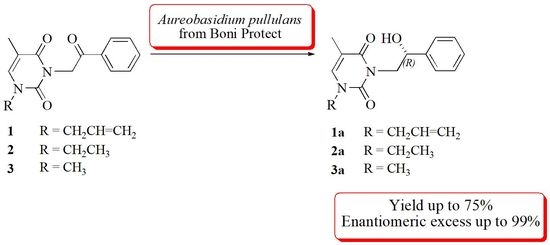
Enantioselective Bioreduction of Prochiral Pyrimidine Base Derivatives by Boni Protect Fungicide Containing Live Cells of Aureobasidium pullulans
Abstract
:1. Introduction
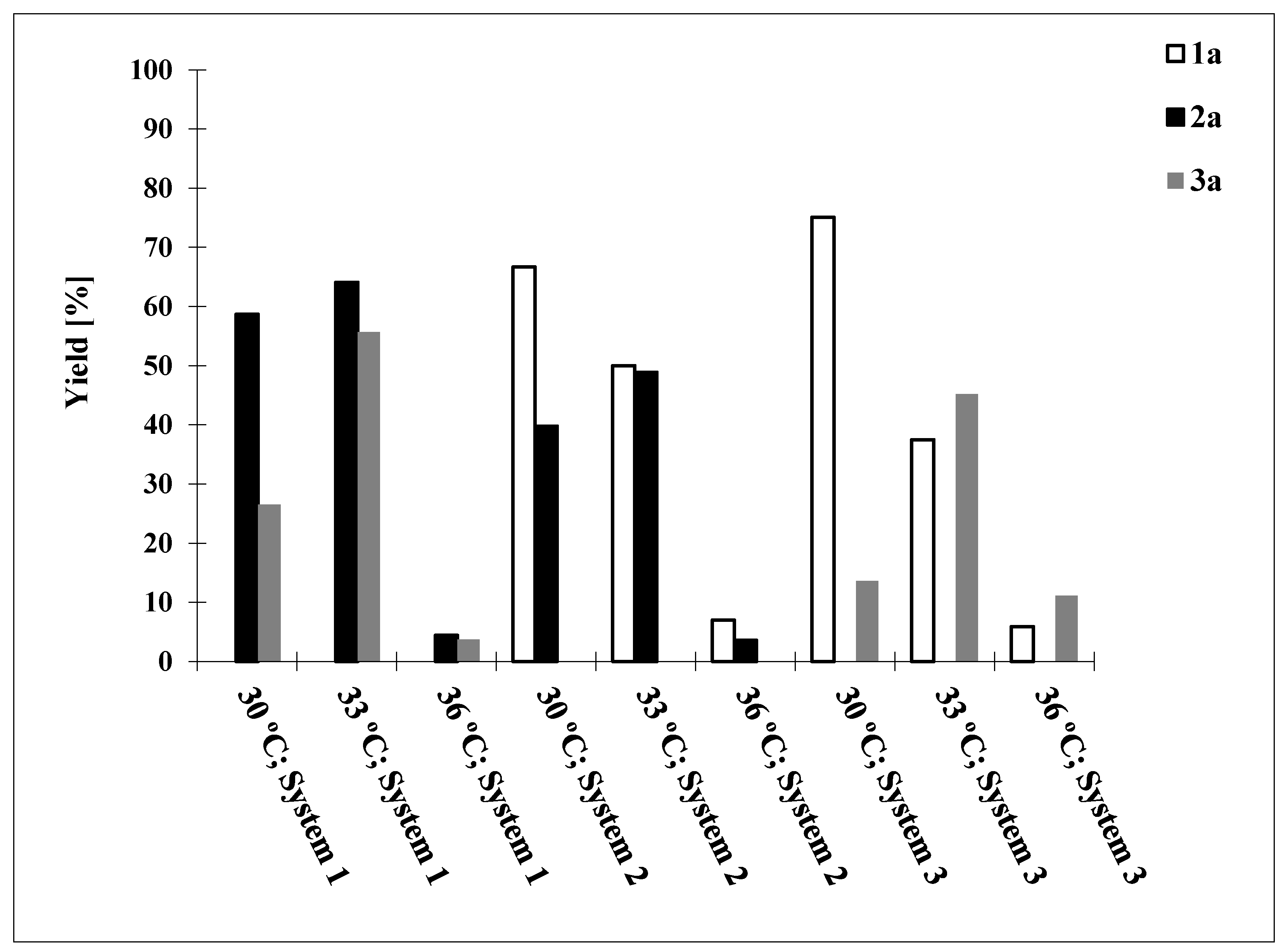
2. Results and Discussion
3. Materials and Methods
3.1. Analytical Methods
3.2. Reagents and Solvents
3.3. General Procedure of Asymmetric Reduction by Aureobasidium pullulans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakamura, K.T.; Yamanaka, R.; Mastuda, T.; Harada, T. Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron Asymmetry 2003, 14, 2659–2681. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Pan, J.; Ma, B.-D.; Xu, J.-H. Efficient biocatalytic synthesis of chiral chemicals. Adv. Biochem. Eng. Biotechnol. 2016, 155, 55–106. [Google Scholar] [CrossRef] [PubMed]
- Gašo-Sokač, D.; Nujić, M.; Bušić, V.; Habuda-Stanić, M. Biocatalytic reductions by plant tissue-green alternative to alcohol production. Croat. J. Food Sci. Technol. 2014, 6, 51–60. [Google Scholar]
- Patel, R.N. Synthesis of chiral pharmaceutical intermediates by biocatalysis. Coord. Chem. Rev. 2008, 252, 659–701. [Google Scholar] [CrossRef]
- Patel, R.N. Biocatalytic synthesis of chiral alcohols and amino acids for development of pharmaceuticals. Biomolecules 2013, 3, 741–777. [Google Scholar] [CrossRef] [PubMed]
- Mastuda, T.; Yamanaka, R.; Nakamura, K. Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetry 2009, 20, 513–557. [Google Scholar] [CrossRef]
- Csuk, R.; Glaenzer, B.I. Baker’s yeast mediated transformations in organic chemistry. Chem. Rev. 1991, 91, 49–97. [Google Scholar] [CrossRef]
- Schubert, T.; Hummel, W.; Kula, M.-R.; Müller, M. Enantioselective synthesis of both enantiomers of various propargylic alcohols by use of two oxidoreductases. Eur. J. Org. Chem. 2001, 22, 4181–4187. [Google Scholar] [CrossRef]
- Nakamura, K.; Takenaka, K.; Fujii, M.; Ida, Y. Asymmetric synthesis of both enantiomers of secondary alcohols by reduction with a single microbe. Tetrahedron Lett. 2002, 43, 3629–3631. [Google Scholar] [CrossRef]
- Patel, R.N.; McNamee, C.G.; Banerjee, A.; Howell, J.M.; Robison, R.S.; Szarka, L. Stereoselective reduction of β-keto esters by Geotrichum candidum. J. Enzyme Microb. Technol. 1992, 14, 731–738. [Google Scholar] [CrossRef]
- Goswami, A.; Bezbaruah, R.L.; Goswami, J.; Borthakur, N.; Dey, D.; Hazarika, A.K. Microbial reduction of ω-bromoacetophenones in the presence of surfactants. Tetrahedron Asymmetry 2000, 11, 3701–3709. [Google Scholar] [CrossRef]
- Goswami, J.; Bezbaruah, R.L.; Goswami, A.; Borthakur, N. A convenient stereoselective synthesis of (R)-(−)-denopamine and (R)-(−)-salmeterol. Tetrahedron Asymmetry 2002, 12, 3343–3348. [Google Scholar] [CrossRef]
- Roy, S.; Alexandre, V.; Neuwels, M.; Le Texier, L. Asymmetric bioreduction of a bulky ketone: 1-phenyl-1-(2-phenylthiazol-5-yl)-methanone. Adv. Synth. Catal. 2001, 343, 738–743. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, G.S.K.K.; Sabitha, G.; Krishna, A.D.; Prasad, A.R.; Rahaman, H.-U.-R.; Rao, K.V.; Rao, A.B. Daucus carota and baker’s yeast mediated bio-reduction of prochiral ketones. Tetrahedron Asymmetry 2007, 18, 717–723. [Google Scholar] [CrossRef]
- Omori, A.T.; Lobo, F.G.; Gonçalves do Amaral, A.C.; de Oliveira, C.S. Purple carrots: Better biocatalysts for the enantioselective reduction of acetophenones than common orange carrots (D. carota). J. Mol. Catal. B Enzym. 2016, 127, 93–97. [Google Scholar] [CrossRef]
- Lakshmi, C.S.; Reddy, G.R.; Rao, A.B. Asymmetric reduction of heteroaryl methyl ketones using Daucus carota. Green Sustain. Chem. 2011, 1, 117–122. [Google Scholar] [CrossRef]
- Yadav, J.S.; Nanda, S.; Reddy, P.T.; Rao, A.B. Efficient enantioselective reduction of ketones with Daucus carota root. J. Org. Chem. 2002, 67, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Kozai, S.; Maruyama, T.; Kimura, T.; Yamamoto, I. Synthesis and hypnotic activities of 4-thio analogues of N3-substituted uridines. Chem. Pharm. Bull. 2001, 49, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yao, S.; Watanabe, K.; Kondo, S.; Ho, I.K.; Yamamoto, I. Hypnotic action of N3-substituted arabinofuranosyluracils on mice. Chem. Pharm. Bull. 2001, 49, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.N. Enzymatic synthesis of chiral intermediates for drug development. Adv. Synth. Catal. 2001, 343, 527–546. [Google Scholar] [CrossRef]
- Patel, R.N.; Chu, L.; Chidambaram, R.; Zhu, J.; Kant, J. Enantioselective microbial reduction of 2-oxo-2-(1′,2′,3′,4′-tetrahydro-1′,1′,4′,4′-tetramethyl-6′-naphthalenyl)acetic acid and its ethyl ester. Tetrahedron Asymmetry 2002, 13, 349–355. [Google Scholar] [CrossRef]
- Zhang, F.; Ni, Y.; Sun, Z.; Zheng, P.; Lin, W.; Zhu, P.; Ju, N. Asymmetric reduction of ethyl 4-chloro-3-oxobutanoate to ethyl (S)-4-chloro-3-hydroxybutanoate catalyzed by Aureobasidium pullulans in an aqueous/ionic liquid biphase system. Chin. J. Catal. 2008, 29, 577–582. [Google Scholar] [CrossRef]
- He, J.-Y.; Sun, Z.-H.; Ruan, W.-Q.; Xu, Y. Biocatalytic synthesis of ethyl (S)-4-chloro-3hydroxy-butanoate in an aqueous-organic solvent biphasic system using Aureobasidium pullulans CGMCC 1244. Process Biochem. 2006, 41, 244–249. [Google Scholar] [CrossRef]
- Ravía, S.P.; Carrera, I.; Seoane, G.A.; Vero, S.; Gamenara, D. Novel fungi-catalyzed reduction of α-alkyl-β-keto esters. Tetrahedron Asymmetry 2009, 20, 1393–1397. [Google Scholar] [CrossRef]
- Kołodziejska, R.; Wróblewski, M.; Studzińska, R.; Karczmarska-Wódzka, A.; Grela, I.; Augustyńska, B.; Modzelewska-Banachiewicz, B. Aureobasidium pullulans as a key for the preparation of optical purity (R)-2-(anthracen-9-yl)-2-methoxyacetic acid—The chiral auxiliary reagent in determination of absolute configuration. J. Mol. Catal. B Enzym. 2015, 121, 28–31. [Google Scholar] [CrossRef]
- Kołodziejska, R.; Studzińska, R.; Kwit, M.; Jelecki, M.; Tafelska-Kaczmarek, A. Microbiological bio-reduction of prochiral carbonyl compounds by antimycotic agent Boni Protect. Catal. Commun. 2017, 101, 81–84. [Google Scholar] [CrossRef]
- Górecki, M.; Karczmarska-Wódzka, A.; Kołodziejska, R.; Dramiński, M.; Frelek, J. Determination of the stereostructure of pyrimidine nucleoside derivatives with a combination of various chiroptical methods. Eur. J. Org. Chem. 2014, 5204–5213. [Google Scholar] [CrossRef]
- Köhler, J.; Wünsch, B. Lipase catalyzed enantioselective desymmetrization of a prochiral pentane-1,3,5-triol derivative. Tetrahedron Asymmetry 2006, 17, 3091–3099. [Google Scholar] [CrossRef]

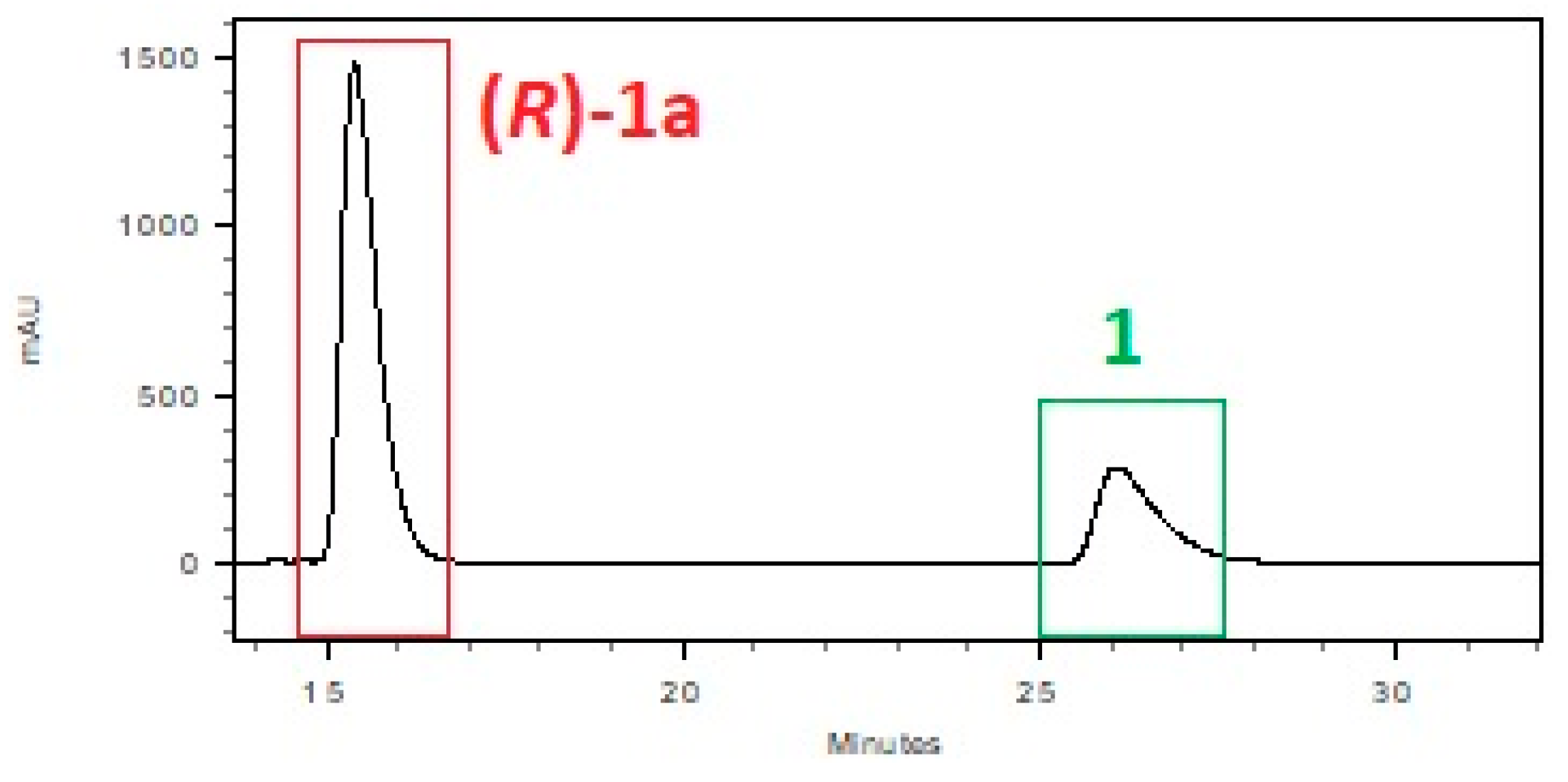
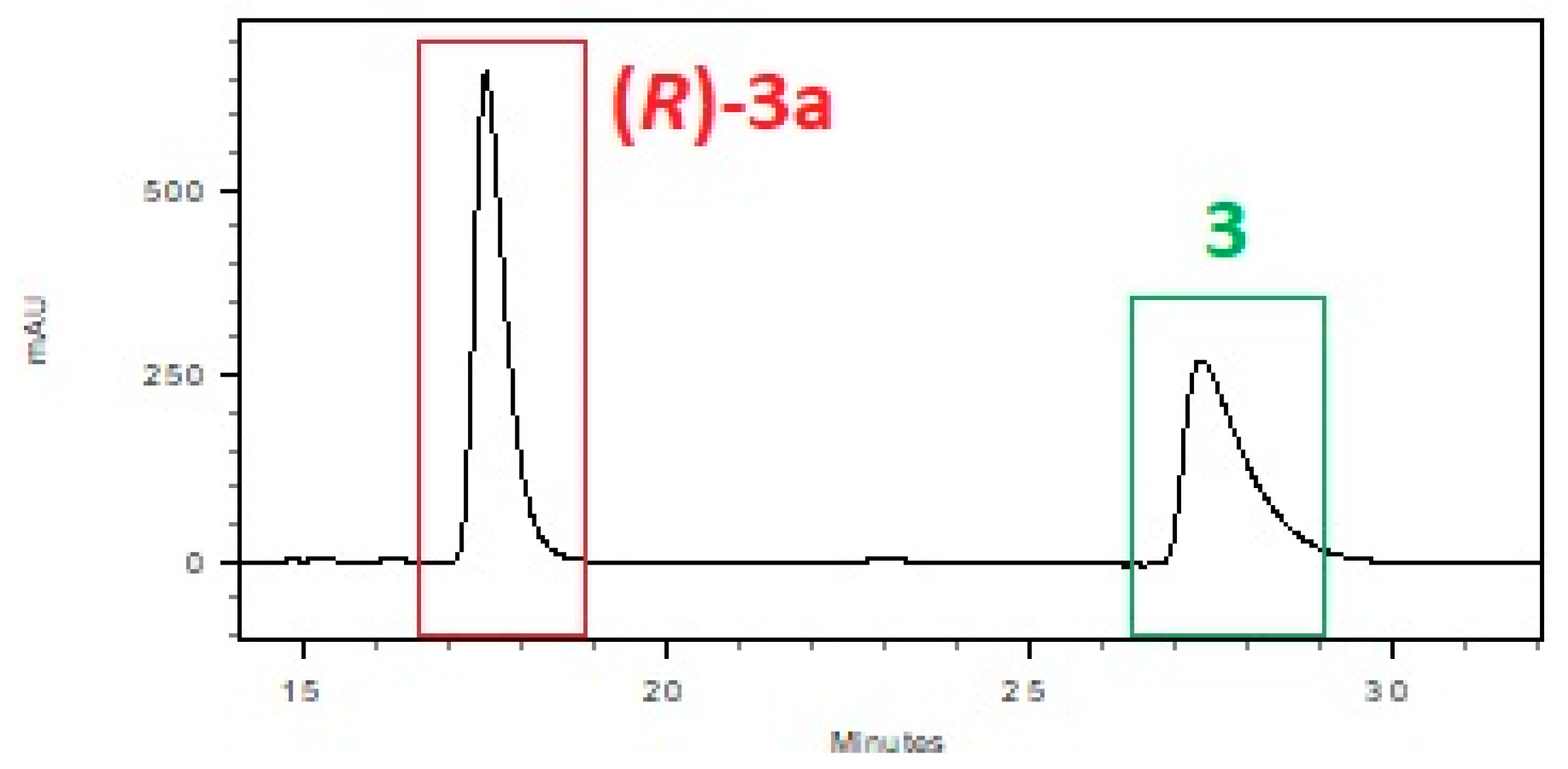
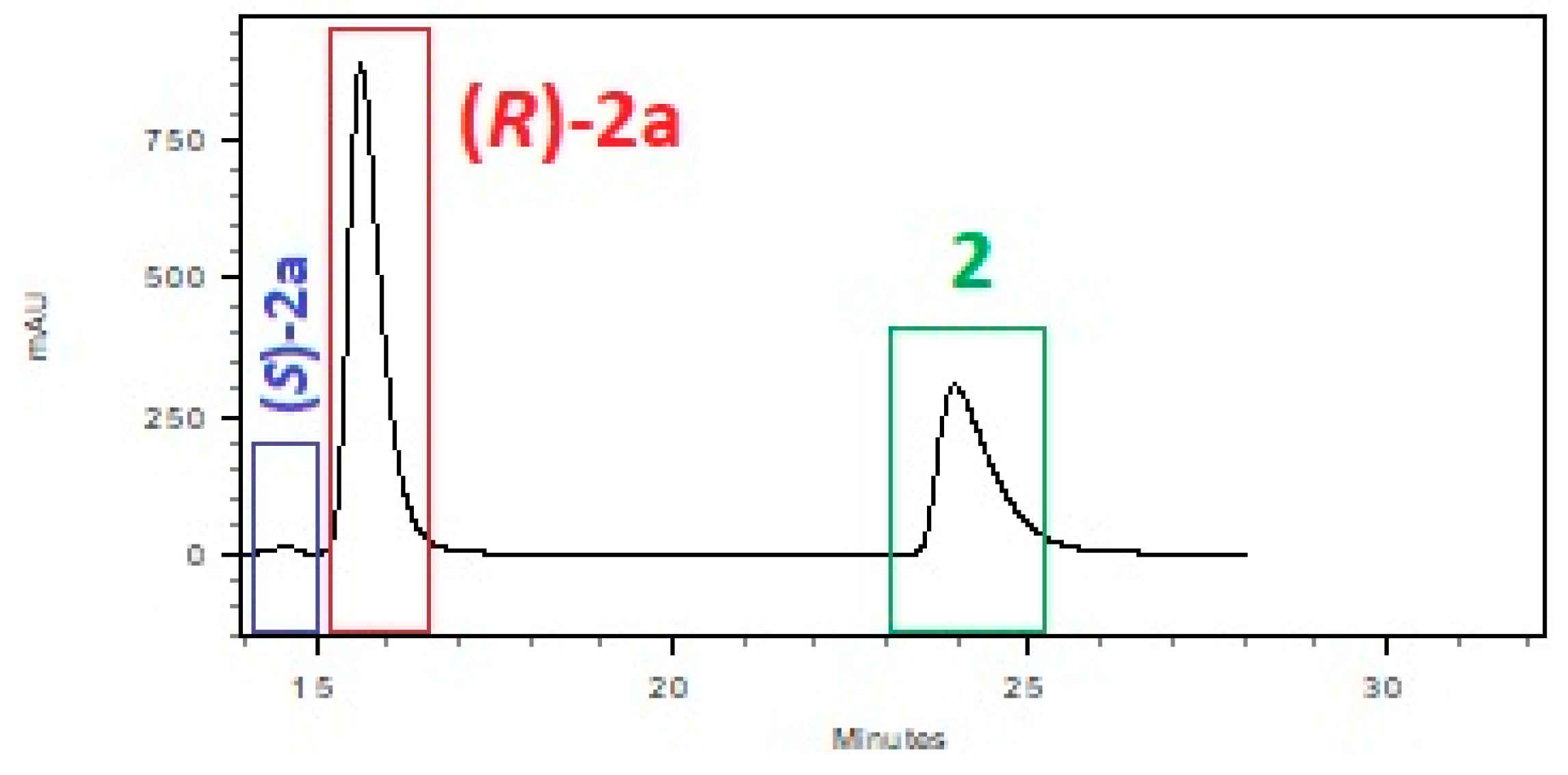
| System: Phosphate Buffer | T [h] | 1 [%] a | 1a [%] a | ee [%] a |
|---|---|---|---|---|
| pH = 7.0, glucose | 72 | 64.6 | 35.4 | 99 |
| pH = 7.0, glucose | 120 | 51.8 | 48.2 | 99 |
| pH = 7.0, glucose | 144 | 39.6 | 60.4 | 99 |
| pH = 7.0:hexane 1:1 (v/v), glucose | 144 | 100 | - | - |
| pH = 7.0:hexane 4:1 (v/v), glucose | 144 | 95.9 | 4.1 | 94 |
| pH = 7.0:hexane 88:2 (v/v), glucose | 144 | 90.0 | 10.0 | 99 |
| pH = 7.0:TBME 88:2 (v/v), glucose | 144 | 96.9 | 3.1 | 99 |
| pH = 7.0:acetonitrile 88:2 (v/v), glucose | 144 | 98.4 | 1.6 | 99 |
| pH = 7.0:THF 88:2 (v/v), glucose | 144 | 98.8 | 1.2 | 99 |
| pH = 7.0:propan-2-ol 88:2 (v/v), glucose | 144 | 93.3 | 6.7 | 99 |
| pH = 7.0:[BMIM][PF6] 88:2 (v/v), glucose | 144 | 90.3 | 9.7 | 99 |
| pH = 7.0:[BMIM][BF4] 88:2 (v/v), glucose | 144 | 88.4 | 11.6 | 99 |
| pH = 6.5, glucose | 144 | 33.3 | 66.7 | 99 |
| pH = 7.0, sucrose | 144 | 24.4 | 75.6 | 99 |
| pH = 6.5, sucrose | 144 | 57.3 | 42.7 | 99 |
| System: Phosphate Buffer | 2 [%] a,b | 2a [%] a,b | ee [%] a | 3 [%] a,b | 3a [%] a,b | ee [%] a |
|---|---|---|---|---|---|---|
| pH = 7.0, glucosec | 41.3 | 58.7 | 99 | 73.5 | 26.5 | 96 |
| pH = 6.5, glucosec | 60.4 | 39.6 | 99 | 91.6 | 8.4 | 99 |
| pH = 7.0, sucrosec | 76.8 | 23.2 | 99 | 86.6 | 13.4 | 99 |
| pH = 6.5, sucrosec | 85.3 | 14.7 | 96 | 94.1 | 5.9 | 96 |
| System | T [°C] | 1a [%] a | ee [%] a | 2a [%] a | ee [%] a | 3a [%] a | ee [%] a |
|---|---|---|---|---|---|---|---|
| Phosphate buffer pH = 7.0, glucose | 33 °C | Nd b | Nd b | 64.1 | 98 | 55.7 | 99 |
| 36 °C | Nd b | Nd b | 4.4 | 99 | 3.7 | 99 | |
| Phosphate buffer pH = 6.5, glucose | 33 °C | 50.0 | 99 | 48.9 | 99 | Nd b | Nd b |
| 36 °C | 7.0 | 96 | 3.6 | 99 | Nd b | Nd b | |
| Phosphate buffer pH = 7.0, sucrose | 33 °C | 37.5 | 99 | Nd b | Nd b | 45.2 | 99 |
| 36 °C | 5.9 | 98 | Nd b | Nd b | 11.1 | 99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodziejska, R.; Studzińska, R.; Pawluk, H.; Karczmarska-Wódzka, A.; Woźniak, A. Enantioselective Bioreduction of Prochiral Pyrimidine Base Derivatives by Boni Protect Fungicide Containing Live Cells of Aureobasidium pullulans. Catalysts 2018, 8, 290. https://doi.org/10.3390/catal8070290
Kołodziejska R, Studzińska R, Pawluk H, Karczmarska-Wódzka A, Woźniak A. Enantioselective Bioreduction of Prochiral Pyrimidine Base Derivatives by Boni Protect Fungicide Containing Live Cells of Aureobasidium pullulans. Catalysts. 2018; 8(7):290. https://doi.org/10.3390/catal8070290
Chicago/Turabian StyleKołodziejska, Renata, Renata Studzińska, Hanna Pawluk, Aleksandra Karczmarska-Wódzka, and Alina Woźniak. 2018. "Enantioselective Bioreduction of Prochiral Pyrimidine Base Derivatives by Boni Protect Fungicide Containing Live Cells of Aureobasidium pullulans" Catalysts 8, no. 7: 290. https://doi.org/10.3390/catal8070290
APA StyleKołodziejska, R., Studzińska, R., Pawluk, H., Karczmarska-Wódzka, A., & Woźniak, A. (2018). Enantioselective Bioreduction of Prochiral Pyrimidine Base Derivatives by Boni Protect Fungicide Containing Live Cells of Aureobasidium pullulans. Catalysts, 8(7), 290. https://doi.org/10.3390/catal8070290