Selective Conversion of Furfural to Cyclopentanone or Cyclopentanol Using Co-Ni Catalyst in Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization Studies
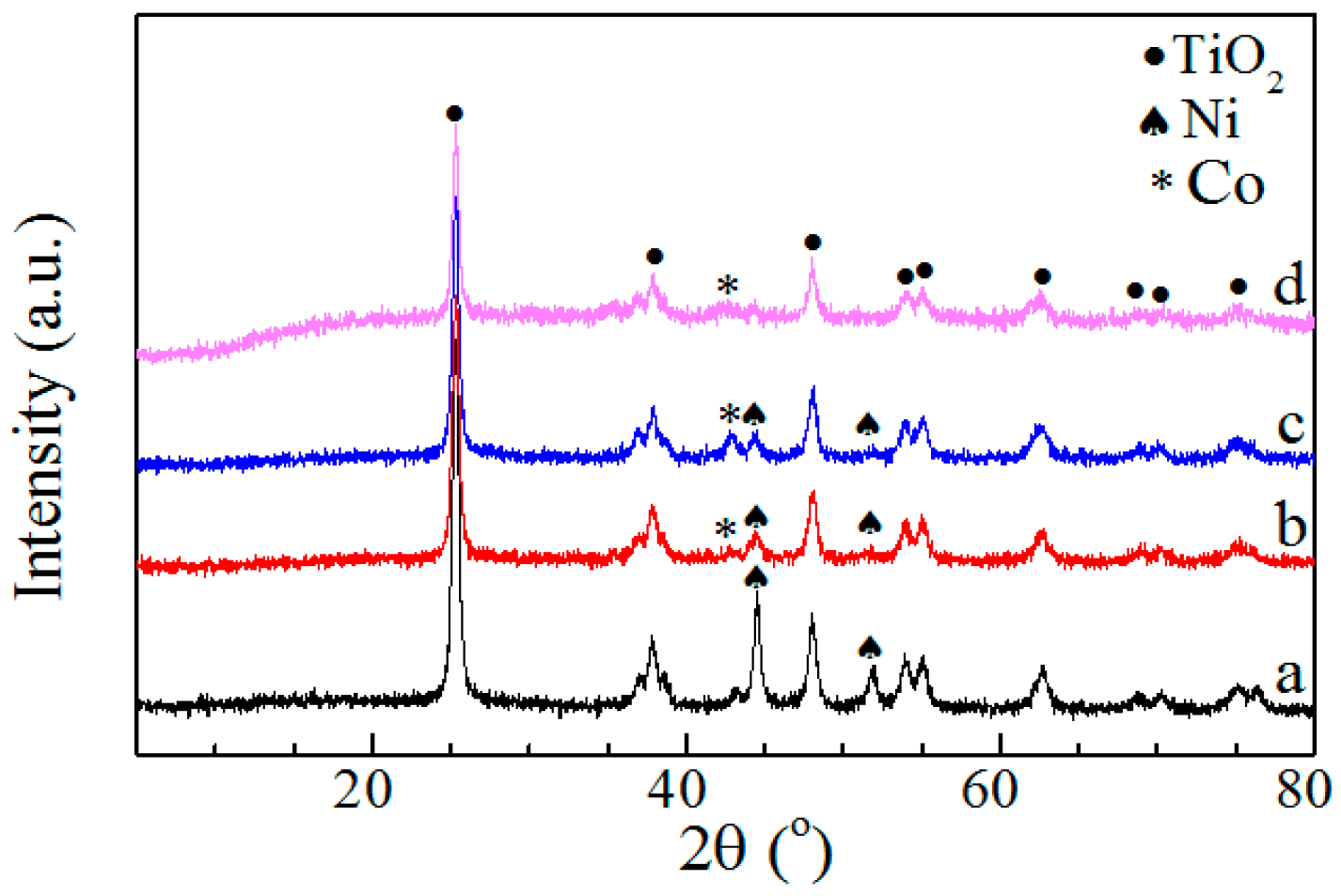
2.1.1. XRD
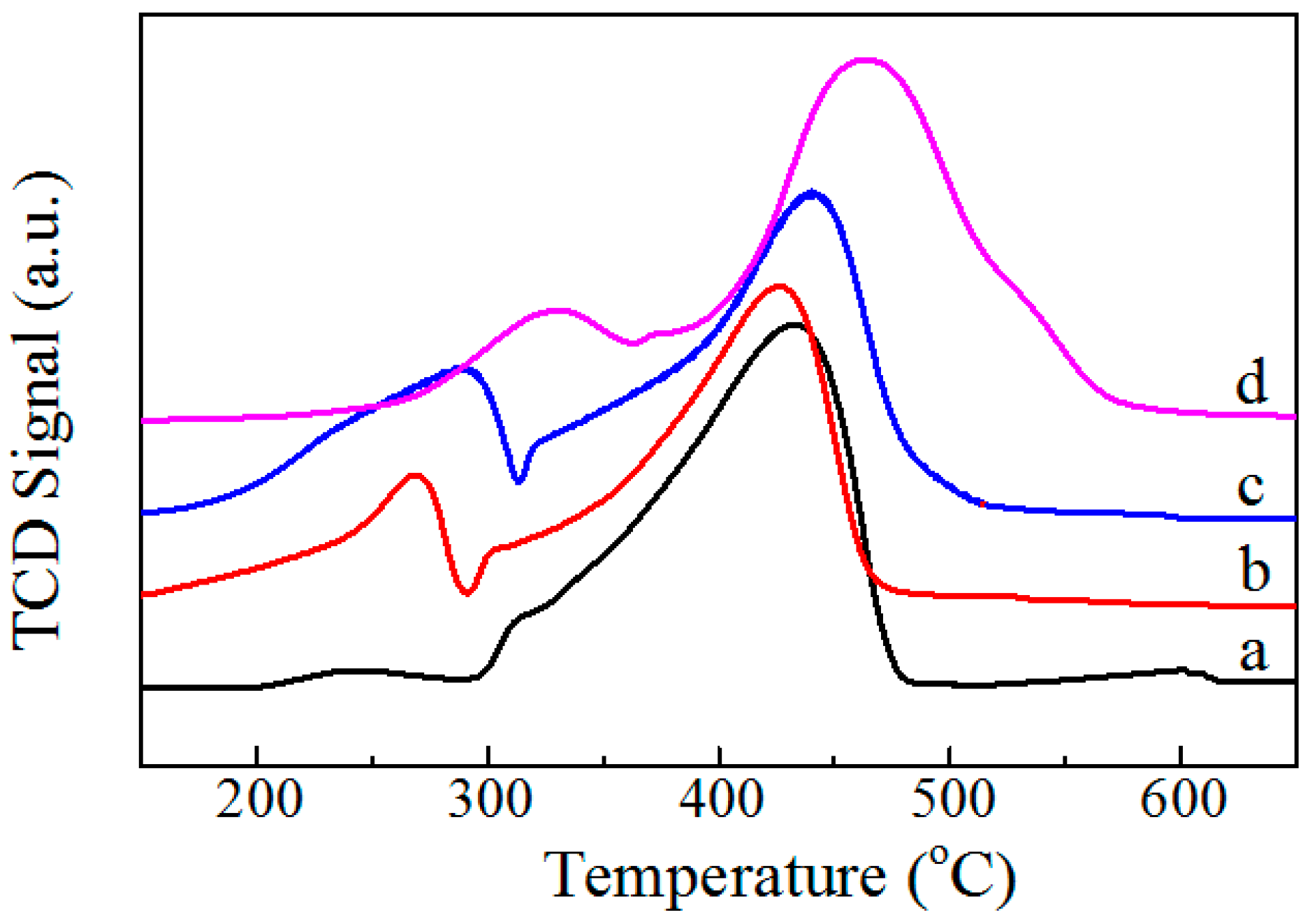
2.1.2. H2-TPR
2.1.3. SEM
2.2. Screening of Catalysts for the Conversion of Furfural
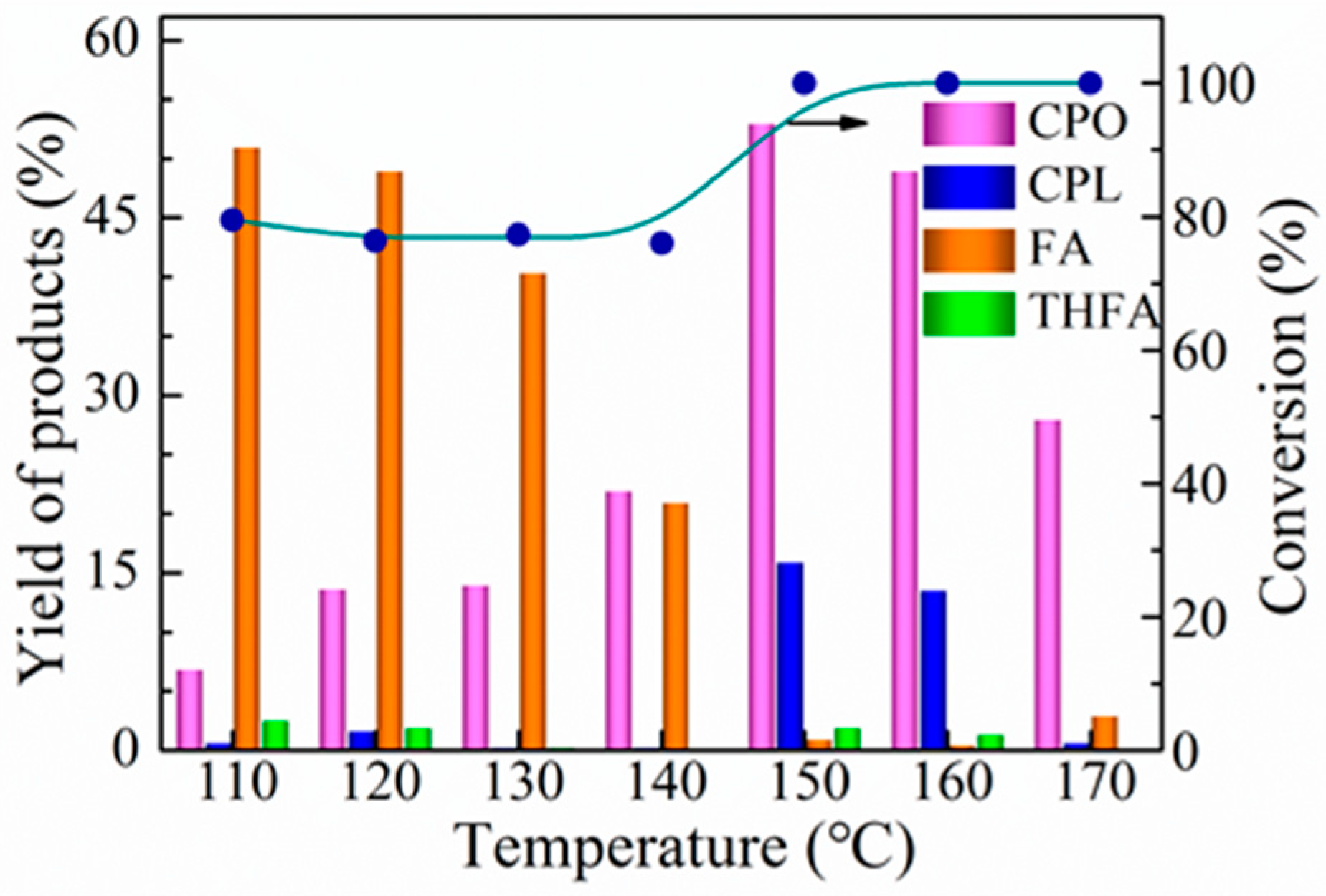
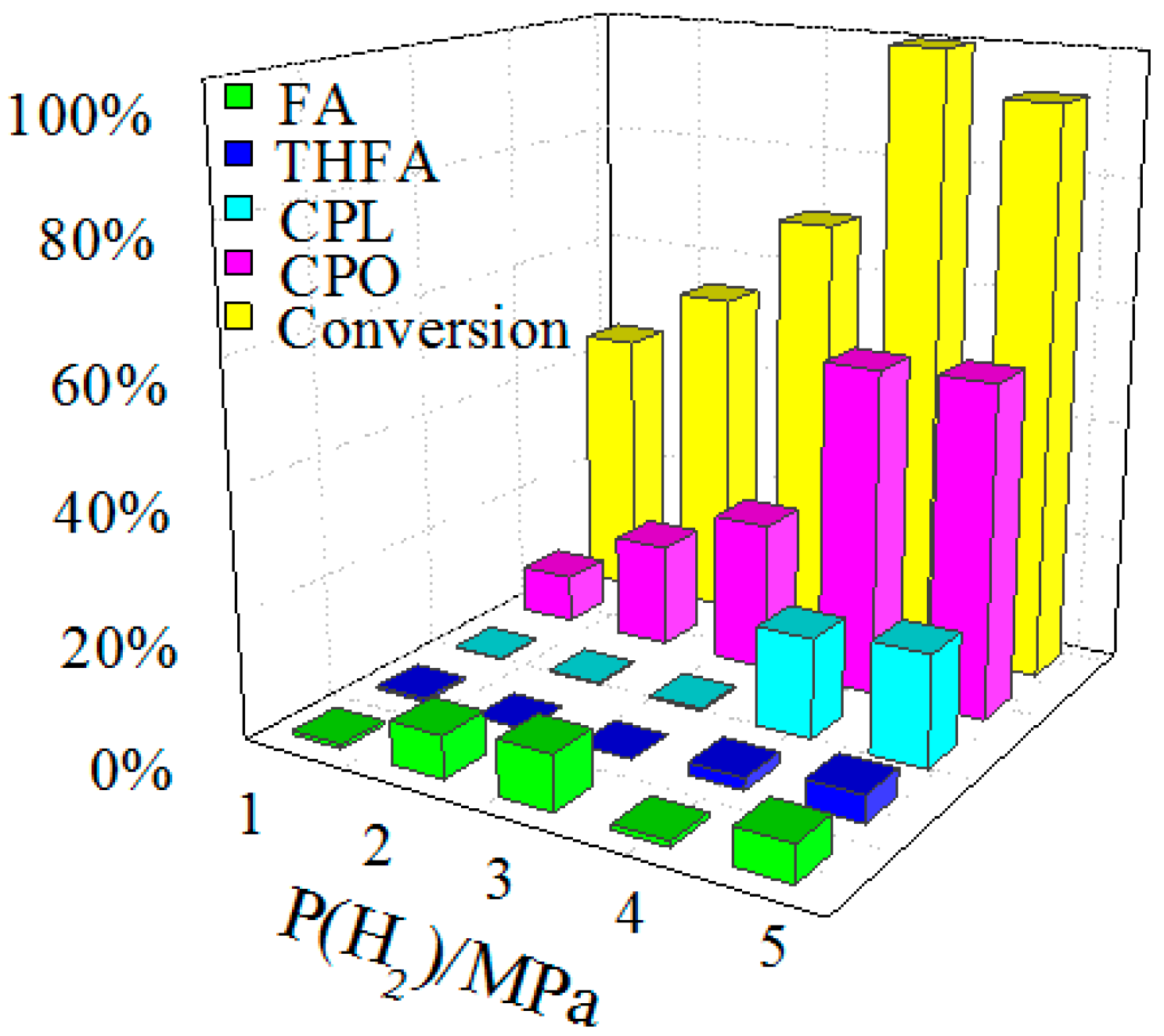
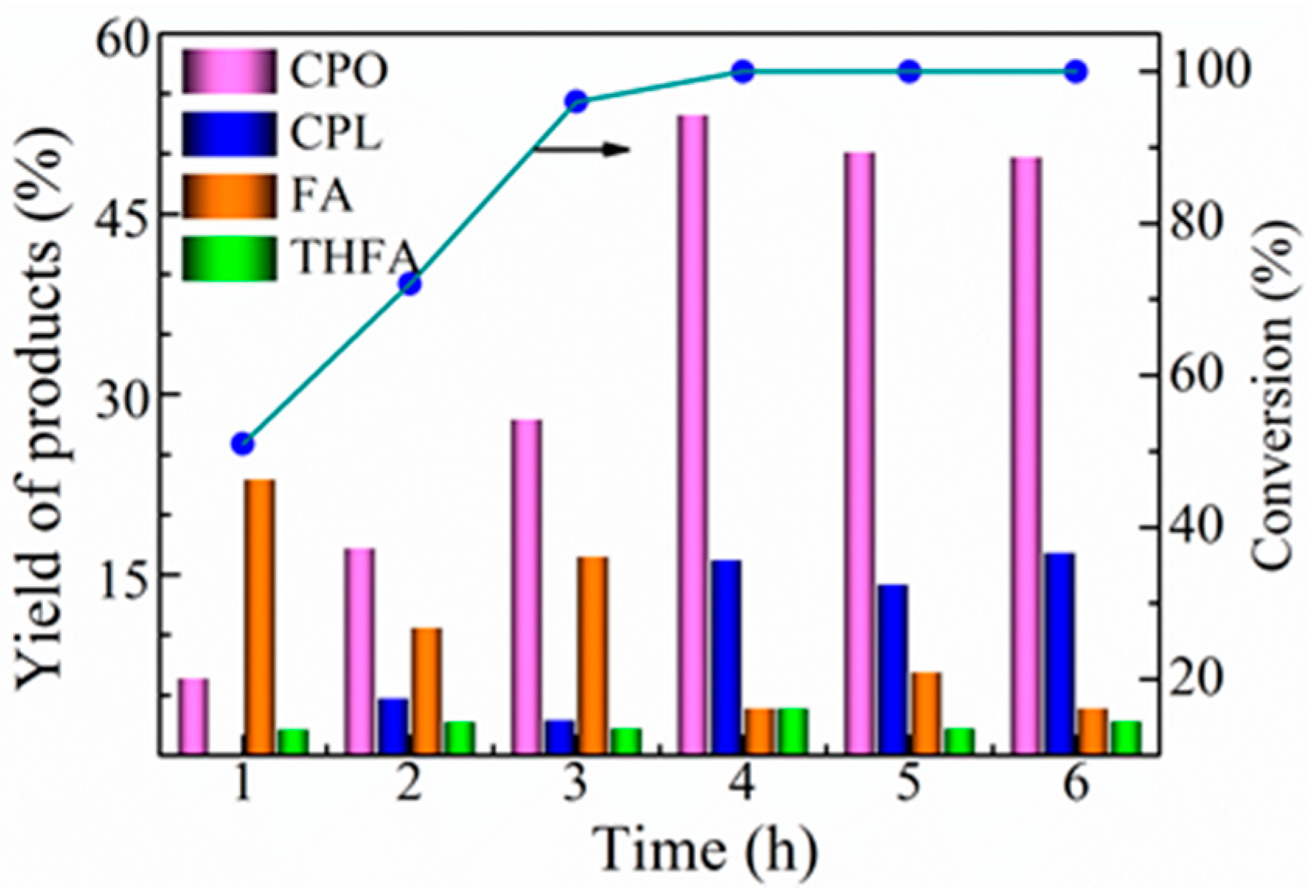
2.3. Optimization of Reaction Parameters
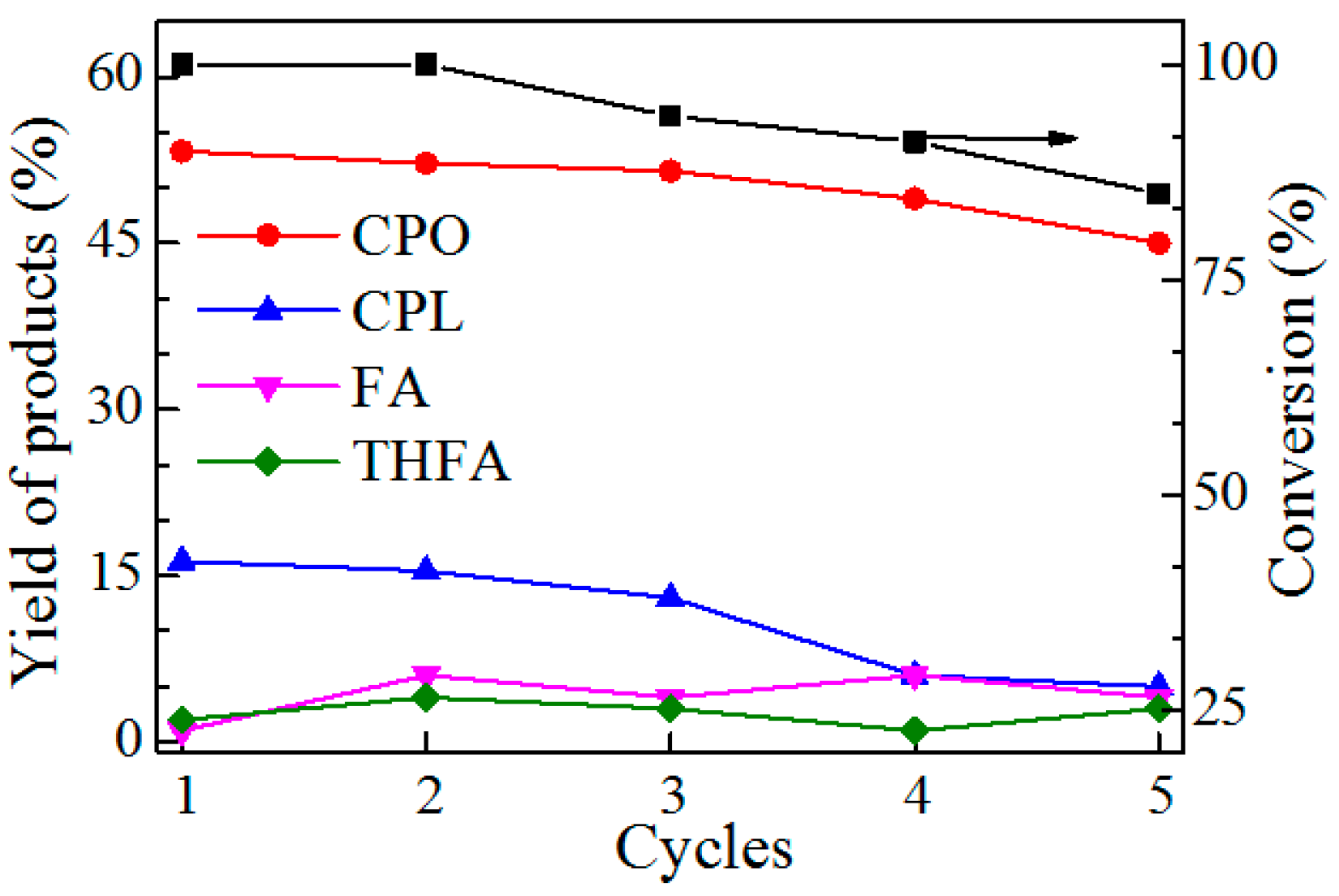
2.4. Catalyst Recycling
3. Experimental Section
3.1. Catalyst Materials and Preparation
3.2. Characterization of Catalysts
3.3. Typical Procedure for Catalytic Conversion of Furfural
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádabaa, I.; López Granados, M. Efficient hydrogenation of concentrated aqueous furfural solutions into furfuryl alcohol under ambient conditions in presence of PtCo bimetallic catalyst. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Wang, X.; Liang, Y.; Yang, X.; Wang, Z. Highly selective and efficient rearrangement of biomass-derived furfural to cyclopentanone over interface-active Ru/CNTs catalyst in water. ACS Sustain. Chem. Eng. 2017, 5, 744–751. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhu, M.M.; Zhang, Q.; Liu, Y.M.; He, H.; Cao, Y. Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem. 2016, 18, 2155–2164. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtárová, K.; Vávra, I.; Soták, T.; Dobročka, E.; Mičušík, M. Carbon supported Pd–Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl. Catal. B Environ. 2016, 181, 210–219. [Google Scholar] [CrossRef]
- Andw, L.; Aqner, A. Reactions catalyzed by potassium fluoride. II. the conversion of adipic acid to cyclopentanone. J. Org. Chem. 1962, 27, 1034–1035. [Google Scholar]
- Akashi, T.; Sato, S.; Takahashi, R.; Sodesawa, T.; Inui, K. Catalytic vapor-phase cyclization of 1,6-hexanediol into cyclopentanone. Catal. Commun. 2003, 4, 411–416. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Katta, L.; Thimurthulu, G.; Reddy, B.M. Vapor phase synthesis of cyclopentanone over nanostructured ceria–zirconia solid solution catalysts. J. Ind. Eng. Chem. 2013, 19, 1517–1524. [Google Scholar] [CrossRef]
- Velarde, R.R.; Grigoropoulos, A.; Perret, N.; Zanella, M.; Katsoulidis, A.P.; Manning, T.D.; Claridge, J.B.; Rosseinsky, M.J. Selective conversion of 5-hydroxymethylfurfural to cyclopentanone derivatives over Cu-Al2O3 and Co-Al2O3 catalysts in water. Green Chem. 2017, 19, 1701–1713. [Google Scholar]
- Liu, X.; Zhang, B.; Fei, B.; Chen, X.; Zhang, J.; Mu, X. Tunable and selective hydrogenation of furfural to furfuryl alcohol and cyclopentanone over Pt supported on biomass-derived porous heteroatom doped carbon. Faraday Discuss. 2017, 202, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Hronec, M.; Fulajtárova, K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Schouten, J.C.; Van der Schaaf, J.; Nijhuis, T.A. Biphasic single-reactor process for dehydration of xylose and hydrogenation of produced furfural. Appl. Catal. A 2013, 451, 6–13. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, H.Y.; Niu, L.; Xiao, G.M.; Xiao, R. Catalytic hydroprocessing of furfural to cyclopentanol over Ni/CNTs catalysts: Model reaction for upgrading of bio-oil. Catal. Lett. 2014, 144, 235–241. [Google Scholar] [CrossRef]
- Guo, J.H.; Xu, G.Y.; Han, Z.; Zhang, Y.; Fu, Y.; Guo, Q.X. Selective conversion of furfural to cyclopentanone with CuZnAl catalysts. ACS Sustain. Chem. Eng. 2014, 2, 2259–2266. [Google Scholar] [CrossRef]
- Yang, Y.L.; Du, Z.T.; Huang, Y.H.; Lu, F.; Wang, F.; Gao, J.; Xu, J. Conversion of furfural into cyclopentanone over Ni–Cu bimetallic catalysts. Green Chem. 2013, 15, 1932–1940. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, S.; Zhu, W.; Gao, L.; Xiao, G. CuNi@C catalysts with high activity derived from metal–organic frameworks precursor for conversion of furfural to cyclopentanone. Chem. Eng. J. 2016, 299, 104–111. [Google Scholar] [CrossRef]
- Ma, Y.F.; Wang, H.; Xu, G.Y.; Liu, X.H.; Zhang, Y.; Fu, Y. Selective conversion of furfural to cyclopentanol over cobalt catalysts in one step. Chin. Chem. Lett. 2017, 1153–1158. [Google Scholar] [CrossRef]
- Li, X.L.; Deng, J.; Shi, J.; Pan, T.; Yu, C.G.; Xu, H.J.; Fu, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu–Co catalysts. Green Chem. 2015, 17, 1038–1046. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K.I. Titania-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2005, 232, 268–275. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Kadier, A.; Takriff, M.S.; Hassan, N.S.M. One-pot sol-gel synthesis of Ni/TiO2, catalysts for methane decomposition into COx free hydrogen and multiwalled carbon nanotubes. Int. J. Hydrog. Energy 2017, 42, 16495–16513. [Google Scholar] [CrossRef]
- Jin, S.; Xiao, Z.; Li, C.; Chen, X.; Wang, L.; Xing, J.; Li, W.; Liang, C. Catalytic hydrodeoxy genation of anisole as lignin model compound over supported nickel catalysts. Catal. Today 2014, 234, 125–132. [Google Scholar] [CrossRef]
- Batista, M.S.; Santos, R.K.S.; Assaf, E.M.; Assaf, J.M.; Ticianelli, E.A. Characterization of the activity and stability of supported cobalt catalysts for the steam reforming of ethanol. J. Power Source 2003, 124, 99–103. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Yang, Q.; Zhao, D.; Zhang, K.; Yao, J.; Li, G.; Zhou, H.; Zuo, X. Controllable synthesis and magnetic properties of hydrothermally synthesised NiCo2O4, nano-spheres. Ceram. Int. 2017, 43, 8585–8589. [Google Scholar] [CrossRef]
- Gonzalezdelacruz, V.M.; Pereñiguez, R.; Ternero, F.; Holgado, J.P.; Caballero, A. In situ xas study of synergic effects on Ni–Co/ZrO2 methane reforming catalysts. J. Phys. Chem. C 2012, 116, 2919–2926. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Q.; Wu, R. Effect of Co–Ni ratio on the activity and stability of Co–Ni bimetallic aerogel catalyst for methane Oxy-CO2 reforming. Int. J. Hydrog. Energy 2011, 36, 2128–2136. [Google Scholar] [CrossRef]
- Kang, S.H.; Ryu, J.H.; Kim, J.H.; Prasad, P.S.S.; Bae, J.W.; Cheon, J.Y.; Jun, K.W. ZSM-5 supported cobalt catalyst for the direct production of gasoline range hydrocarbons by fischer–tropsch synthesis. Catal. Lett. 2011, 141, 1464–1471. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Kumar, V.S.; Shasikala, V.; Padmasri, A.H.; Sreedhar, B.; Raju, B.D.; Rao, K.S.R. A highly efficient Cu/MgO catalyst for vapour phase hydrogenation of furfural to furfuryl alcohol. Catal. Commun. 2003, 4, 287–293. [Google Scholar] [CrossRef]

| Catalyst | Conv. (%) | Product Selectivity (%) | |||||
|---|---|---|---|---|---|---|---|
| CPO | CPL | FA | THFA | 1,2-PeD | 1,5-PeD | ||
| 5%Ni-15%Co/H-ZSM5 | 59 | 14 | 23 | 1.7 | 2.4 | 0.4 | 0 |
| 5%Ni-15%Co/HPO | 63 | 18 | 3 | 6.1 | 4.6 | 0 | 0 |
| 5%Ni-15%Co/γ-Al2O3 | 89 | 6.7 | 33 | 13 | 24 | 0 | 0 |
| 5%Ni-15%Co/ZrO2 | 84 | 15 | 3 | 21 | 0 | 0.9 | 0 |
| 5%Ni-15%Co/TiO2 | 100 | 42.1 | 11.5 | 13.2 | 0.4 | 3.4 | 1.4 |
| Entry | Catalyst | Conv. (%) | Product Selectivity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CPO | CPL | FA | THFA | 1,2-PeD | 1,5-PeD | Others | |||
| 1 | TiO2 | 23.5 | 0 | 0 | 0.36 | 0.68 | 0 | 0 | 98.9 |
| 2 | 20%Co/TiO2 a | 100 | 1.0 | 45.4 | 10.6 | 2.7 | 0.4 | 0.6 | 39.3 |
| 3 | 5%Ni-15%Co/TiO2 a | 100 | 42.1 | 11.5 | 13.2 | 0.4 | 3.4 | 1.4 | 28 |
| 4 | 10%Ni-10% Co/TiO2 a | 100 | 53.3 | 16.3 | 1.6 | 2.3 | 5.3 | 2.2 | 19 |
| 5 | 15%Ni-5%Co/TiO2 a | 100 | 49.6 | 15.2 | 1.2 | 1.7 | 4.3 | 1.8 | 26.2 |
| 6 | 20%Ni/TiO2 a | 100 | 12.2 | 39.4 | 0.5 | 4.5 | 2 | 0 | 41.4 |
| 7 | 10%Co-10%Ni/TiO2-A b | 100 | 0 | 0.2 | 0.3 | 71.1 | 0.3 | 0.5 | 27.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Guo, X.; Liu, D.; Mu, X.; Chen, X.; Shi, Y. Selective Conversion of Furfural to Cyclopentanone or Cyclopentanol Using Co-Ni Catalyst in Water. Catalysts 2018, 8, 193. https://doi.org/10.3390/catal8050193
Li Y, Guo X, Liu D, Mu X, Chen X, Shi Y. Selective Conversion of Furfural to Cyclopentanone or Cyclopentanol Using Co-Ni Catalyst in Water. Catalysts. 2018; 8(5):193. https://doi.org/10.3390/catal8050193
Chicago/Turabian StyleLi, Yaru, Xingcui Guo, Daosheng Liu, Xindong Mu, Xiufang Chen, and Yan Shi. 2018. "Selective Conversion of Furfural to Cyclopentanone or Cyclopentanol Using Co-Ni Catalyst in Water" Catalysts 8, no. 5: 193. https://doi.org/10.3390/catal8050193
APA StyleLi, Y., Guo, X., Liu, D., Mu, X., Chen, X., & Shi, Y. (2018). Selective Conversion of Furfural to Cyclopentanone or Cyclopentanol Using Co-Ni Catalyst in Water. Catalysts, 8(5), 193. https://doi.org/10.3390/catal8050193






