Magnetic Microreactors with Immobilized Enzymes—From Assemblage to Contemporary Applications
Abstract
1. Introduction
2. Microfluidics in Enzyme Biotechnology
2.1. Advantages of Microfluidics for Biocatalysis
2.2. Fabrication of Enzymatic Microreactors
3. Magnetic Microreactors
3.1. Overview
3.2. Enzyme Immobilization on Magnetic Particles
4. Selected Examples
4.1. Exploring the Optimal System Configuration
4.2. Utilizing Multiple-Enzyme Systems
4.3. Emphasizing on the Immobilization Support
4.4. Optimizing Process Parameters for Scaling-Up
4.5. Other Applications
5. Concluding Remarks and Future Prospects
Acknowledgments
Conflicts of Interest
References
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow Bioreactors as Complementary Tools for Biocatalytic Process Intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, R.; Plazl, I.; Žnidaršič-Plazl, P.; Gernaey, K.V.; Woodley, J.M. Microscale technology and biocatalytic processes: Opportunities and challenges for synthesis. Trends Biotechnol. 2015, 33, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Wiesbauer, J.; Nidetzky, B. Biotransformations in microstructured reactors: More than flowing with the stream? Trends Biotechnol. 2011, 29, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Marcy, Y.; Ishoey, T.; Lasken, R.S.; Stockwell, T.B.; Walenz, B.P.; Halpern, A.L.; Beeson, K.Y.; Goldberg, S.M.D.; Quake, S.R. Nanoliter reactors improve multiple displacement amplification of genomes from single cells. PLoS Genet. 2007, 3, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Pastre, J.C.; Browne, D.L.; Ley, S.V. Flow chemistry syntheses of natural products. Chem. Soc. Rev. 2013, 42, 8849–8869. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.T.; deMello, A.J.; Di Carlo, D.; Doyle, P.S.; Hansen, C.; Maceiczyk, R.M.; Wootton, R.C.R. Small but Perfectly Formed? Successes, Challenges, and Opportunities for Microfluidics in the Chemical and Biological Sciences. Chem 2017, 2, 201–223. [Google Scholar] [CrossRef]
- Meller, K.; Szumski, M.; Buszewski, B. Sensors and Actuators B: Chemical Microfluidic reactors with immobilized enzymes—Characterization, dividing, perspectives. Sens. Actuators B. Chem. 2017, 244, 84–106. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Patila, M.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Graphene-based nanobiocatalytic systems: Recent advances and future prospects. Trends Biotechnol. 2014, 32, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Kecskemeti, A.; Gaspar, A. Particle-based immobilized enzymatic reactors in microfluidic chips. Talanta 2018, 180, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.-H.; Gyak, K.-W.; Kim, D.-P. Emerging microreaction systems based on 3D printing techniques and separation technologies. J. Flow Chem. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Takenaga, S.; Schneider, B.; Erbay, E.; Biselli, M.; Schnitzler, T.; Schöning, M.J.; Wagner, T. Fabrication of biocompatible lab-on-chip devices for biomedical applications by means of a 3D-printing process. Phys. Status Solidi Appl. Mater. Sci. 2015, 212, 1347–1352. [Google Scholar] [CrossRef]
- Au, A.K.; Bhattacharjee, N.; Horowitz, L.F.; Chang, T.C.; Folch, A. 3D-printed microfluidic automation. Lab Chip 2015, 15, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.; Yang, L. Capillary electrophoresis-integrated immobilized enzyme reactors. Rev. Anal. Chem. 2016, 35, 115–131. [Google Scholar] [CrossRef]
- Boehm, C.R.; Freemont, P.S.; Ces, O. Design of a prototype flow microreactor for synthetic biology in vitro. Lab Chip 2013, 13, 3426–3432. [Google Scholar] [CrossRef] [PubMed]
- Tzialla, A.A.; Pavlidis, I.V.; Felicissimo, M.P.; Rudolf, P.; Gournis, D.; Stamatis, H. Lipase immobilization on smectite nanoclays: Characterization and application to the epoxidation of α-pinene. Bioresour. Technol. 2010, 101, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, B.I.V.; Tsoufis, T.; Enotiadis, A.; Gournis, D.; Stamatis, H. Functionalized Multi-Wall Carbon Nanotubes for Lipase Immobilization. Adv. Eng. Mater. 2010, 1, 179–183. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M.; Al-Busoul, M.; Penchev, I.; Orfali, W. Immobilized enzymes bioreactors utilizing a magnetic field: A review. Biochem. Eng. J. 2017, 121, 94–106. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M.; Al-Bosoul, M.; Penchev, I.; Al-Ahmadi, H.; Al-Qodah, K. On the performance of immobilized cell bioreactors utilizing a magnetic field. Rev. Chem. Eng. 2018, 34, 385–408. [Google Scholar] [CrossRef]
- Frost, C.G.; Mutton, L. Heterogeneous catalytic synthesis using microreactor technology. Green Chem. 2010, 12, 1687–1703. [Google Scholar] [CrossRef]
- Digigow, R.G.; Dechézelles, J.-F.; Kaufmann, J.; Vanhecke, D.; Knapp, H.; Lattuada, M.; Rothen-Rutishauser, B.; Petri-Fink, A. Magnetic microreactors for efficient and reliable magnetic nanoparticle surface functionalization. Lab Chip 2014, 14, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Shen, S.; Wu, D. Progress of recyclable magnetic particles for biomedical applications. J. Mater. Chem. B 2018, 6, 366–380. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Wagner, H.; Rapp, B.E.; Franzreb, M. Optimization of enzyme immobilization on magnetic microparticles using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) as a crosslinking agent. Anal. Methods 2015, 7, 10291–10298. [Google Scholar] [CrossRef]
- Mehta, R.V. Synthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnology. Mater. Sci. Eng. C 2017, 79, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on methods and easy separated support materials for enzymes immobilization. Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Huo, J.; Aguilera-Sigalat, J.; El-Hankari, S.; Bradshaw, D. Magnetic MOF microreactors for recyclable size-selective biocatalysis. Chem. Sci. 2015, 6, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Lu, T.; Zeng, R.; Bi, Y. Preparation and highlighted applications of magnetic microparticles and nanoparticles: A review on recent advances. Microchim. Acta 2016, 183, 2655–2675. [Google Scholar] [CrossRef]
- Koneracká, M.; Kopčanský, P.; Timko, M.; Ramchand, C.N.; Saiyed, Z.M.; Trevan, M.; de Sequeira, A. Immobilization of Enzymes on Magnetic Particles; Humana Press: New York, NY, USA, 2006; pp. 217–228. [Google Scholar]
- Hajba, L.; Guttman, A. Continuous-flow biochemical reactors: Biocatalysis, bioconversion, and bioanalytical applications utilizing immobilized microfluidic enzyme reactors. J. Flow Chem. 2016, 6, 8–12. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Patila, M.; Polydera, A.C.; Gournis, D.; Stamatis, H. Immobilization of Enzymes and other Biomolecules on Graphene. In Functionalization of Graphene; Wiley-VCH Verlag: Weinheim, Germany, 2014; pp. 139–172. ISBN 9783527672790. [Google Scholar]
- Orfanakis, G.; Patila, M.; Catzikonstantinou, A.V.; Lyra, K.; Kouloumpis, A.; Spyrou, K.; Katapodis, P. Hybrid Nanomaterials of Magnetic Iron Nanoparticles and Graphene Oxide as Matrices for the Immobilization of β-Glucosidase: Synthesis, Characterization, and Biocatalytic Properties. Front. Mater. 2018, 5. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Vakhshiteh, F.; Barkhi, M.; Baharifar, H.; Poor-Akbar, E.; Zari, N.; Stamatis, H.; Bordbar, A.K. Immobilization of cellulase enzyme onto magnetic nanoparticles: Applications and recent advances. Mol. Catal. 2017, 442, 66–73. [Google Scholar] [CrossRef]
- Myung, S.; Zhang, Y.H.P. Non-Complexed Four Cascade Enzyme Mixture: Simple Purification and Synergetic Co-stabilization. PLoS ONE 2013, 8, e61500. [Google Scholar] [CrossRef] [PubMed]
- France, S.P.; Hepworth, L.J.; Turner, N.J.; Flitsch, S.L. Constructing Biocatalytic Cascades: In Vitro and in Vivo Approaches to de Novo Multi-Enzyme Pathways. ACS Catal. 2017, 7, 710–724. [Google Scholar] [CrossRef]
- Schmidt-Dannert, C.; Lopez-Gallego, F. A roadmap for biocatalysis–functional and spatial orchestration of enzyme cascades. Microb. Biotechnol. 2016, 9, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; De Oliveira, D. Nanomaterials for biocatalyst immobilization-state of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Lungu, M.; Neculae, A.; Bunoiu, M.; Biris, C. Nanoparticles’ promises and risks: Characterization, manipulation, and potential hazards to humanity and the environment. In Nanoparticles’ Promises and Risks; Springer International Publishing: Basel, Switzerland, 2015; ISBN 9783319117287. [Google Scholar]
- Ema, T.; Miyazaki, Y.; Kozuki, I.; Sakai, T.; Hashimoto, H.; Takada, J. Highly active lipase immobilized on biogenous iron oxide via an organic bridging group: The dramatic effect of the immobilization support on enzymatic function. Green Chem. 2011, 13, 3187–3195. [Google Scholar] [CrossRef]
- Hashimoto, H.; Kobayashi, G.; Sakuma, R.; Fujii, T.; Hayashi, N.; Suzuki, T.; Kanno, R.; Takano, M.; Takada, J. Bacterial nanometric amorphous Fe-based oxide: A potential lithium-ion battery anode material. ACS Appl. Mater. Interfaces 2014, 6, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Miyazaki, Y.; Murakami, A.; Sakamoto, N.; Ema, T.; Hashimoto, H.; Furutani, M.; Nakanishi, M.; Fujii, T.; Takada, J. Chemical modification of biogenous iron oxide to create an excellent enzyme scaffold. Org. Biomol. Chem. 2010, 8, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Mandai, K.; Fukuda, T.; Miyazaki, Y.; Hashimoto, H.; Mandai, H.; Ema, T.; Takada, J.; Suga, S. Magnetic Attachment of Lipase Immobilized on Bacteriogenic Iron Oxide Inside a Microtube Reactor for the Kinetic Resolution of Secondary Alcohols. Synlett 2017, 28, 805–810. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, W.; Chen, Y.; Guo, L.; Yang, L. A replaceable dual-enzyme capillary microreactor using magnetic beads and its application for simultaneous detection of acetaldehyde and pyruvate. Electrophoresis 2012, 33, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, Z.; Zhao, L.; Wang, X.; Yu, P.; Su, L.; Mao, L. A non-oxidative electrochemical approach to online measurements of dopamine release through laccase-catalyzed oxidation and intramolecular cyclization of dopamine. Biosens. Bioelectron. 2010, 25, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Ramana, P.; Schejbal, J.; Houthoofd, K.; Martens, J.; Adams, E.; Augustijns, P.; Glatz, Z.; Van Schepdael, A. An improved design to capture magnetic microparticles for capillary electrophoresis based immobilized microenzyme reactors. Electrophoresis 2018, 39, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.P.; Wang, X.N.; Liu, C.M.; Meng, X.Y.; Qiu, J.D. Construction of graphene oxide magnetic nanocomposites-based on-chip enzymatic microreactor for ultrasensitive pesticide detection. J. Chromatogr. A 2013, 1315, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Šalić, A.; Pindrić, K.; Hojnik Podrepšek, G.; Novosel, N.; Leitgeb, M.; Zelić, B. NADH oxidation in a microreactor with an oscillating magnetic field. J. Flow Chem. 2016, 6, 27–32. [Google Scholar] [CrossRef]
- Bataille, J.; Viodé, A.; Pereiro, I.; Lafleur, J.P.; Varenne, F.; Descroix, S.; Becher, F.; Kutter, J.P.; Roesch, C.; Poüs, C.; et al. On-a-chip tryptic digestion of transthyretin: A step toward an integrated microfluidic system for the follow-up of familial transthyretin amyloidosis. Analyst 2018, 143, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Schejbal, J.; Řemínek, R.; Zeman, L.; Mádr, A.; Glatz, Z. On-line coupling of immobilized cytochrome P450 microreactor and capillary electrophoresis: A promising tool for drug development. J. Chromatogr. A 2016, 1437, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Hübner, J.; Brenner-weiß, G. Compartmented microfluidic bioreactor system using magnetic enzyme immobilisates for fast small-scale biotransformation studies. Eng. Life Sci. 2015, 15, 721–726. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Qi, D.; Deng, C.; Yang, P.; Zhang, X. On-chip enzymatic microreactor using trypsin-immobilized superparamagnetic nanoparticles for highly efficient proteolysis. J. Chromatogr. A 2007, 1176, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Peschke, T.; Skoupi, M.; Burgahn, T.; Gallus, S.; Ahmed, I.; Rabe, K.S.; Niemeyer, C.M. Self-Immobilizing Fusion Enzymes for Compartmentalized Biocatalysis. ACS Catal. 2017, 7, 7866–7872. [Google Scholar] [CrossRef]
- Jussen, D.; Soltner, H.; Stute, B.; Wiechert, W.; von Lieres, E.; Pohl, M. μMORE: A microfluidic magnetic oscillation reactor for accelerated parameter optimization in biocatalysis. J. Biotechnol. 2016, 231, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Zhang, L.; Lei, J.; Ju, H. Fabrication of tunable microreactor with enzyme modified magnetic nanoparticles for microfluidic electrochemical detection of glucose. Anal. Chim. Acta 2012, 709, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Weiser, D.; Bencze, L.C.; Bánõczi, G.; Ender, F.; Kiss, R.; Kõkai, E.; Szilágyi, A.; Vértessy, B.G.; Farkas, Ö.; Paizs, C.; et al. Phenylalanine Ammonia-Lyase-Catalyzed Deamination of an Acyclic Amino Acid: Enzyme Mechanistic Studies Aided by a Novel Microreactor Filled with Magnetic Nanoparticles. ChemBioChem 2015, 16, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yu, P.; Mao, L. A multi-enzyme microreactor-based online electrochemical system for selective and continuous monitoring of acetylcholine. Analyst 2015, 140, 3781–3787. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]

| Entry | Type of Reactor | Immobilization Support | Enzyme | Application | Ref. |
|---|---|---|---|---|---|
| 1 | fused-silica capillary microreactor | superparamagnetic beads | alcohol dehydrogenase (ADH)lactate dehydrogenase (LDH) | determination of acetaldeyde and pyruvate content | [43] |
| 2 | fused-silica capillary microreactor | magnetite nanoparticles | laccase from Trametes versicolor | online recording dopamine (DA) release in the rat brain | [44] |
| 3 | fused-silica capillary microreactor | superparamagnetic silica microparticles | human flavin-containing monooxygenase 3 (hFMO3) | drug metabolism | [45] |
| 4 | cross-type PDMS microchip | GO/Fe3O4 magnetic nanocomposites | acetylcholinesterase (AChe) | determination of organophosphorus pesticides | [46] |
| 5 | glass tubular microreactor | maghemite (γ-Fe2O3) nanoparticles | alcohol dehydrogenase (ADH) | NADH oxidation | [47] |
| 6 | micro-fluidized PDMS chip | carboxyl-functionalized magnetic beads | trypsin from bovine pancreas | tryptic digestion of transthyretin | [48] |
| 7 | fused-silica cpillary microreactor | magnetic SiMAG-carboxyl microparticles | cytochrome P450 2C9 | on-line kinetic and inhibition studies of clinically and pharmacologically important CYP2C9 | [49] |
| 8 | fluorinated ethylene propylene capillary microreactor | polyvinyl alcohol–magnetite composite microparticles | horseradish peroxidase (HRP) Type VI | kinetic and recycling studies of immobilized HRP | [50] |
| 9 | fused-silica microchip | superparamagnetic nanoparticles | trypsin from bovine pancreas | protein digestion | [51] |
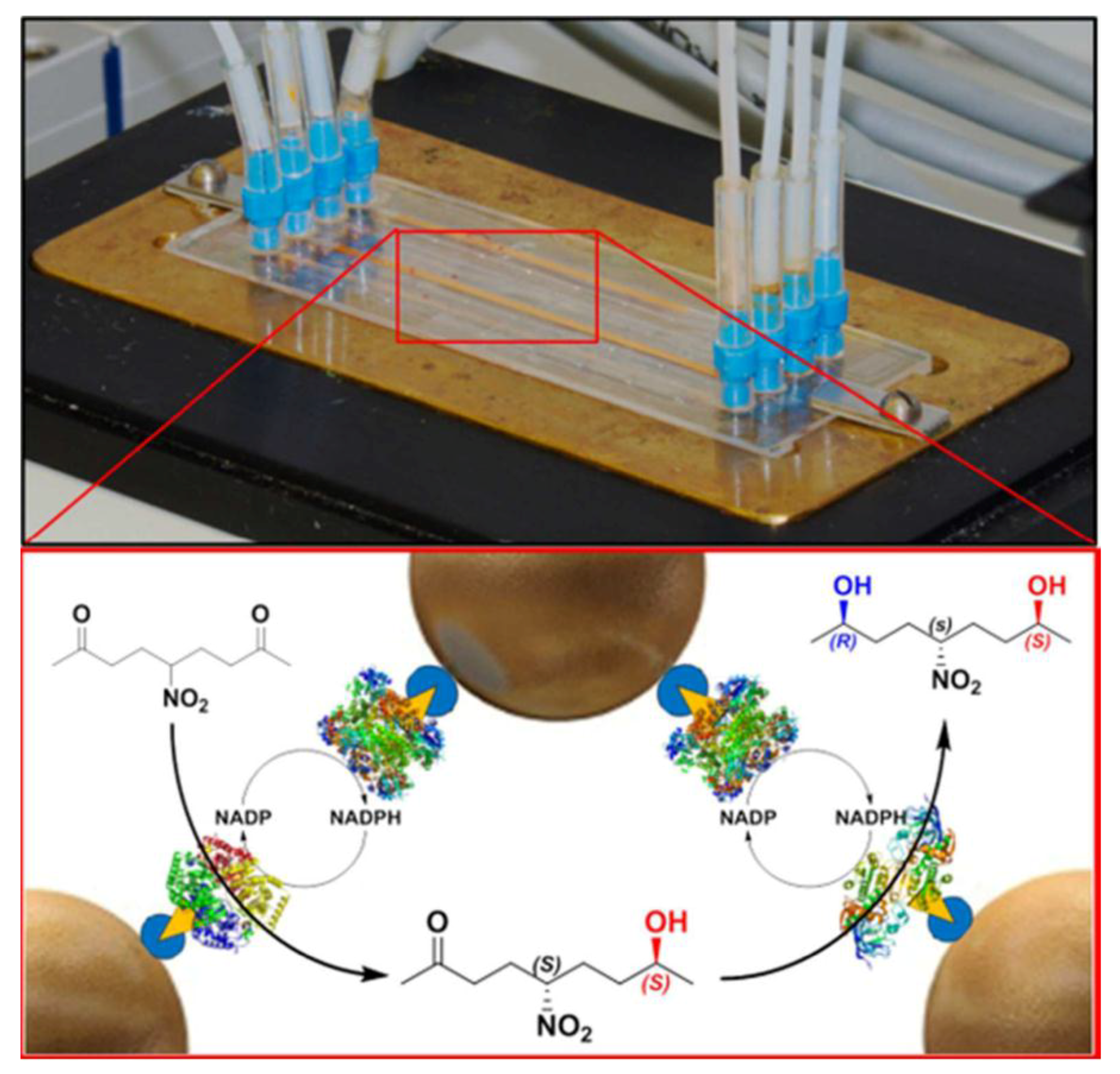
| 10 | four-channel PMMA chip | STV-functionalized superparagnetic microbeads | (R)-selective alcohol dehydrogenase (LbADH) (S)-selective methylglyoxal reductase (Gre2p) glucose 1-dehydrogenase (GDH) | stereoselective multi-step reactions | [52] |
| 11 | magnetic oscillation microfluidic chip | magnetic beads | benzoylformate decarboxylase from Pseudomonas putida (BFD) | stereoselective biocatalytic synthesis of chiral 2-hydroxy ketones | [53] |
| 12 | Fused-silica capillary microreacror | hydroxyl group modified superparamagnetic nanospheres | Glucose oxidase (GOx) from Aspergillus niger | quantitative detection of glucose in human serum samples | [54] |
| 13 | PTFE microtube reactor | Silicate-covered bacteriogenic iron oxide nanoparticles | lipase from Burkholderia cepacia (BCL) | kinetic resolution of secondary alcohols | [42] |
| 14 | Magne-Chip with multiple magnetic cells | epoxy-magnetic nanoparticles | phenylalanine ammonia-lyase (PAL) | ammonia elimination | [55] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkantzou, E.; Patila, M.; Stamatis, H. Magnetic Microreactors with Immobilized Enzymes—From Assemblage to Contemporary Applications. Catalysts 2018, 8, 282. https://doi.org/10.3390/catal8070282
Gkantzou E, Patila M, Stamatis H. Magnetic Microreactors with Immobilized Enzymes—From Assemblage to Contemporary Applications. Catalysts. 2018; 8(7):282. https://doi.org/10.3390/catal8070282
Chicago/Turabian StyleGkantzou, Elena, Michaela Patila, and Haralambos Stamatis. 2018. "Magnetic Microreactors with Immobilized Enzymes—From Assemblage to Contemporary Applications" Catalysts 8, no. 7: 282. https://doi.org/10.3390/catal8070282
APA StyleGkantzou, E., Patila, M., & Stamatis, H. (2018). Magnetic Microreactors with Immobilized Enzymes—From Assemblage to Contemporary Applications. Catalysts, 8(7), 282. https://doi.org/10.3390/catal8070282



_Stamatis.png)