 2018; 8(8):304. https://doi.org/10.3390/catal8080304
Chicago/Turabian StyleZhang, Zhibo, Bao-hua Xu, Jianquan Luo, Nicolas Von Solms, Hongyan He, Yaqin Zhang, Manuel Pinelo, and Suojiang Zhang. 2018. "Ionic Liquids as Bifunctional Cosolvents Enhanced CO2 Conversion Catalysed by NADH-Dependent Formate Dehydrogenase" Catalysts 8, no. 8: 304. https://doi.org/10.3390/catal8080304
APA StyleZhang, Z., Xu, B.-h., Luo, J., Solms, N. V., He, H., Zhang, Y., Pinelo, M., & Zhang, S. (2018). Ionic Liquids as Bifunctional Cosolvents Enhanced CO2 Conversion Catalysed by NADH-Dependent Formate Dehydrogenase. Catalysts, 8(8), 304. https://doi.org/10.3390/catal8080304







