Geocatalytic Uptake of Ozone onto Natural Mineral Dust
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Section
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Chameides, W.; Fehsenfeld, F.; Rodgers, M.; Cardelino, C.; Martinez, J.; Parrish, D.; Lonneman, W.; Lawson, D.; Rasmussen, R.; Zimmerman, P. Ozone precursor relationships in the ambient atmosphere. J. Geophys. Res. Atmos. 1992, 97, 6037–6055. [Google Scholar] [CrossRef]
- Vingarzan, R. A review of surface ozone background levels and trends. Atmos. Environ. 2004, 38, 3431–3442. [Google Scholar] [CrossRef]
- Bocci, V. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Br. J. Biomed. Sci. 1999, 56, 9–270. [Google Scholar]
- Baysan, A.; Lynch, E. The use of ozone in dentistry and medicine. Prim. Dent. Care 2005, 12, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Thevenet, F.; Sivachandiran, L.; Guaitella, O.; Barakat, C.; Rousseau, A. Plasma-catalyst coupling for volatile organic compound removal and indoor air treatment: A review. J. Phys. D Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Roland, U.; Holzer, F.; Kopinke, F.D. Combination of non-thermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds: Part 2. Ozone decomposition and deactivation of γ-Al2O3. Appl. Catal. B Environ. 2005, 58, 217–226. [Google Scholar] [CrossRef]
- Mehandjiev, D.; Naidenov, A. Ozone decomposition on α-Fe2O3 catalyst. J. Int. Ozone Assoc. 1992, 14, 277–282. [Google Scholar]
- Klimovskii, A.; Bavin, A.; Tkalich, V.; Lisachenko, A. Interaction of ozone with γ-Al2O3 surface. React. Kinet. Catal. Lett. 1983, 23, 95–98. [Google Scholar] [CrossRef]
- Bulanin, K.; Lavalley, J.; Tsyganenko, A. Ir spectra of adsorbed ozone. Colloids Surf. A Physicochem. Eng. Asp. 1995, 101, 153–158. [Google Scholar] [CrossRef]
- Thomas, K.; Hoggan, P.; Mariey, L.; Lamotte, J.; Lavalley, J. Experimental and theoretical study of ozone adsorption on alumina. Catal. Lett. 1997, 46, 77–82. [Google Scholar] [CrossRef]
- Sullivan, R.; Thornberry, T.; Abbatt, J. Ozone decomposition kinetics on alumina: Effects of ozone partial pressure, relative humidity and repeated oxidation cycles. Atmos. Chem. Phys. 2004, 4, 1301–1310. [Google Scholar] [CrossRef]
- Dhandapani, B.; Oyama, S.T. Kinetics and mechanism of ozone decomposition on a manganese oxide catalyst. Chem. Lett. 1995, 24, 413–414. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Calderbank, P.; Lewis, J. Ozone-decomposition catalysis. Chem. Eng. Sci. 1976, 31, 1216. [Google Scholar] [CrossRef]
- Alebić-Juretić, A.; Cvitaš, T.; Klasinc, L. Ozone destruction on powders. Ber. Bunsenges. Phys. Chem. 1992, 96, 493–495. [Google Scholar] [CrossRef]
- Bulanin, K.; Alexeev, A.; Bystrov, D.; Lavalley, J.; Tsyganenko, A. IR study of ozone adsorption on SiO2. J. Phys. Chem. 1994, 98, 5100–5103. [Google Scholar] [CrossRef]
- Bulanin, K.; Lavalley, J.; Tsyganenko, A. Infrared study of ozone adsorption on CaO. J. Phys. Chem. B 1997, 101, 2917–2922. [Google Scholar] [CrossRef]
- Bulanin, K.; Lavalley, J.; Tsyganenko, A. Infrared study of ozone adsorption on TiO2 (anatase). J. Phys. Chem. 1995, 99, 10294–10298. [Google Scholar] [CrossRef]
- Berlier, G.; Yamamoto, T.; Spoto, G.; Lamberti, C.; Gribov, E.; Zecchina, A. IR spectra of ozone adsorbed on MgO. Phys. Chem. Chem. Phys. 2002, 4, 3872–3875. [Google Scholar] [CrossRef]
- Pangilinan, C.D.C.; Kurniawan, W.; Salim, C.; Hinode, H. Effect of Ag/TiO2 catalyst preparation on gas-phase benzene decomposition using non-thermal plasma driven catalysis under oxygen plasma. React. Kinet. Mech. Catal. 2016, 117, 103–118. [Google Scholar] [CrossRef]
- Perego, C.; Villa, P. Catalyst preparation methods. Catal. Today 1997, 34, 281–305. [Google Scholar] [CrossRef]
- Winterton, N. Chemistry for Sustainable Technologies: A Foundation; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Tang, M.; Cziczo, D.J.; Grassian, V.H. Interactions of water with mineral dust aerosol: Water adsorption, hygroscopicity, cloud condensation, and ice nucleation. Chem. Rev. 2016, 116, 4205–4259. [Google Scholar] [CrossRef] [PubMed]
- Michel, A.; Usher, C.; Grassian, V. Reactive uptake of ozone on mineral oxides and mineral dusts. Atmos. Environ. 2003, 37, 3201–3211. [Google Scholar] [CrossRef]
- Usher, C.; Michel, A.; Stec, D.; Grassian, V. Laboratory studies of ozone uptake on processed mineral dust. Atmos. Environ. 2003, 37, 5337–5347. [Google Scholar] [CrossRef]
- Hanisch, F.; Crowley, J. Ozone decomposition on saharan dust: An experimental investigation. Atmos. Chem. Phys. 2003, 3, 119–130. [Google Scholar] [CrossRef]
- Schoonen, M.A.; Xu, Y.; Strongin, D.R. An introduction to geocatalysis. J. Geochem. Explor. 1998, 62, 201–215. [Google Scholar] [CrossRef]
- Romanias, M.N.; Ourrad, H.; Thevenet, F.; Riffault, V. Investigating the heterogeneous interaction of VOCs with natural atmospheric particles: Adsorption of limonene and toluene on saharan mineral dusts. J. Phys. Chem. A 2016, 120, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Goudie, A.; Middleton, N. Saharan dust storms: Nature and consequences. Earth Sci. Rev. 2001, 56, 179–204. [Google Scholar] [CrossRef]
- Carlos-Cuellar, S.; Li, P.; Christensen, A.; Krueger, B.; Burrichter, C.; Grassian, V. Heterogeneous uptake kinetics of volatile organic compounds on oxide surfaces using a knudsen cell reactor: Adsorption of acetic acid, formaldehyde, and methanol on α-Fe2O3, α-Al2O3, and SiO2. J. Phys. Chem. A 2003, 107, 4250–4261. [Google Scholar] [CrossRef]
- Crowley, J.; Ammann, M.; Cox, R.; Hynes, R.; Jenkin, M.E.; Mellouki, A.; Rossi, M.; Troe, J.; Wallington, T. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume v–heterogeneous reactions on solid substrates. Atmos. Chem. Phys. 2010, 10, 9059–9223. [Google Scholar] [CrossRef]
- Kolb, C.; Cox, R.; Abbatt, J.; Ammann, M.; Davis, E.; Donaldson, D.; Garrett, B.C.; George, C.; Griffiths, P.; Hanson, D. An overview of current issues in the uptake of atmospheric trace gases by aerosols and clouds. Atmos. Chem. Phys. 2010, 10, 10561–10605. [Google Scholar] [CrossRef]
- Tang, M.; Huang, X.; Lu, K.; Ge, M.; Li, Y.; Cheng, P.; Zhu, T.; Ding, A.; Zhang, Y.; Gligorovski, S. Heterogeneous reactions of mineral dust aerosol: Implications for tropospheric oxidation capacity. Atmos. Chem. Phys. 2017, 17, 11727–11777. [Google Scholar] [CrossRef]
- Stephens, S.; Rossi, M.J.; Golden, D.M. The heterogeneous reaction of ozone on carbonaceous surfaces. Int. J. Chem. Kinet. 1986, 18, 1133–1149. [Google Scholar] [CrossRef]
- Sheehy, D.P. A perspective on desertification of grazingland ecosystems in North China. Ambio 1992, 21, 303–307. [Google Scholar]
- Romanias, M.N.; Zeineddine, M.N.; Gaudion, V.; Lun, X.; Thevenet, F.; Riffault, V. Heterogeneous interaction of isopropanol with natural gobi dust. Environ. Sci. Technol. 2016, 50, 11714–11722. [Google Scholar] [CrossRef] [PubMed]
- Zeineddine, M.N.; Romanias, M.N.; Gaudion, V.; Riffault, V.; Thévenet, F. Heterogeneous interaction of isoprene with natural gobi dust. ACS Earth Space Chem. 2017, 1, 236–243. [Google Scholar] [CrossRef]
- Romanias, M.N.; Zeineddine, M.N.; Riffault, V.; Thevenet, F. Isoprene heterogeneous uptake and reactivity on TiO2: A kinetic and product study. Int. J. Chem. Kinet. 2017, 49, 773–788. [Google Scholar] [CrossRef]
- Thevenet, F.; Olivier, L.; Batault, F.; Sivachandiran, L.; Locoge, N. Acetaldehyde adsorption on TiO2: Influence of NO2 preliminary adsorption. Chem. Eng. J. 2015, 281, 126–133. [Google Scholar] [CrossRef]
- Batault, F.; Thevenet, F.; Hequet, V.; Rillard, C.; Le Coq, L.; Locoge, N. Acetaldehyde and acetic acid adsorption on TiO2 under dry and humid conditions. Chem. Eng. J. 2015, 264, 197–210. [Google Scholar] [CrossRef]
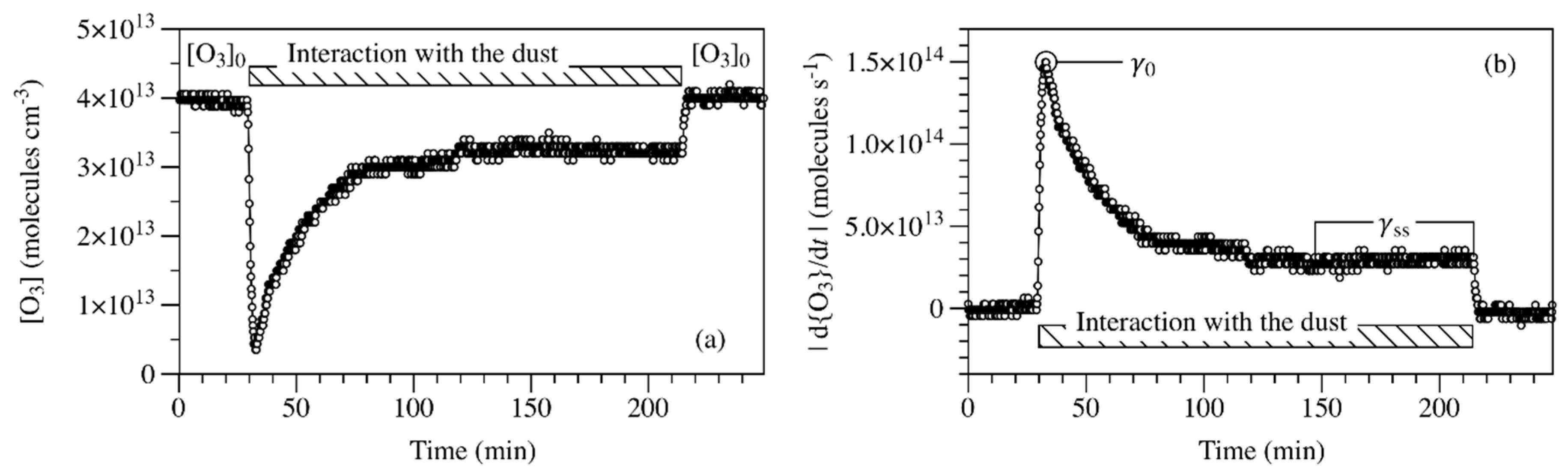
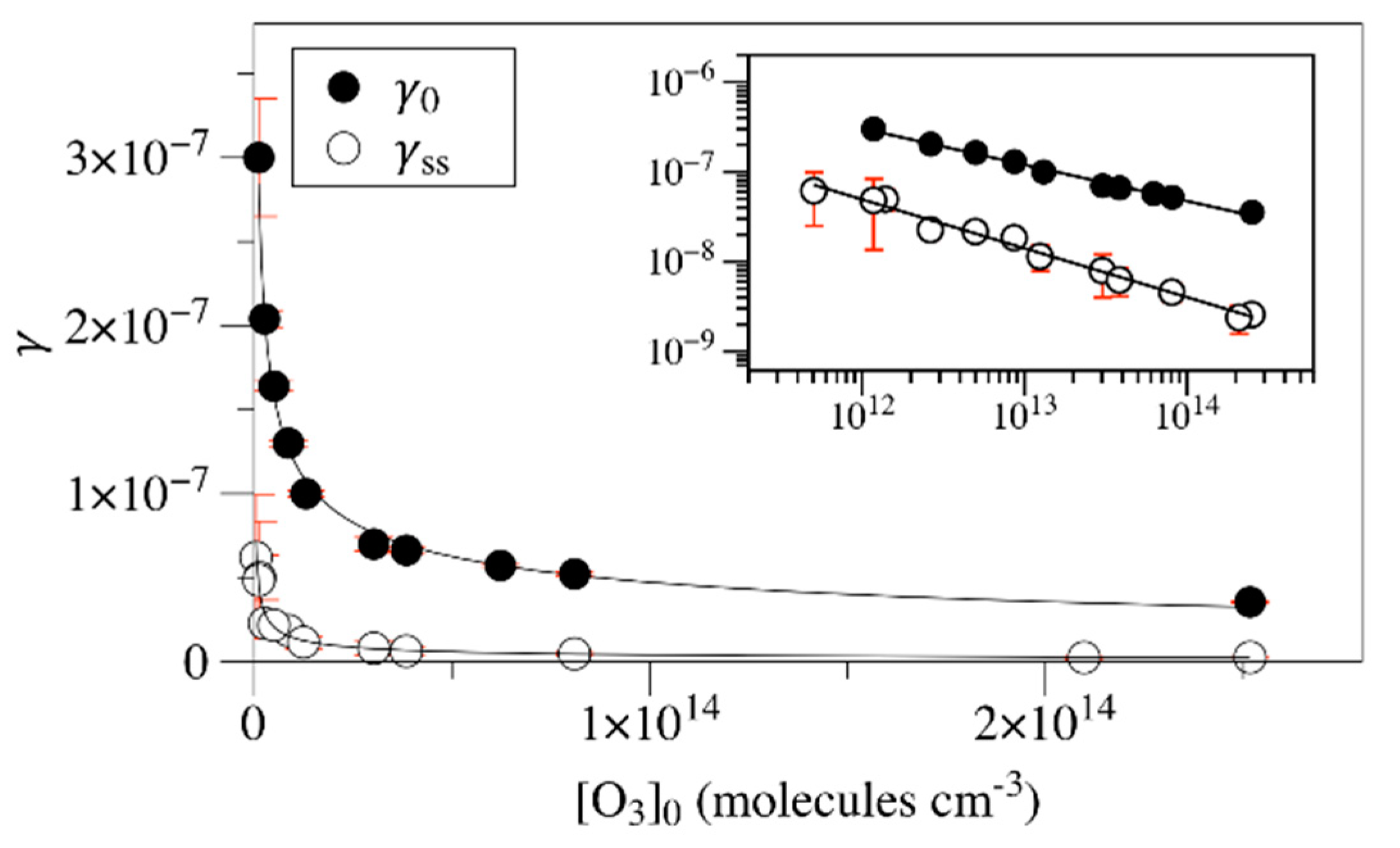
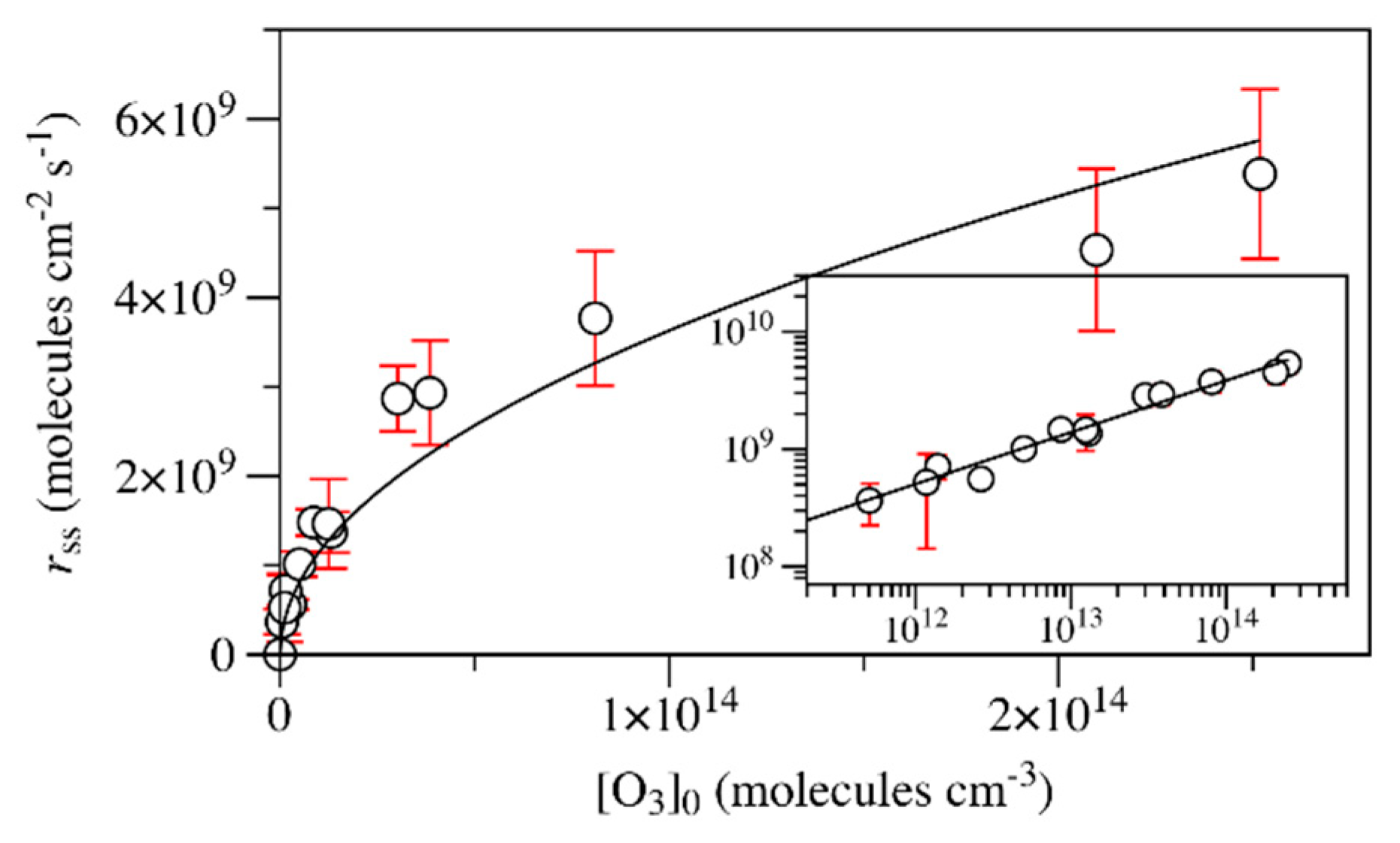
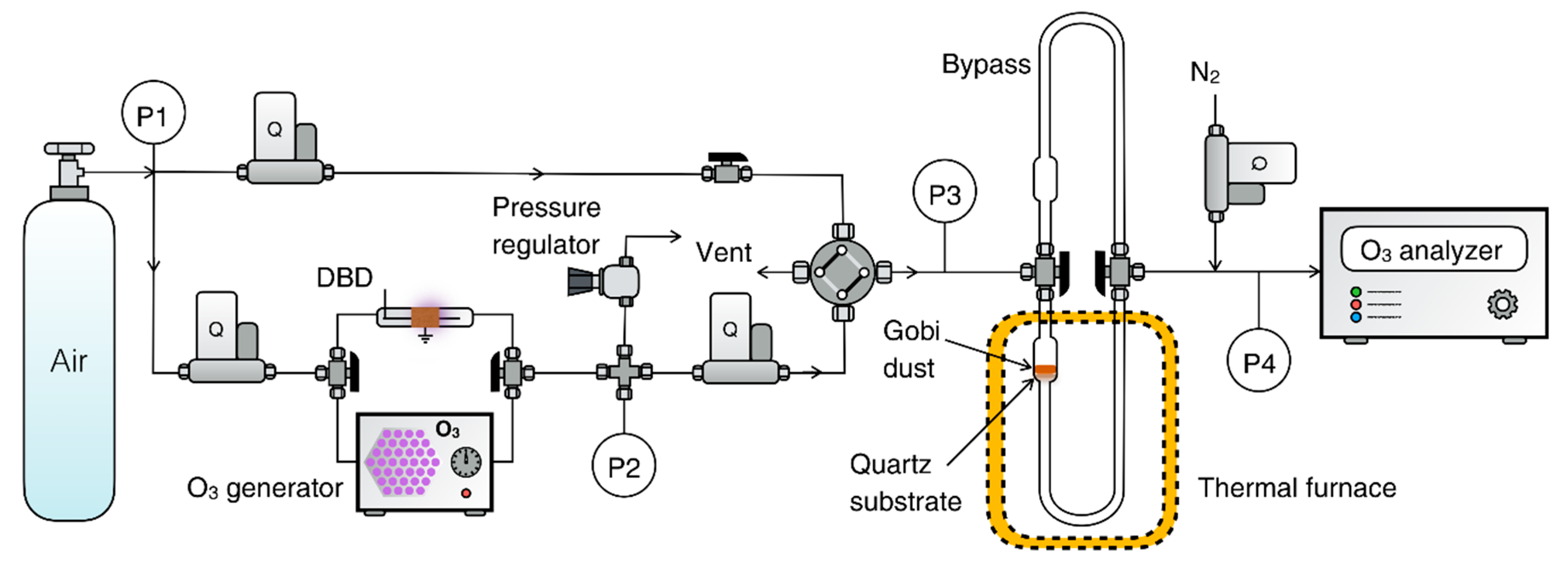
| Materials | γss | rss (Molecules cm−2 s−1) | Ref. |
|---|---|---|---|
| SiO2 | 3.74 × 10−10 | 7.82 × 106 | [15] |
| Al2O3 | 3.79 × 10−9 | 8.53 × 107 | [15] |
| CaCO3 | <<10−10 | <<2.40 × 106 | [15] |
| Saharan dust | 0 | 0 | [15] |
| Gobi dust | 2.28 × 10−8 | 5.60 × 108 | This work |
| Materials | γss | rss (Molecules cm−2 s−1) | Ref. |
|---|---|---|---|
| α-MnO2 | >2.5 × 10−8 | >7.9 × 1010 | [13] |
| β-MnO2 | 8.1 × 10−8 | 2.6 × 1011 | [13] |
| γ-MnO2 | 2.6 × 10−8 | 8.2 × 1010 | [13] |
| Gobi dust | 2.6 × 10−9 | 5.8 × 109 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Romanias, M.N.; Thévenet, F.; Rousseau, A. Geocatalytic Uptake of Ozone onto Natural Mineral Dust. Catalysts 2018, 8, 263. https://doi.org/10.3390/catal8070263
Wang X, Romanias MN, Thévenet F, Rousseau A. Geocatalytic Uptake of Ozone onto Natural Mineral Dust. Catalysts. 2018; 8(7):263. https://doi.org/10.3390/catal8070263
Chicago/Turabian StyleWang, Xianjie, Manolis N. Romanias, Frédéric Thévenet, and Antoine Rousseau. 2018. "Geocatalytic Uptake of Ozone onto Natural Mineral Dust" Catalysts 8, no. 7: 263. https://doi.org/10.3390/catal8070263
APA StyleWang, X., Romanias, M. N., Thévenet, F., & Rousseau, A. (2018). Geocatalytic Uptake of Ozone onto Natural Mineral Dust. Catalysts, 8(7), 263. https://doi.org/10.3390/catal8070263





