Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology and Formation of TiO2/CuxOy NTs
2.2. XRD Analysis
2.3. XPS Analysis
2.4. UV-Vis Spectra and Photoluminescence Properties
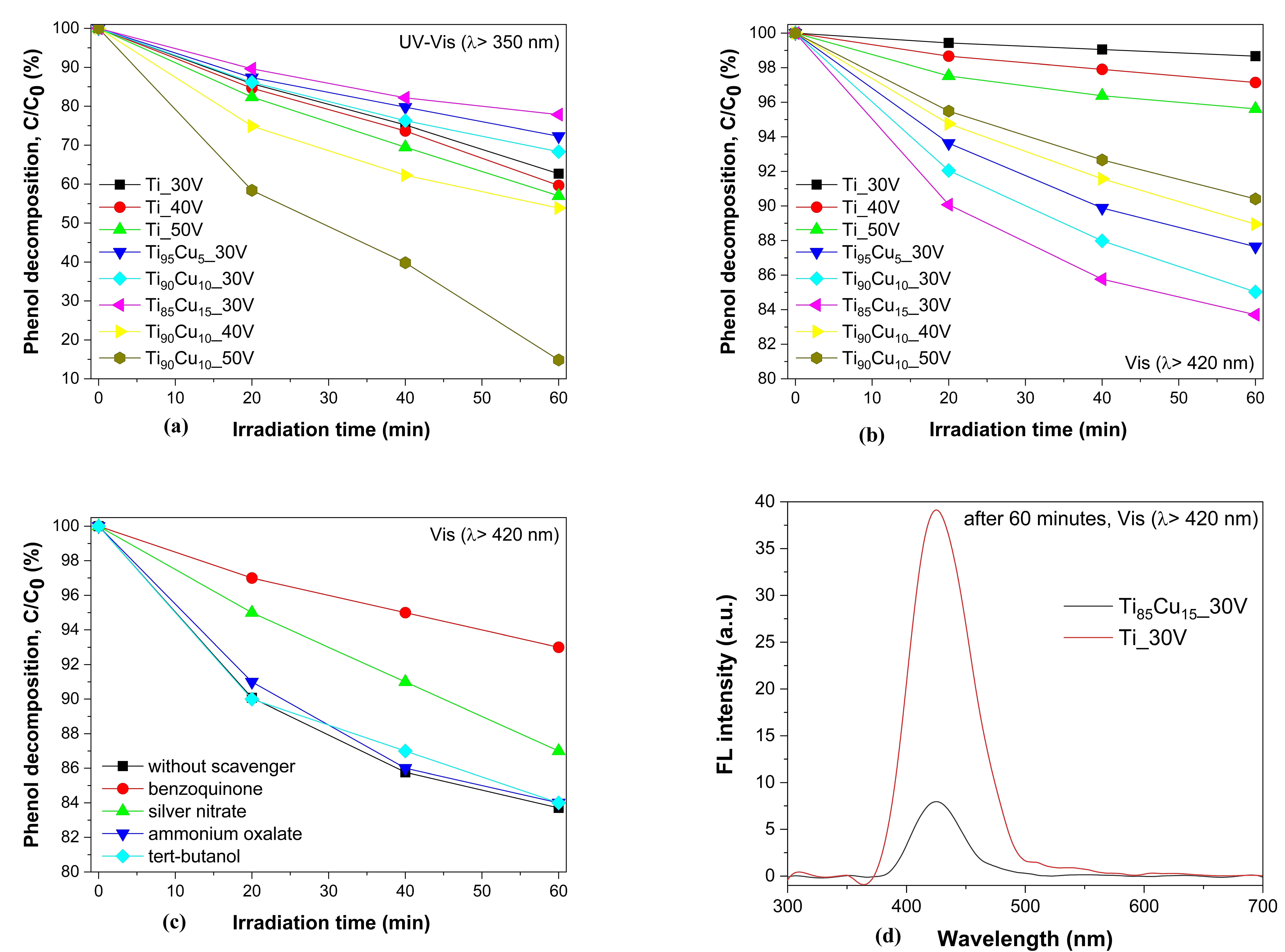
2.5. Photodegradation Ability in Aqueous Phase
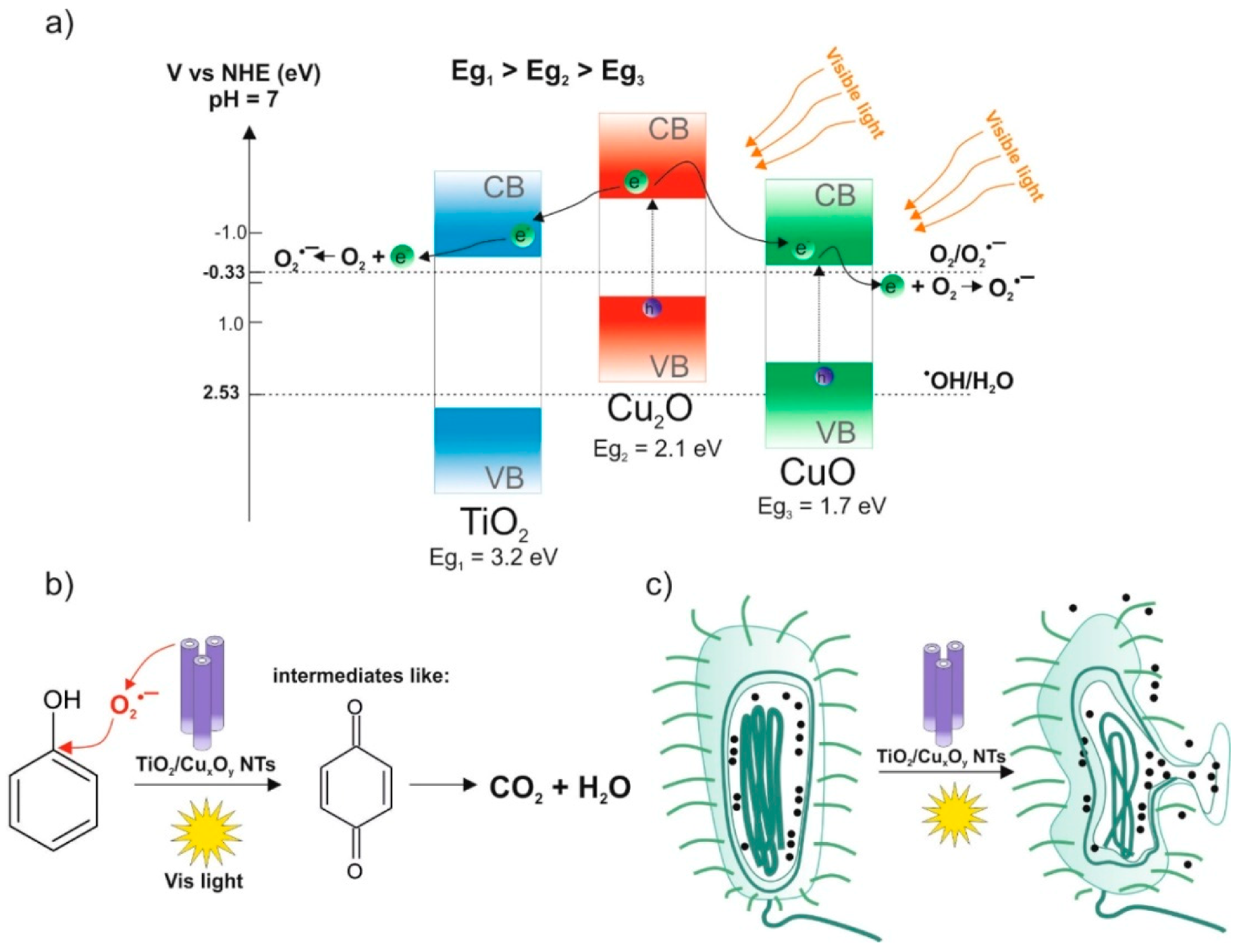
2.6. The Excitation Mechanism of TiO2-CuxOy NTs
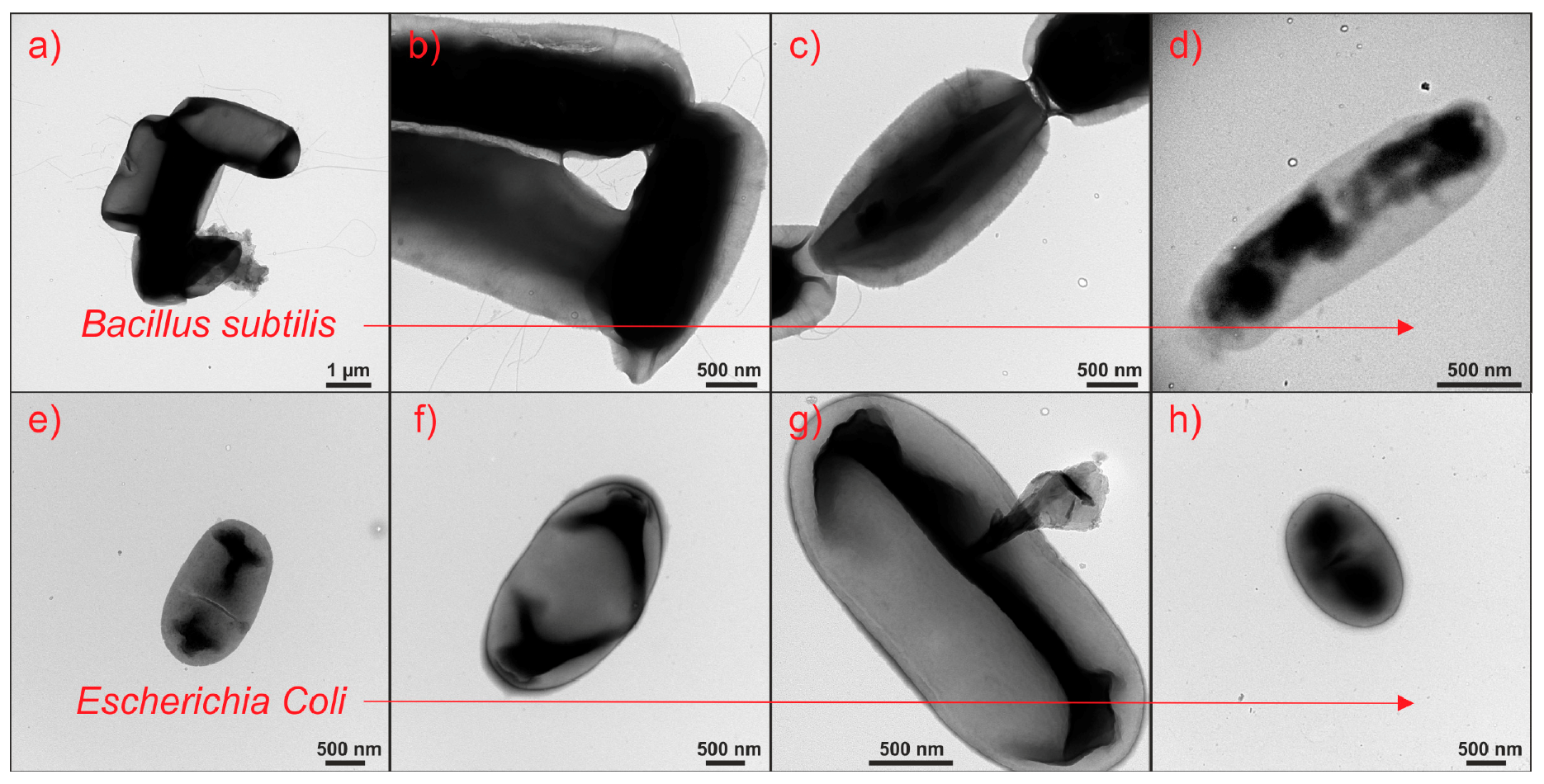
2.7. Assessment of Antibacterial Properties of TiO2/CuxOy NTs
2.8. Suggested Mechanism of Bacteria Inactivation
3. Materials and Methods
3.1. Materials
3.2. Preparation of NTs
3.3. Characterization Systems
3.4. Photocatalytic Activity
3.4.1. Phenol Degradation Process
3.4.2. Microorganisms Inactivation Process
3.4.3. Measurement of Copper Cu2+ Influence on Growth of Bacterial Cultures
3.4.4. Measurement of Hydroxyl Radicals
3.4.5. Investigation of Photodegradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2 : A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Peng, F.; Zhang, H.; Liu, H.; Zhao, H. Electrodeposition of polyhedral Cu2O on TiO2 nanotube arrays for enhancing visible light photocatalytic performance. Electrochem. Commun. 2011, 13, 861–864. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.; Kobylański, M.; Mazierski, P.; Wółkiewicz, J.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.; Zaleska-Medynska, A. Self-Organized TiO2–MnO2 Nanotube Arrays for Efficient Photocatalytic Degradation of Toluene. Molecules 2017, 22, 564. [Google Scholar] [CrossRef]
- Daraio, C.; Jin, S. Synthesis and Patterning Methods for Nanostructures Useful for Biological Applications. In Nanotechnology for Biology and Medicine; Silva, G.A., Parpura, V., Eds.; Springer: New York, NY, USA, 2012; pp. 27–44. ISBN 978-0-387-31282-8. [Google Scholar]
- Nevárez-Martínez, M.; Mazierski, P.; Kobylański, M.; Szczepańska, G.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.; Zaleska-Medynska, A. Growth, Structure, and Photocatalytic Properties of Hierarchical V2O5–TiO2 Nanotube Arrays Obtained from the One-step Anodic Oxidation of Ti–V Alloys. Molecules 2017, 22, 580. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface Modifications of Titanium Materials for developing Corrosion Behavior in Human Body Environment: A Review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef]
- Jung, M.; Scott, J.; Ng, Y.H.; Jiang, Y.; Amal, R. CuO x dispersion and reducibility on TiO2 and its impact on photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 12499–12506. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Macak, J.M.; Schmuki, P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim. Acta 2006, 52, 1258–1264. [Google Scholar] [CrossRef]
- Xie, Z.B.; Blackwood, D.J. Effects of anodization parameters on the formation of titania nanotubes in ethylene glycol. Electrochim. Acta 2010, 56, 905–912. [Google Scholar] [CrossRef]
- Erjavec, B.; Tišler, T.; Tchernychova, E.; Plahuta, M.; Pintar, A. Self-Doped Cu-Deposited Titania Nanotubes as Efficient Visible Light Photocatalyst. Catal. Lett. 2017, 147, 1686–1695. [Google Scholar] [CrossRef]
- Hai, Z.; EL Kolli, N.; Chen, J.; Remita, H. Radiolytic synthesis of Au–Cu bimetallic nanoparticles supported on TiO2: Application in photocatalysis. New J. Chem. 2014, 38, 5279–5286. [Google Scholar] [CrossRef]
- Lalitha, K.; Sadanandam, G.; Kumari, V.D.; Subrahmanyam, M.; Sreedhar, B.; Hebalkar, N.Y. Highly Stabilized and Finely Dispersed CuO/TiO: A Promising Visible Sensitive Photocatalyst for Continuous Production of Hydrogen from Glycerol:Water Mixtures. J. Phys. Chem. C 2010, 114, 22181–22189. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, Y.; Yuan, X.; Huo, M.; Zhao, Y.; Lu, Y.; Qiu, Y. Incorporation of Cu2O nanocrystals into TiO2 photonic crystal for enhanced UV–visible light driven photocatalysis. J. Alloys Compd. 2015, 644, 734–741. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; Muñoz-Bonilla, A., Cerrada, M., Fernández-García, M., Eds.; Polymer Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 978-1-84973-807-1. [Google Scholar]
- Chen, L.; Luo, T.; Yang, S.; Xu, J.; Liu, Z.; Wu, F. Efficient metoprolol degradation by heterogeneous copper ferrite/sulfite reaction. Environ. Chem. Lett. 2018, 16, 599–603. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Kaczmarek, D.; Poniedziałek, A.; Osękowska, M. An impact of the copper additive on photocatalytic and bactericidal properties of TiO2 thin films. Mater. Sci. Pol. 2017, 35. [Google Scholar] [CrossRef]
- Reilche, H.; Dunn, W.W.; Bard, J.A. Allen Heterogeneous photocatalytic and photosynthetic deposition of copper on TiO2 and WO3 powders. J. Phys. Chem. 1979, 83, 2248–2251. [Google Scholar]
- Moniz, S.J.A.; Tang, J. Charge Transfer and Photocatalytic Activity in CuO/TiO2 Nanoparticle Heterojunctions Synthesised through a Rapid, One-Pot, Microwave Solvothermal Route. ChemCatChem 2015, 7, 1659–1667. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, J.; Li, G.; Chen, J.; Zhang, Z.; Deng, Z.; Jiang, Y.; Guo, W.; Cao, Y. Effective water splitting using CuO x/TiO2 composite films: Role of Cu species and content in hydrogen generation. Appl. Surf. Sci. 2016, 369, 201–206. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, S.J.; Weng, L.Q.; Liu, Y.; Liu, B. Growth, structure and photocatalytic properties of hierarchical Cu–Ti–O nanotube arrays by anodization. J. Alloys Compd. 2010, 501, 333–338. [Google Scholar] [CrossRef]
- Gao, L.; Qiu, Z.; Gan, W.; Zhan, X.; Li, J.; Qiang, T. Negative Oxygen Ions Production by Superamphiphobic and Antibacterial TiO2/Cu2O Composite Film Anchored on Wooden Substrates. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, S.; Chen, R.; Zhu, X.; Liao, Q.; Huang, Y. Copper-decorated TiO2 nanorod thin films in optofluidic planar reactors for efficient photocatalytic reduction of CO2. Int. J. Hydrogen Energy 2017, 42, 9722–9732. [Google Scholar] [CrossRef]
- He, X.; Zhang, G.; Wang, X.; Hang, R.; Huang, X.; Qin, L.; Tang, B.; Zhang, X. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017, 43, 16185–16195. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A Chem. 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Wei, Z.; Siuzdak, K.; Kouame, N.A.; Kowalska, E.; Remita, H.; Zaleska-Medynska, A. Enhanced photocatalytic, electrochemical and photoelectrochemical properties of TiO2 nanotubes arrays modified with Cu, AgCu and Bi nanoparticles obtained via radiolytic reduction. Appl. Surf. Sci. 2016, 387, 89–102. [Google Scholar] [CrossRef]
- Zong, M.; Bai, L.; Liu, Y.; Wang, X.; Zhang, X.; Huang, X.; Hang, R.; Tang, B. Antibacterial ability and angiogenic activity of Cu-Ti-O nanotube arrays. Mater. Sci. Eng. C 2017, 71, 93–99. [Google Scholar] [CrossRef]
- Kim, D.; Fujimoto, S.; Schmuki, P.; Tsuchiya, H. Nitrogen doped anodic TiO2 nanotubes grown from nitrogen-containing Ti alloys. Electrochem. Commun. 2008, 10, 910–913. [Google Scholar] [CrossRef]
- He, J.-B.; Lu, D.-Y.; Jin, G.-P. Potential dependence of cuprous/cupric duplex film growth on copper electrode in alkaline media. Appl. Surf. Sci. 2006, 253, 689–697. [Google Scholar] [CrossRef]
- Jiang, X.; Herricks, T.; Xia, Y. CuO Nanowires Can Be Synthesized by Heating Copper Substrates in Air. Nano Lett. 2002, 2, 1333–1338. [Google Scholar] [CrossRef]
- Mazierski, P.; Nadolna, J.; Lisowski, W.; Winiarski, M.J.; Gazda, M.; Nischk, M.; Klimczuk, T.; Zaleska-Medynska, A. Effect of irradiation intensity and initial pollutant concentration on gas phase photocatalytic activity of TiO2 nanotube arrays. Catal. Today 2017, 284, 19–26. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Grimes, C.A.; Mor, G.K. TiO2 Nanotube Arrays: Synthesis, Properties, and Applications; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2009; ISBN 978-1-4419-0067-8. [Google Scholar]
- Valota, A.; LeClere, D.J.; Hashimoto, T.; Skeldon, P.; Thompson, G.E.; Berger, S.; Kunze, J.; Schmuki, P. The efficiency of nanotube formation on titanium anodized under voltage and current control in fluoride/glycerol electrolyte. Nanotechnology 2008, 19, 355701. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database. 2012. Available online: https://srdata.nist.gov/xps/ (accessed on 4 June 2018).
- Suzuki, S.; Hirabayashi, K.; Mimura, K.; Okabe, T.; Isshiki, M.; Waseda, Y. Surface Layer Formed by Selective Oxidation in High-Purity Copper-Titanium Binary Alloys. Mater. Trans. 2002, 43, 2303–2308. [Google Scholar] [CrossRef]
- Sanguinetti, S.; Guzzi, M.; Gurioli, M. Accessing structural and electronic properties of semiconductor nanostructures via photoluminescence. In Characterization of Semiconductor Heterostructures and Nanostructures; Elsevier: New York, NY, USA, 2008; pp. 175–208. ISBN 978-0-444-53099-8. [Google Scholar]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Garlisi, C.; Szlachetko, J.; Aubry, C.; Fernandes, D.L.A.; Hattori, Y.; Paun, C.; Pavliuk, M.V.; Rajput, N.S.; Lewin, E.; Sá, J.; Palmisano, G. N-TiO2/Cu-TiO2 double-layer films: Impact of stacking order on photocatalytic properties. J. Catal. 2017, 353, 116–122. [Google Scholar] [CrossRef]
- Beltran-Huarac, J.; Guinel, M.J.-F.; Weiner, B.R.; Morell, G. Bifunctional Fe3O4/ZnS:Mn composite nanoparticles. Mater. Lett. 2013, 98, 108–111. [Google Scholar] [CrossRef]
- Mazierski, P.; Malankowska, A.; Kobylański, M.; Diak, M.; Kozak, M.; Winiarski, M.J.; Klimczuk, T.; Lisowski, W.; Nowaczyk, G.; Zaleska-Medynska, A. Photocatalytically Active TiO2/Ag2O Nanotube Arrays Interlaced with Silver Nanoparticles Obtained from the One-Step Anodic Oxidation of Ti–Ag Alloys. ACS Catal. 2017, 7, 2753–2764. [Google Scholar] [CrossRef]
- Park, S.-M.; Razzaq, A.; Park, Y.H.; Sorcar, S.; Park, Y.; Grimes, C.A.; In, S.-I. Hybrid CuxO–TiO2 Heterostructured Composites for Photocatalytic CO2 Reduction into Methane Using Solar Irradiation: Sunlight into Fuel. ACS Omega 2016, 1, 868–875. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P. Highly stable copper oxide composite as an effective photocathode for water splitting via a facile electrochemical synthesis strategy. J. Mater. Chem. 2012, 22, 2456–2464. [Google Scholar] [CrossRef]
- Luna, A.L.; Valenzuela, M.A.; Colbeau-Justin, C.; Vázquez, P.; Rodriguez, J.L.; Avendaño, J.R.; Alfaro, S.; Tirado, S.; Garduño, A.; De la Rosa, J.M. Photocatalytic degradation of gallic acid over CuO–TiO2 composites under UV/Vis LEDs irradiation. Appl. Catal. A Gen. 2016, 521, 140–148. [Google Scholar] [CrossRef]
- Chen, L.; Tang, M.; Chen, C.; Chen, M.; Luo, K.; Xu, J.; Zhou, D.; Wu, F. Efficient Bacterial Inactivation by Transition Metal Catalyzed Auto-Oxidation of Sulfite. Environ. Sci. Technol. 2017, 51, 12663–12671. [Google Scholar] [CrossRef]
- Stephens, C. Bacterial sporulation: A question of commitment? Curr. Biol. 1998, 8, R45–R48. [Google Scholar] [CrossRef]
- Grossman, A.D.; Losick, R. Extracellular control of spore formation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 1988, 85, 4369–4373. [Google Scholar]
- Setlow, P. Germination of Spores of Bacillus Species: What We Know and Do Not Know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef]
- Jacoby, W.A.; Maness, P.C.; Wolfrum, E.J.; Blake, D.M.; Fennell, J.A. Mineralization of Bacterial Cell Mass on a Photocatalytic Surface in Air. Environ. Sci. Technol. 1998, 32, 2650–2653. [Google Scholar] [CrossRef]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B Biol. 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Sunada, K.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Photocatalytic bactericidal effect of TiO2 thin films: Dynamic view of the active oxygen species responsible for the effect. J. Photochem. Photobiol. A Chem. 1997, 106, 51–56. [Google Scholar] [CrossRef]
- Kunicki-Goldfinger, W.; Baj, J.; Markiewicz, Z.; Kobyliński, S. Życie Bakterii; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2008; ISBN 978-83-01-14378-7. [Google Scholar]
- Bubacz, K.; Kusiak-Nejman, E.; Tryba, B.; Morawski, A.W. Investigation of OH radicals formation on the surface of TiO2/N photocatalyst at the presence of terephthalic acid solution. Estimation of optimal conditions. J. Photochem. Photobiol. A Chem. 2013, 261, 7–11. [Google Scholar] [CrossRef]
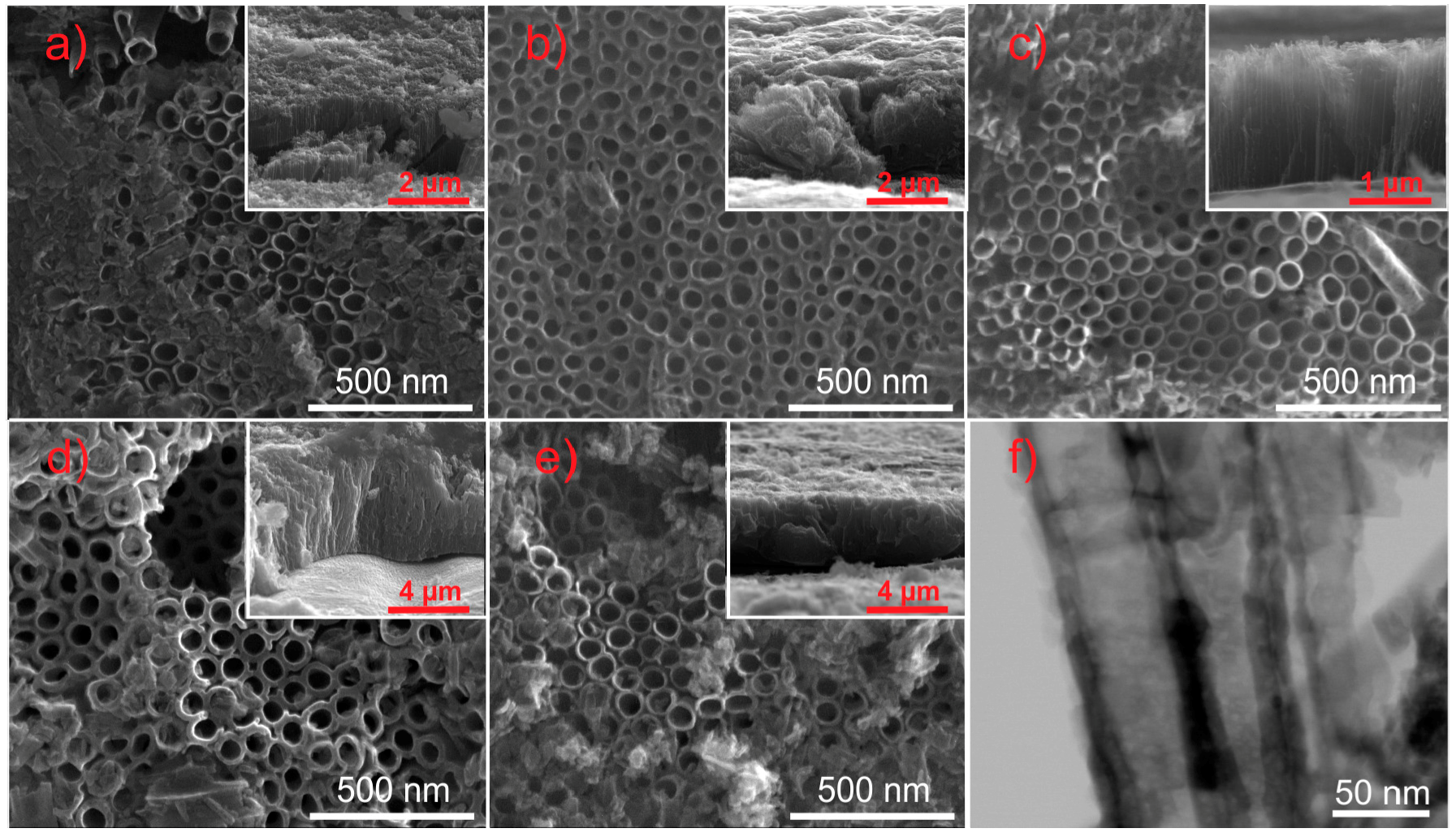
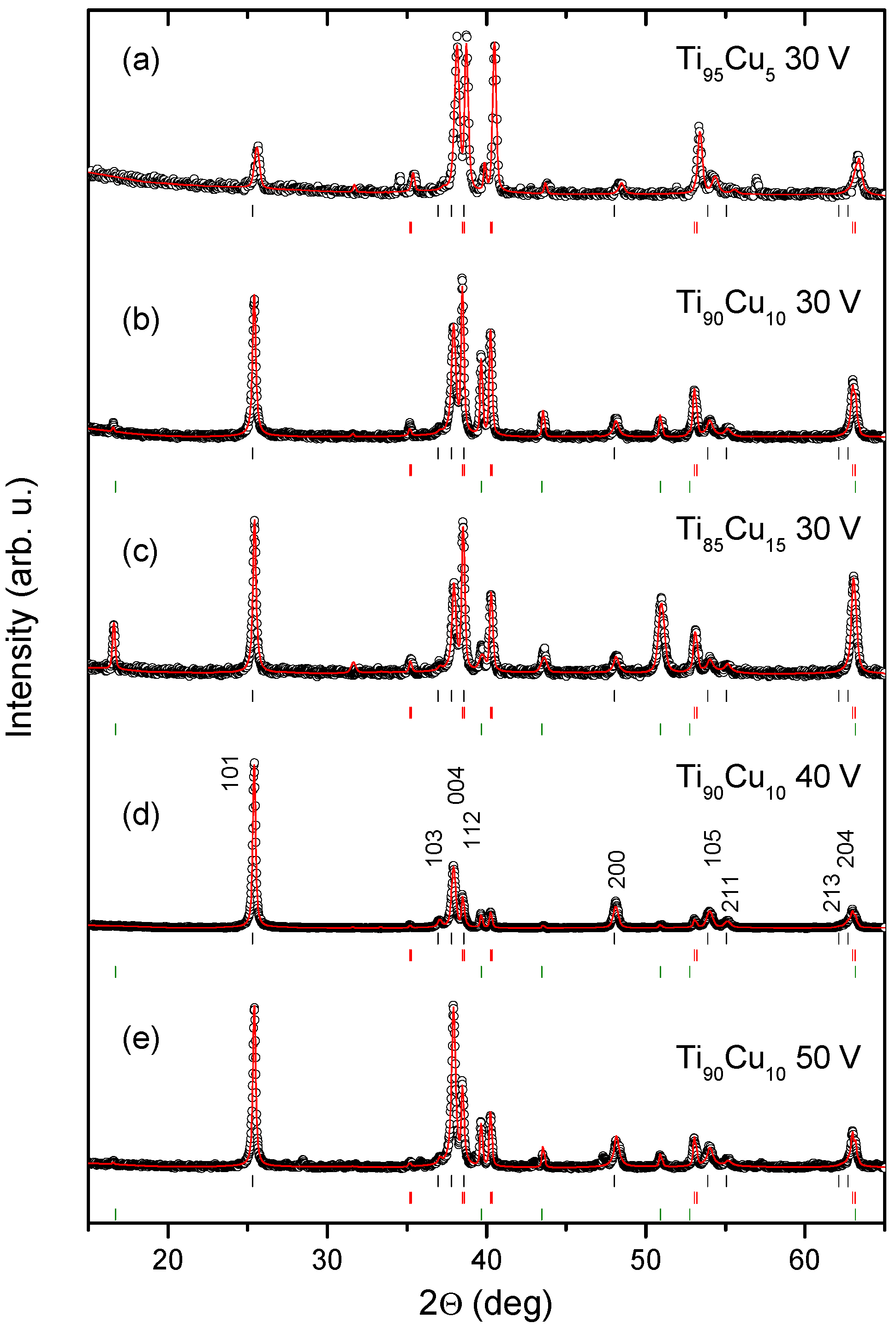
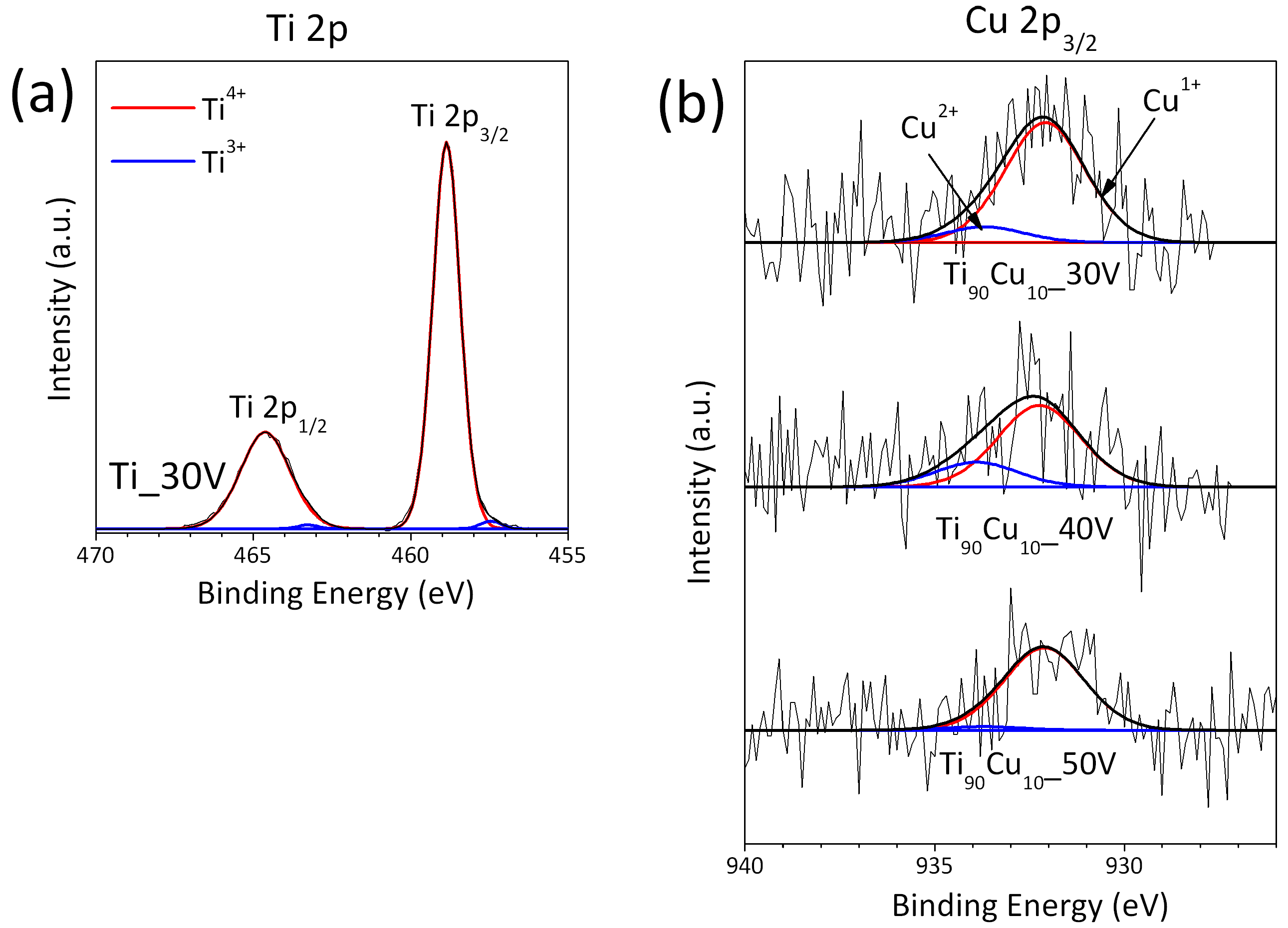
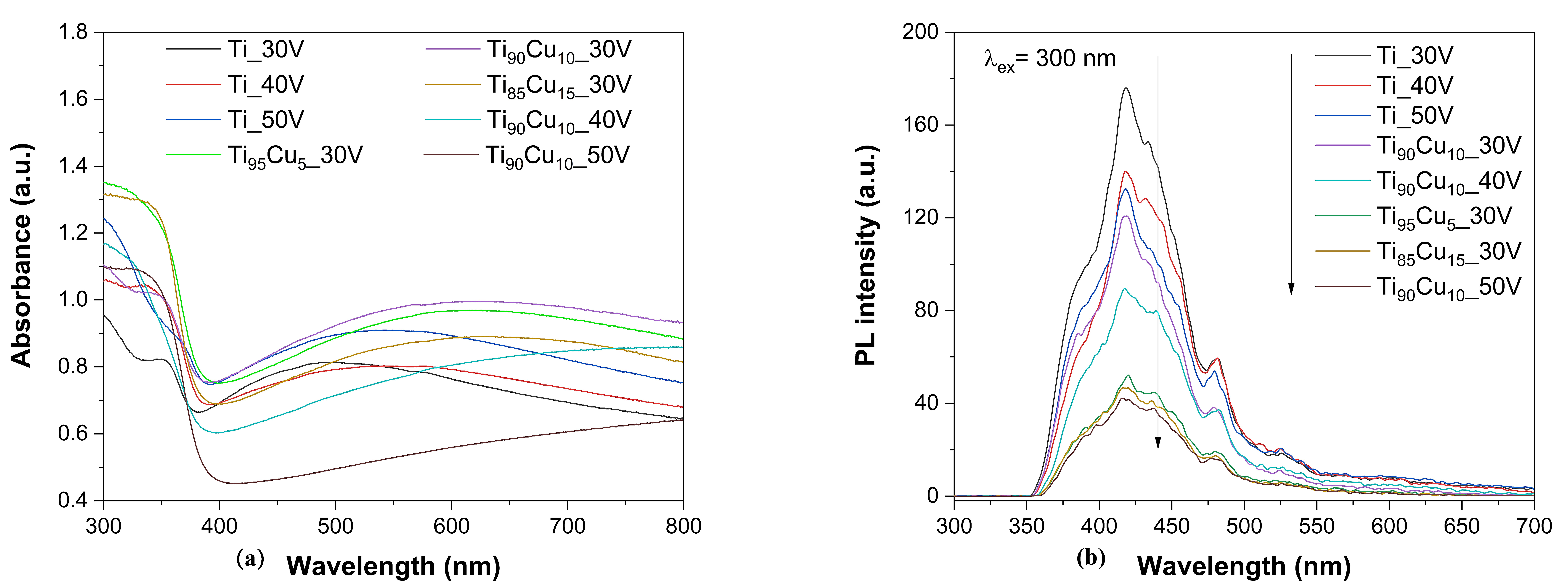
| Sample Label | Material of Working Electrode | Anodization Voltage (V) | External Diameter (nm) | Tubes Length (μm) | Wall Thickness (nm) | Cu Content (wt.%) |
|---|---|---|---|---|---|---|
| Ti_30V | Ti foil | 30 | 80 | 1.5 | 10 | - |
| Ti_40V | 40 | 100 | 3.0 | 13 | - | |
| Ti_50V | 50 | 120 | 6.0 | 18 | - | |
| Ti95Cu5_30V | Ti(95%)/Cu(5%) alloy | 30 | 85 | 1.3 | 12 | 3.57 |
| Ti90Cu10_30V | Ti(90%)/Cu(10%) alloy | 30 | 88 | 1.2 | 11 | 6.20 |
| Ti85Cu15_30V | Ti(85%)/Cu(15%) alloy | 30 | 83 | 1.1 | 15 | 9.45 |
| Ti90Cu10_40V | Ti(90%)/Cu(10%) alloy | 40 | 98 | 2.5 | 14 | 6.25 |
| Ti90Cu10_50V | Ti(90%)/Cu(10%) alloy | 50 | 97 | 3.5 | 14 | 3.44 |
| Sample Label | Average Crystallite Size (nm) | XPS Analysis | Photocatalytic Reaction Rate, r (μmol·dm−3·min−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ∑ Ti (at.%) | Ti4+ 458.7 eV (%) | Ti3+ 457.3 eV (%) | Cu (at.%) | Cu1+ 932.2 eV (%) | Cu2+ 933.8 eV (%) | UV-Vis Light (λ > 350 nm) | Vis Light (λ > 420 nm) | ||
| Ti_30V | 33 | 16.20 | 98.42 | 1.58 | 0 | - | - | 1.25 | 0.04 |
| Ti_40V | 27 | 24.79 | 97.38 | 2.62 | 0 | - | - | 1.35 | 0.13 |
| Ti_50V | 36 | 26.29 | 97.38 | 2.62 | 0 | - | - | 1.44 | 0.15 |
| Ti95Cu5_30V | 25 | 21.79 | 95.89 | 4.11 | 0.13 | 81.27 | 18.73 | 1.02 | 0.41 |
| Ti90Cu10_30V | 36 | 25.32 | 97.49 | 2.51 | 0.11 | 88.38 | 11.62 | 1.16 | 0.51 |
| Ti85Cu15_30V | 39 | 23.99 | 97.30 | 2.70 | 0.14 | 93.28 | 6.72 | 0.81 | 0.55 |
| Ti90Cu10_40V | 46 | 25.41 | 98.07 | 1.93 | 0.11 | 76.61 | 23.39 | 1.62 | 0.37 |
| Ti90Cu10_50V | 46 | 25.06 | 97.16 | 2.84 | 0.08 | 95.78 | 4.22 | 3.31 | 0.32 |
| Bacterial Strain | Experimental Conditions | Efficiency after 60 min |
|---|---|---|
| E. coli − OD = 0.09 STARTING CFU/mL: 3.3 × 102 | Light source: switched on | 97% |
| Bacteria: present | ||
| Photocatalytic layer: present | ||
| Light source: switched off | 12% | |
| Bacteria: present | ||
| Photocatalytic layer: present | ||
| Light source: switched on | 3% | |
| Bacteria: present | ||
| Photocatalytic layer: absent | ||
| B. subtilis − OD = 0.09 STARTING CFU/mL: 2.5 × 102 | Light source: switched on | Did not grow |
| Bacteria: present | ||
| Photocatalytic layer: present | Did not grow | |
| Light source: switched off | ||
| Bacteria: present | ||
| Photocatalytic layer: present | ||
| Light source: switched on | 16% | |
| Bacteria: present | ||
| Photocatalytic layer: absent | ||
| Clostridium sp. − OD = 0.1 STARTING CFU/mL: 3.8 × 102 | Light source: switched on | 98% |
| Bacteria: present | ||
| Photocatalytic layer: present | ||
| Light source: switched off | 0% | |
| Bacteria: present | ||
| Photocatalytic layer: present | ||
| Light source: switched on | 5% | |
| Bacteria: present | ||
| Photocatalytic layer: absent |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, M.; Mazierski, P.; Żebrowska, J.; Kobylański, M.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Nowaczyk, G.; Zaleska-Medynska, A. Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase. Catalysts 2018, 8, 237. https://doi.org/10.3390/catal8060237
Kozak M, Mazierski P, Żebrowska J, Kobylański M, Klimczuk T, Lisowski W, Trykowski G, Nowaczyk G, Zaleska-Medynska A. Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase. Catalysts. 2018; 8(6):237. https://doi.org/10.3390/catal8060237
Chicago/Turabian StyleKozak, Magda, Paweł Mazierski, Joanna Żebrowska, Marek Kobylański, Tomasz Klimczuk, Wojciech Lisowski, Grzegorz Trykowski, Grzegorz Nowaczyk, and Adriana Zaleska-Medynska. 2018. "Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase" Catalysts 8, no. 6: 237. https://doi.org/10.3390/catal8060237
APA StyleKozak, M., Mazierski, P., Żebrowska, J., Kobylański, M., Klimczuk, T., Lisowski, W., Trykowski, G., Nowaczyk, G., & Zaleska-Medynska, A. (2018). Electrochemically Obtained TiO2/CuxOy Nanotube Arrays Presenting a Photocatalytic Response in Processes of Pollutants Degradation and Bacteria Inactivation in Aqueous Phase. Catalysts, 8(6), 237. https://doi.org/10.3390/catal8060237









