Total Synthesis and Biological Evaluation of Phaeosphaerides
Abstract
1. Introduction
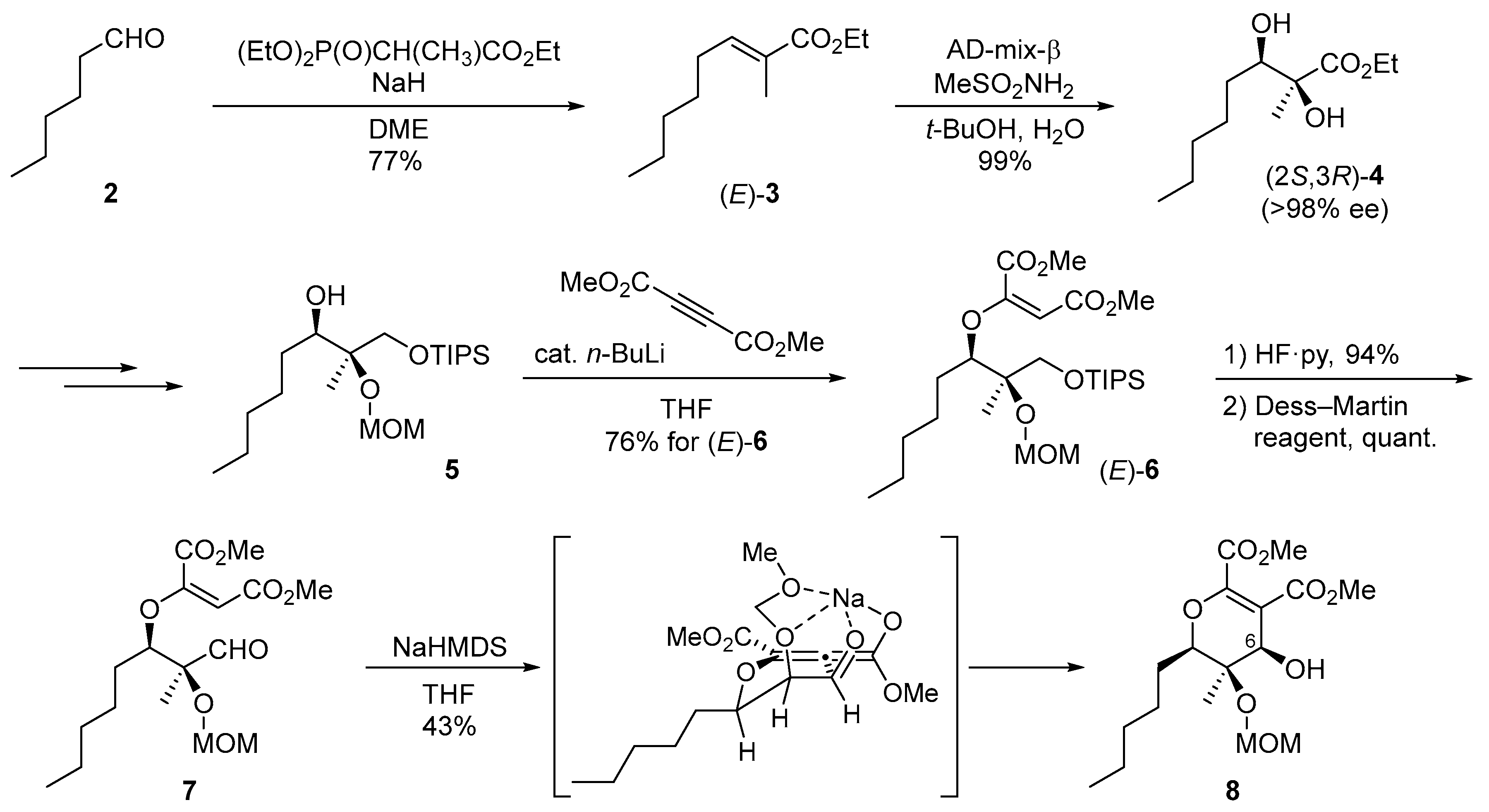
2. Synthetic Approach toward Phaeosphaeride A
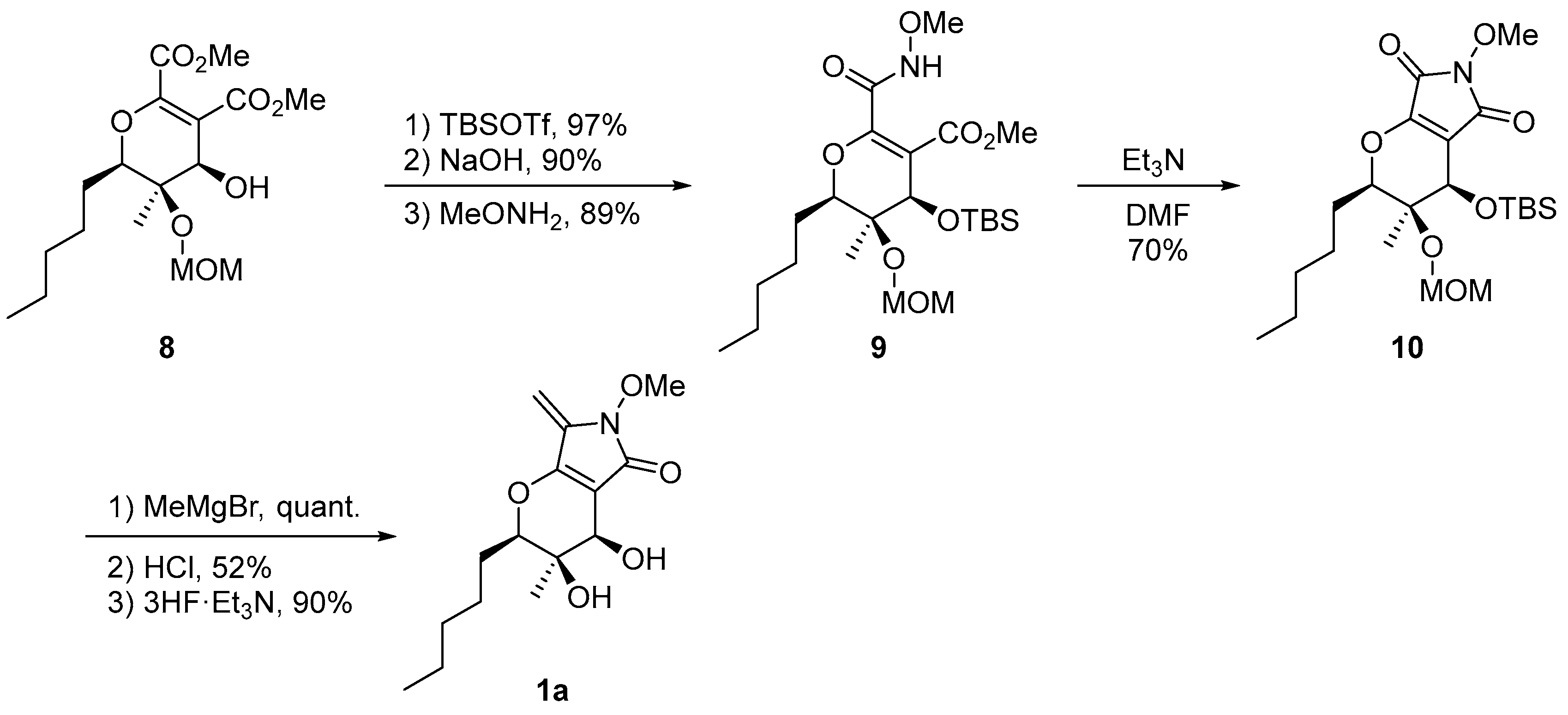
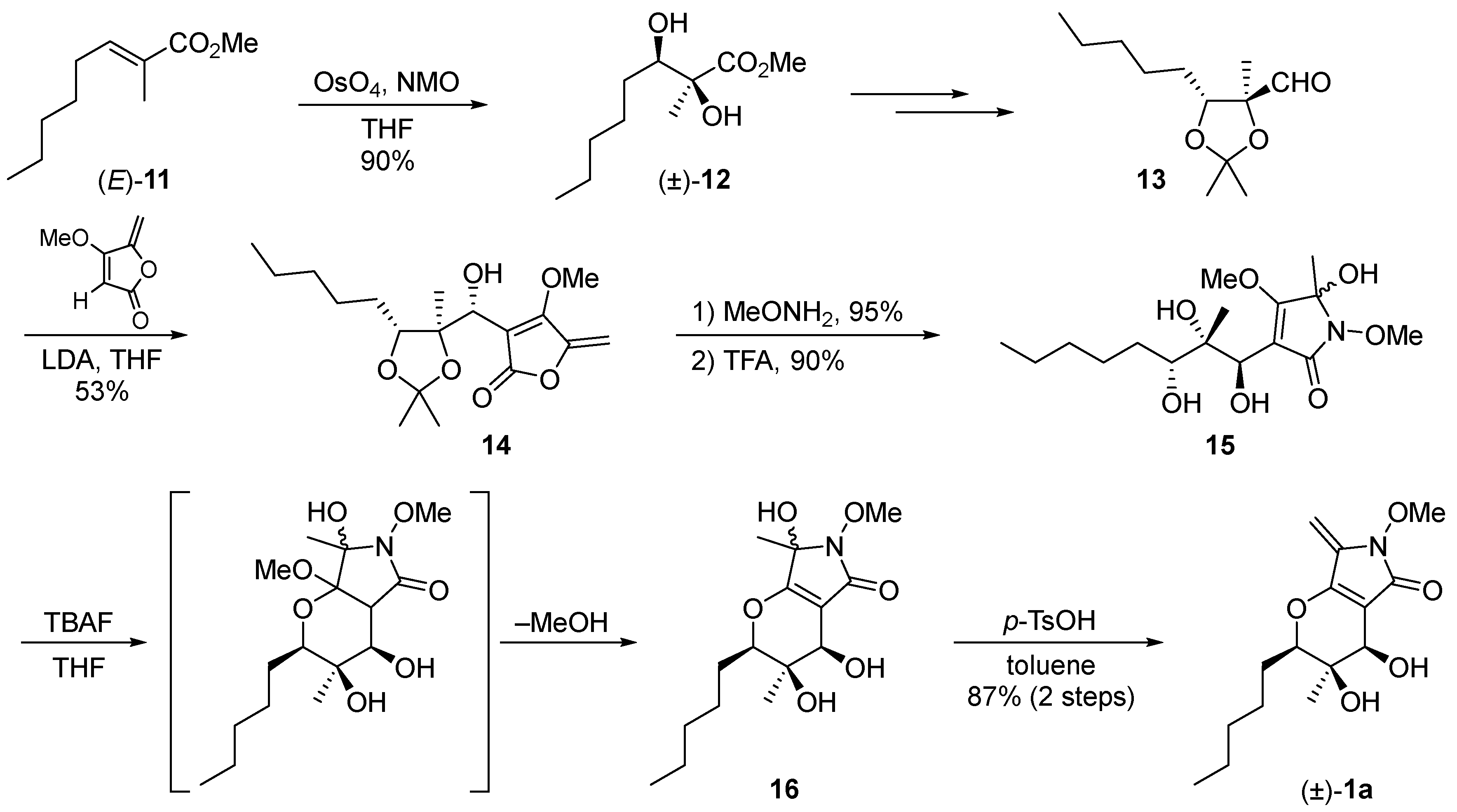
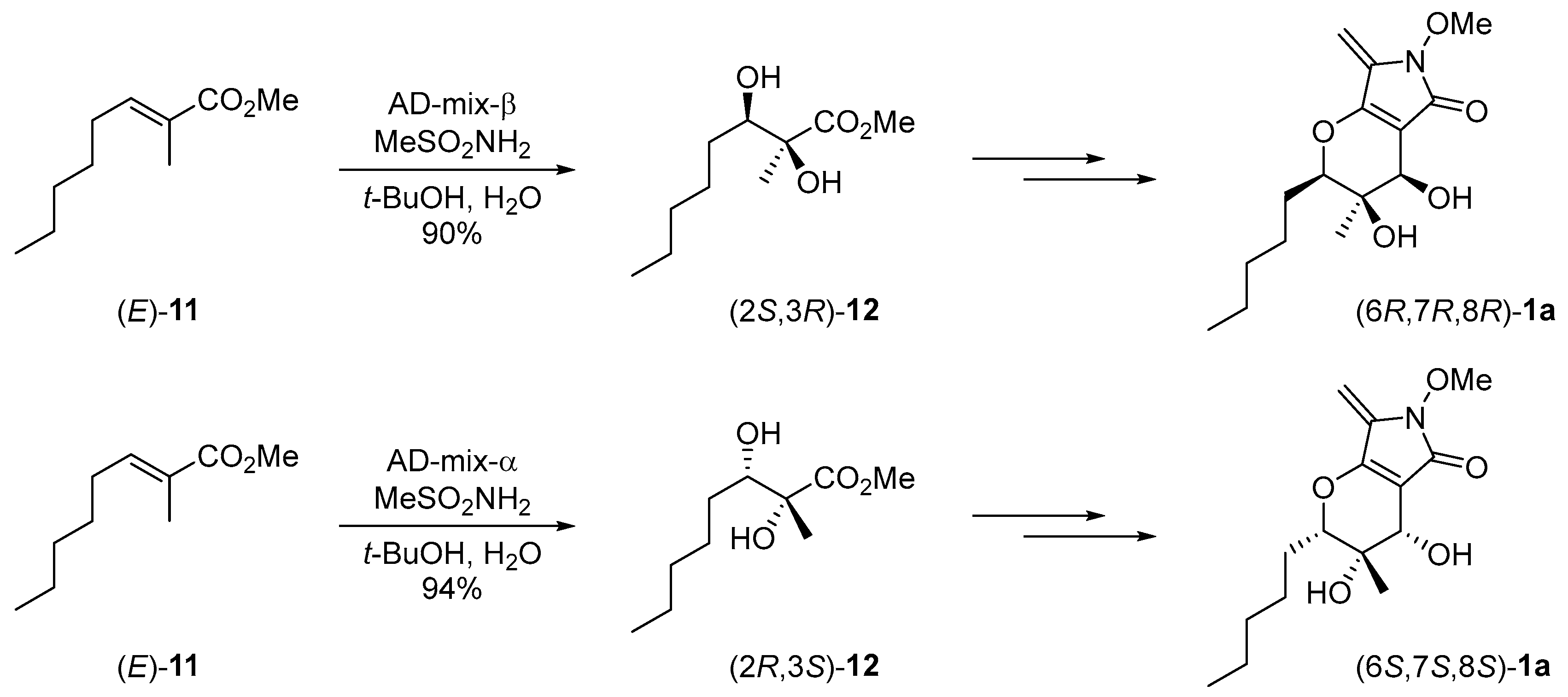
2.1. Synthesis of the Proposed Structure of Phaeosphaeride A
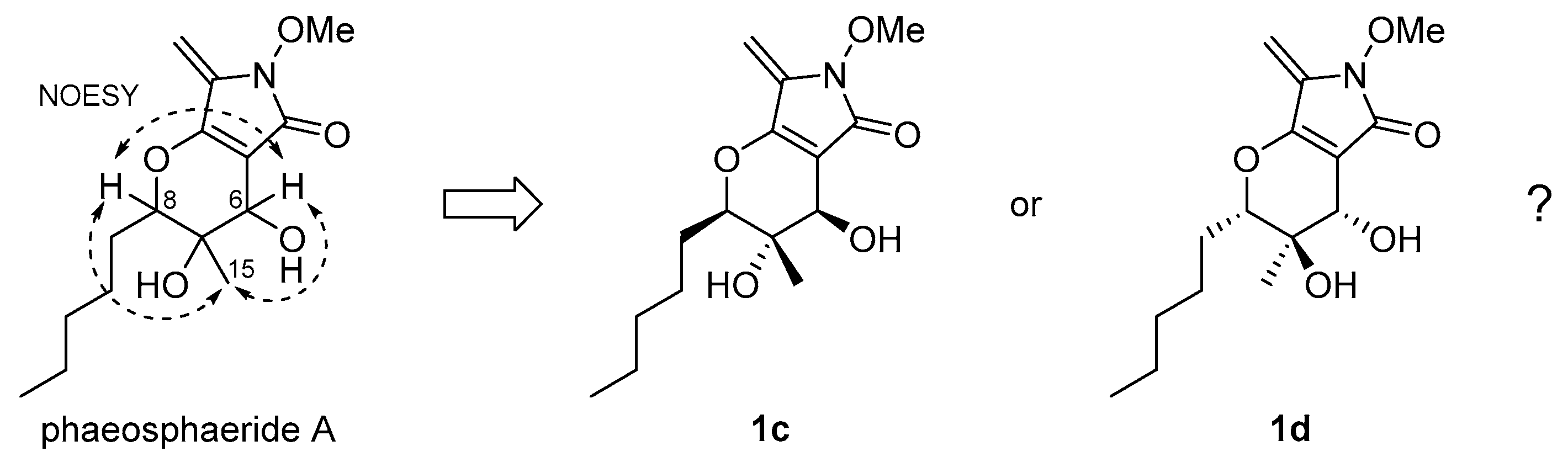
2.2. Stereochemical Determination of Natural (−)-Phaeosphaeride A
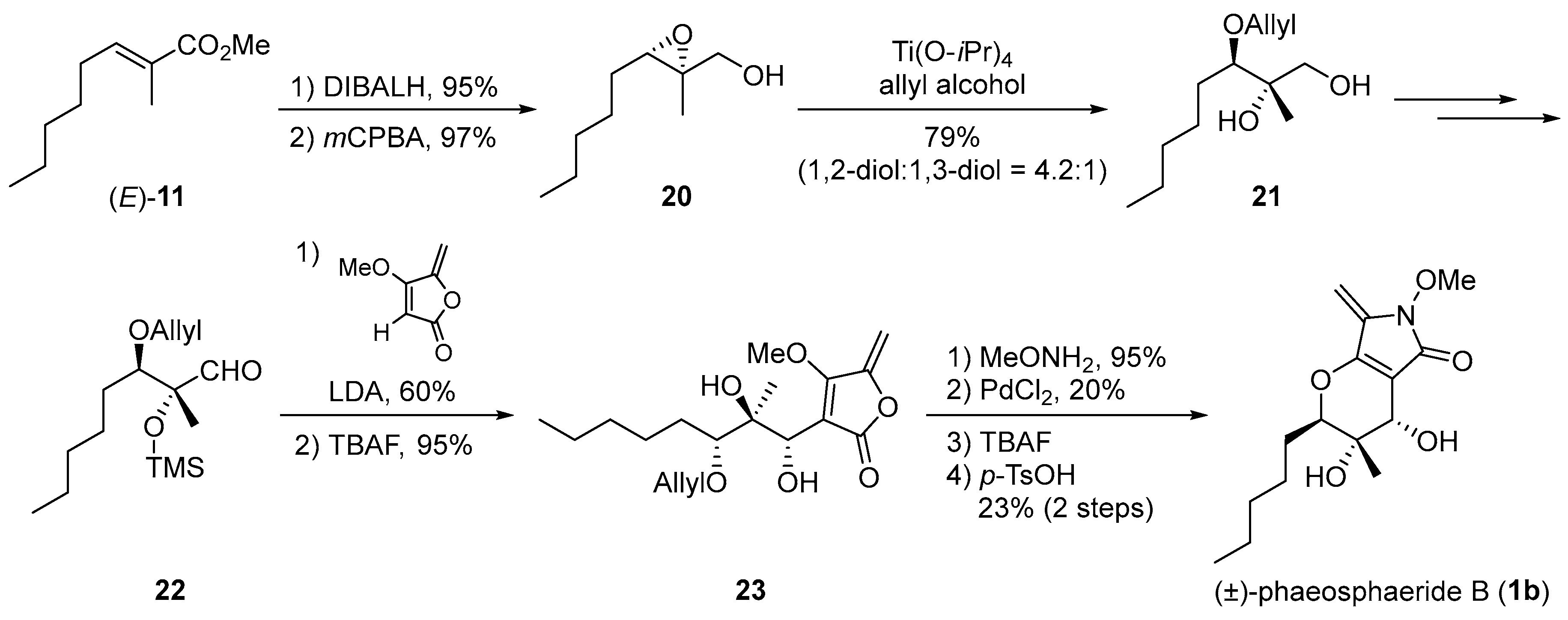
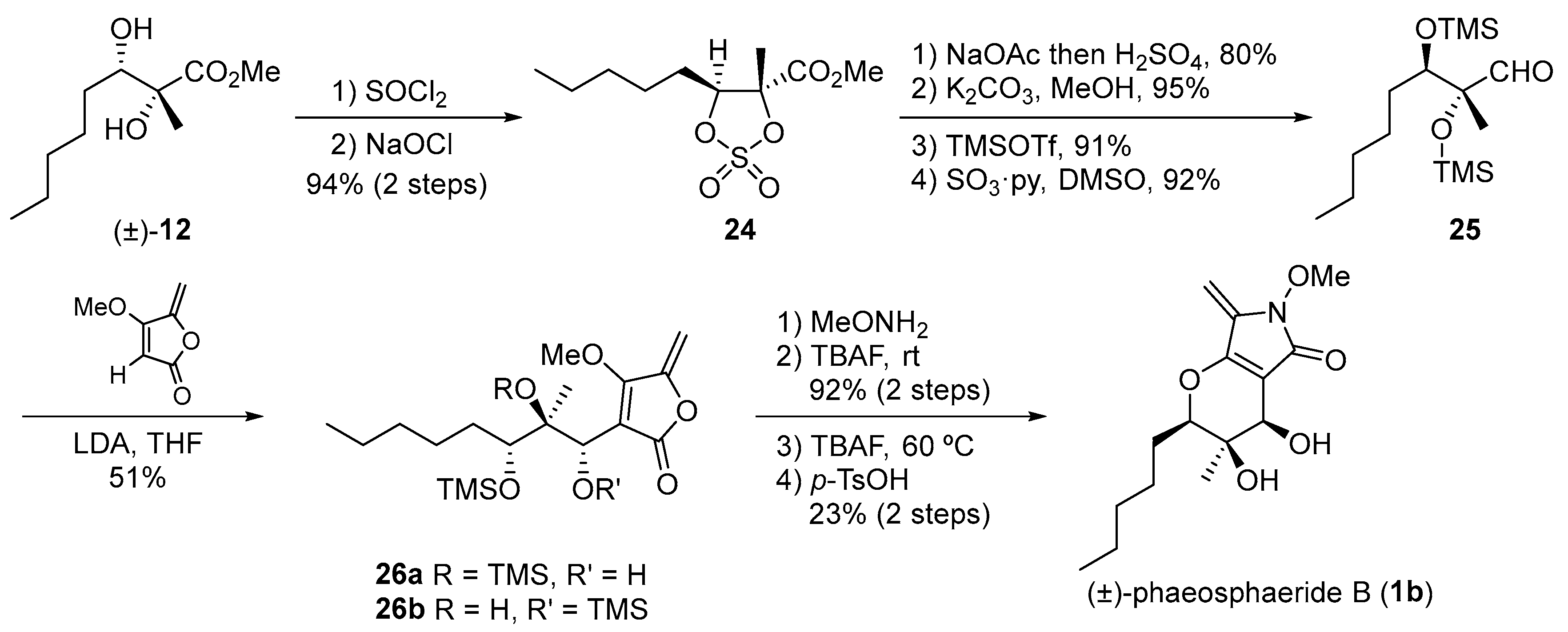
3. Synthetic Approach toward Phaeosphaeride B
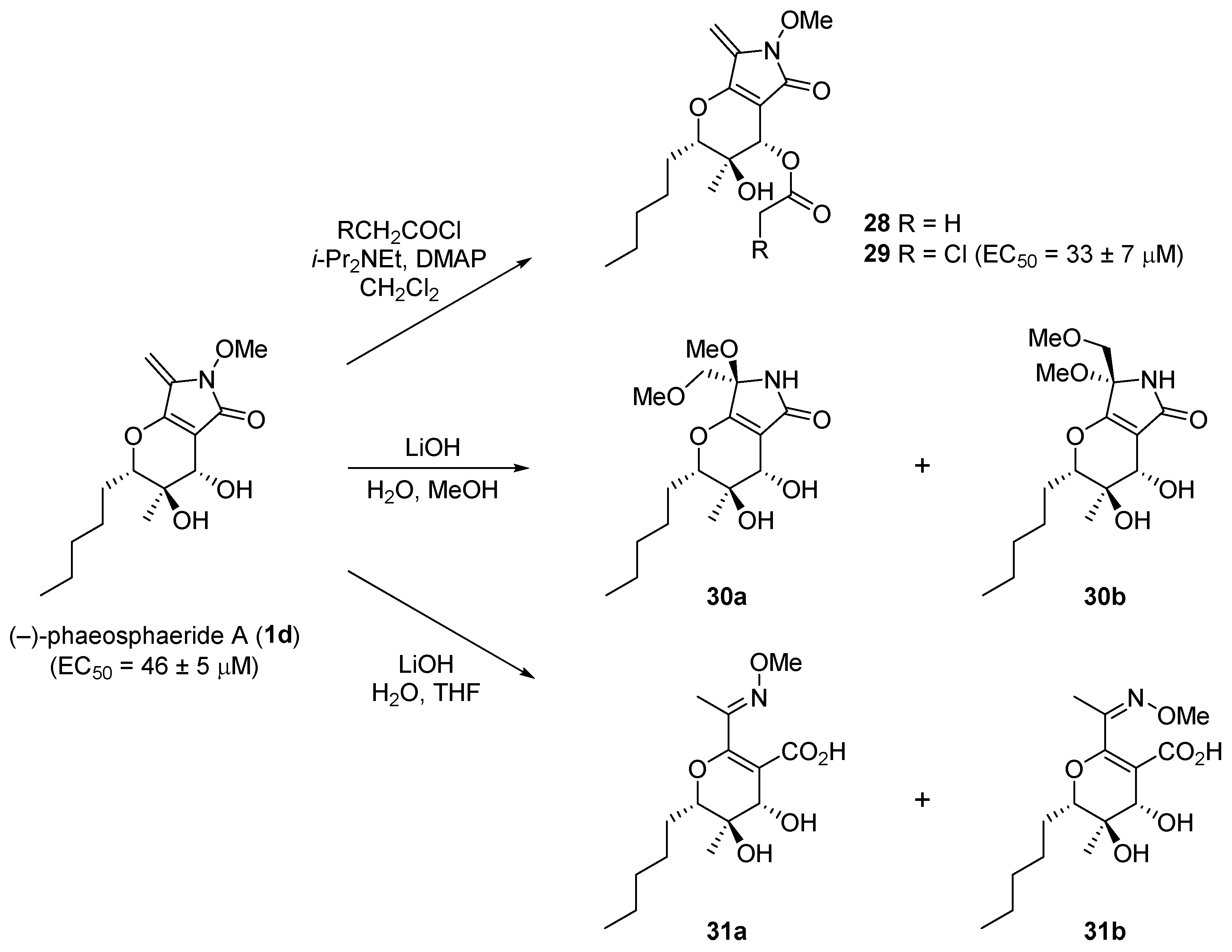
4. Biological Evaluation of Phaeosphaerides and Their Derivatives
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Levy, D.E. Physiological significance of STAT proteins: Investigations through gene disruption in vivo. Cell. Mol. Life Sci. 1999, 55, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E., Jr. STATs and gene regulation. Science 1997, 277, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.E., Jr.; Kerr, I.M.; Stark, G.R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Darnell, J.E., Jr. Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994, 264, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E., Jr. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef]
- Subramaniam, A.; Shanmugam, M.K.; Perumal, E.; Li, F.; Nachiyappan, A.; Dai, X.; Swamy, S.N.; Ahn, K.S.; Kumar, A.P.; Tan, B.K.; et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim. Biophys. Acta Rev. Cancer 2013, 1835, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta Rev. Cancer 2014, 1845, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.N.; Hao, W.; Xu, J.; Gibbons, J.; Hucul, J.; Roll, D.; Brady, S.F.; Schroeder, F.C.; Clardy, J. Phaeosphaeride A, an Inhibitor of STAT3-dependent signaling isolated from an endophytic fungus. Org. Lett. 2006, 8, 4067–4070. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Okamoto, I.; Morita, N.; Kiyotani, T.; Tamura, O. Synthesis of the proposed structure of phaeosphaeride A. Org. Biomol. Chem. 2011, 9, 5825–5832. [Google Scholar] [CrossRef] [PubMed]
- Chatzimpaloglou, A.; Yavropoulou, M.P.; Rooij, K.E.; Biedermann, R.; Mueller, U.; Kaskel, S.; Sarli, V. Total synthesis and biological activity of the proposed structure of phaeosphaeride A. J. Org. Chem. 2012, 77, 9659–9667. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kobayashi, Y.; Nakamura, M.; Tamura, O.; Kogen, H. Establishment of relative and absolute configurations of phaeosphaeride A: Total synthesis of ent-phaeosphaeride A. J. Org. Chem. 2015, 80, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kunimura, R.; Tanaka, K., III; Tamura, O.; Kogen, H. Total synthesis of (–)-phaeosphaeride B by a biomimetic conversion from (−)-phaeosphaeride A. Tetrahedron 2017, 73, 2382–2388. [Google Scholar] [CrossRef]
- Abzianidze, V.V.; Poluektova, E.V.; Bolshakova, K.P.; Panikorovskii, T.L.; Bogachenkov, A.S.; Berestetskiy, A.O. Crystal structure of natural phaeosphaeride A. Acta Crystallogr. 2015, E71, o625–o626. [Google Scholar] [CrossRef] [PubMed]
- Chatzimpaloglou, A.; Kolosov, M.; Eckols, T.K.; Tweardy, D.J.; Sarli, V. Synthetic and biological studies of phaeosphaerides. J. Org. Chem. 2014, 79, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Abzianidze, V.V.; Prokofieva, D.S.; Chisty, L.A.; Bolshakova, K.P.; Berestetskiy, A.O.; Panikorovskii, T.L.; Bogachenkov, A.S.; Holder, A.A. Synthesis of natural phaeosphaeride A derivatives and an in vitro evaluation of their anti-cancer potential. Bioorg. Med. Chem. Lett. 2015, 25, 5566–5569. [Google Scholar] [CrossRef] [PubMed]
- Abzianidze, V.V.; Bolshakova, K.P.; Prokofieva, D.S.; Berestetskiy, A.O.; Kuznetsova, V.A.; Trishin, Y.G. Synthesis of 7-(4-methylphenyl)thiomethyl and 7-morpholylmethyl derivatives of natural phaeosphaeride A and their cytotoxic activity. Mendeleev Commun. 2017, 27, 82–84. [Google Scholar] [CrossRef]
- Abzianidze, V.V.; Efimova, K.P.; Poluektova, E.V.; Trishin, Y.G.; Kuznetsov, V.A. Synthesis of natural phaeosphaeride A and semi-natural phaeosphaeride B derivatives. Mendeleev Commun. 2017, 27, 490–492. [Google Scholar] [CrossRef]
- Li, C.S.; Ding, Y.; Yang, B.J.; Miklossy, G.; Yin, H.Q.; Walker, L.A.; Turkson, J.; Cao, S. A new metabolite with a unique 4-pyranone−γ-lactam−1,4-thiazine moiety from a Hawaiian-plant associated fungus. Org. Lett. 2015, 17, 3556–3559. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Sarotti, A.M.; Huang, P.; Dang, U.T.; Hurdle, J.G.; Kondratyuk, T.P.; Pezzuto, J.M.; Turkson, J.; Cao, S. NF-κB inhibitors, unique γ-pyranol-γ-lactams with sulfide and sulfoxide moieties from Hawaiian plant Lycopodiella cernua derived fungus Paraphaeosphaeria neglecta FT462. Sci. Rep. 2017, 7, 10424–10433. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Cimmino, A.; Andolfi, A.; Vurro, M.; Zonno, M.C.; Cantrell, C.L.; Motta, A. Phyllostictines A–D, oxazatricycloalkenones produced by Phyllosticta cirsii, a potential mycoherbicide for Cirsium arvense biocontrol. Tetrahedron 2008, 64, 1612–1619. [Google Scholar] [CrossRef]
- Trenti, F.; Cox, R.J. Structural revision and biosynthesis of the fungal phytotoxins phyllostictines A and B. J. Nat. Prod. 2017, 80, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.H.; Zeraik, M.L.; Oliveira, C.M.; Teles, H.L.; Trevisan, H.C.; Pfenning, L.H.; Nicolli, C.P.; Young, M.C.M.; Mascarenhas, Y.P.; Abreu, L.M.; et al. Lactone derivatives produced by a Phaeoacremonium sp., an endophytic fungus from Senna spectabilis. J. Nat. Prod. 2017, 80, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Thines, E.; Arendholz, W.-R.; Anke, H. Bunesudon, a new antibiotic fungal metabolite from cultures of Mollisia benesuada (Tul.) Phill. J. Antibiot. 1997, 50, 13–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abraham, W.-R.; Meyer, H.; Abate, D. Curvupallides, a new class of alkaloids from the fungus Curvularia pallescens. Tetrahedron 1995, 51, 4947–4952. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, K.; Tanaka, K.; Kogen, H. Total Synthesis and Biological Evaluation of Phaeosphaerides. Catalysts 2018, 8, 206. https://doi.org/10.3390/catal8050206
Kobayashi K, Tanaka K, Kogen H. Total Synthesis and Biological Evaluation of Phaeosphaerides. Catalysts. 2018; 8(5):206. https://doi.org/10.3390/catal8050206
Chicago/Turabian StyleKobayashi, Kenichi, Kosaku Tanaka, and Hiroshi Kogen. 2018. "Total Synthesis and Biological Evaluation of Phaeosphaerides" Catalysts 8, no. 5: 206. https://doi.org/10.3390/catal8050206
APA StyleKobayashi, K., Tanaka, K., & Kogen, H. (2018). Total Synthesis and Biological Evaluation of Phaeosphaerides. Catalysts, 8(5), 206. https://doi.org/10.3390/catal8050206




