The Activation of Methane on Ru, Rh, and Pd Decorated Carbon Nanotube and Boron Nitride Nanotube: A DFT Study
Abstract
:1. Introduction
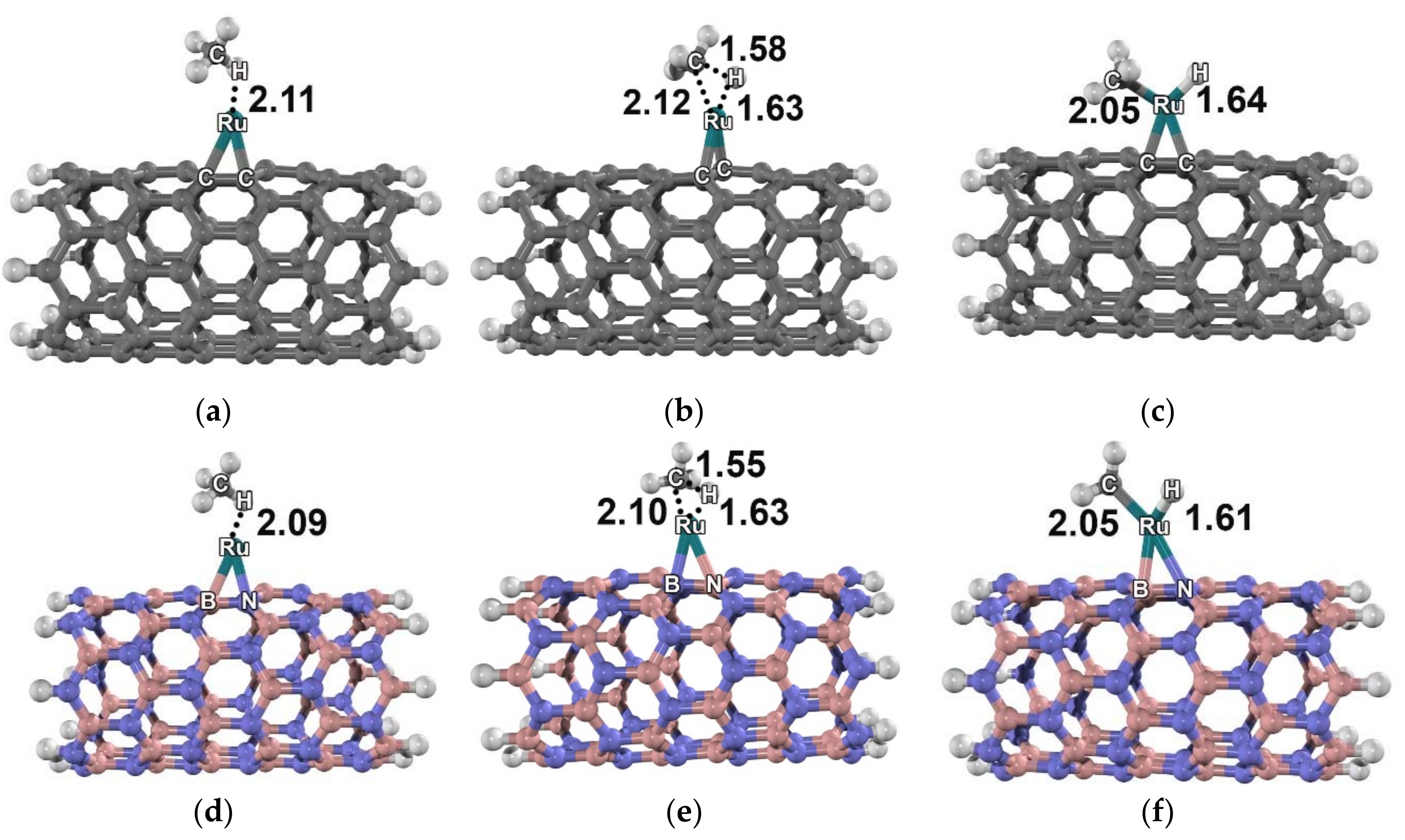
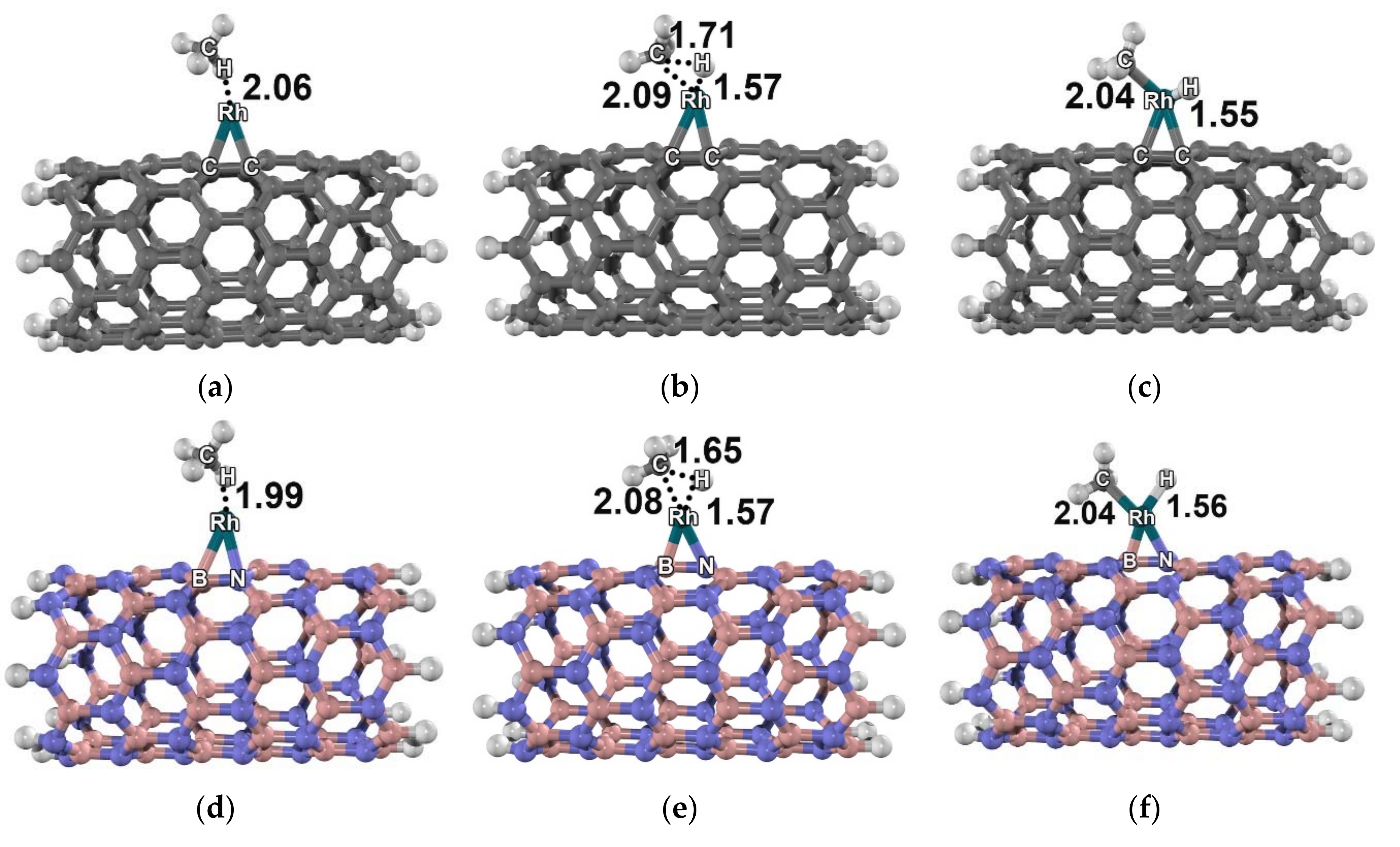
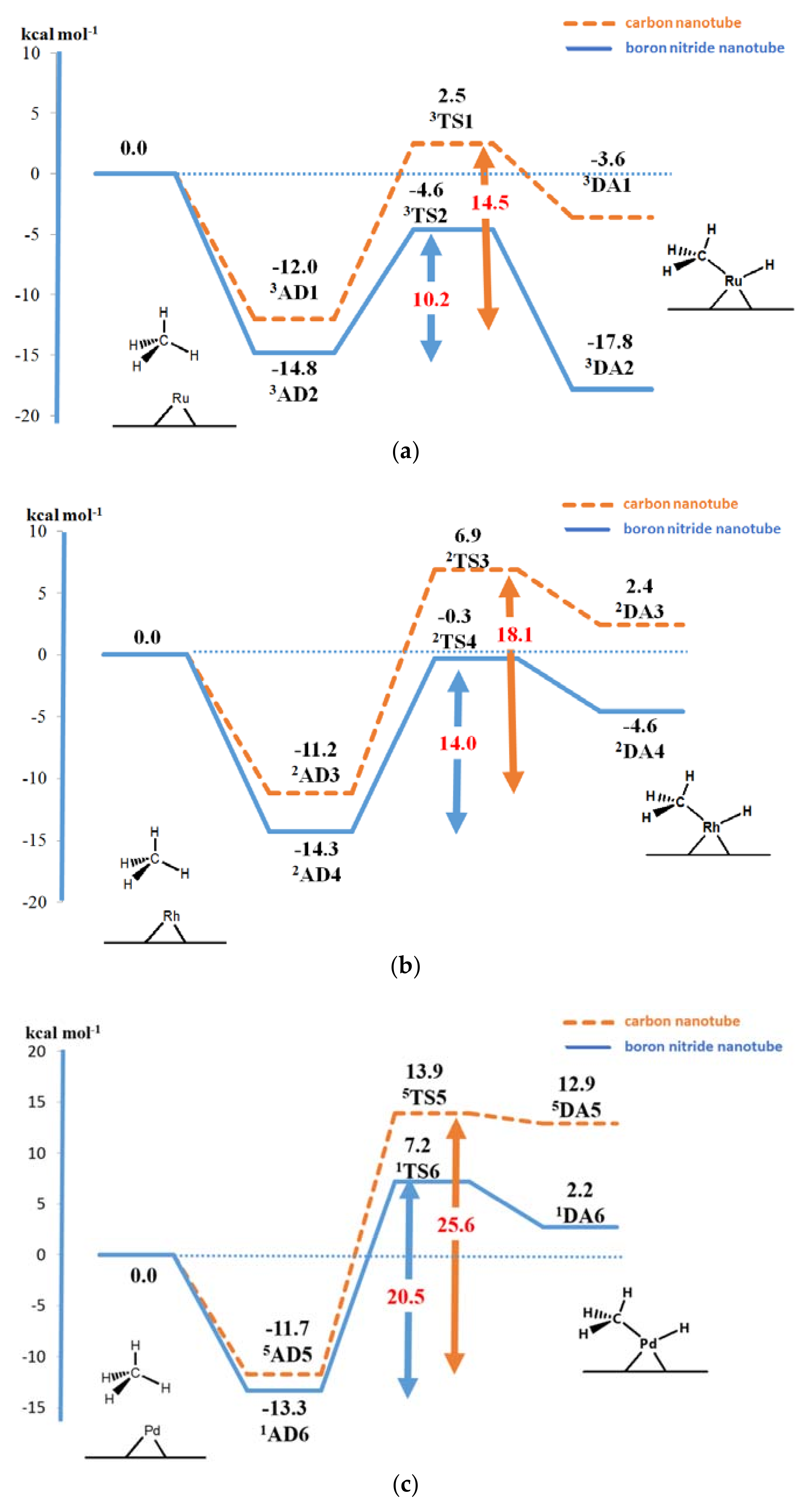
2. Results
3. Models and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hammond, C.; Conrad, S.; Hermans, I. Oxidative methane upgrading. ChemSusChem 2012, 5, 1668–1686. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H. Chemistry with methane: Concepts rather than recipes. Angew. Chem. Int. Ed. Engl. 2011, 50, 10096–10115. [Google Scholar] [CrossRef] [PubMed]
- Wannakao, S.; Warakulwit, C.; Kongpatpanich, K.; Probst, M.; Limtrakul, J. Methane activation in gold cation-exchanged zeolites: A DFT study. ACS Catal. 2012, 2, 986–992. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Mota, N.; Ojeda, M.; Rojas, S.; Navarro, R.M.; Fierro, J.L.G. Direct methane conversion routes to chemicals and fuels. Catal. Today 2011, 171, 15–23. [Google Scholar] [CrossRef]
- Schwarz, H. Activation of Methane. Angew. Chem. Int. Ed. Engl. 1991, 30, 820–821. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’pin, G.B. Activation of C-H bonds by metal complexes. Chem. Rev. 1997, 97, 2879–2932. [Google Scholar] [CrossRef] [PubMed]
- Impeng, S.; Khongpracha, P.; Warakulwit, C.; Jansang, B.; Sirijaraensre, J.; Ehara, M.; Limtrakul, J. Direct oxidation of methane to methanol on Fe-O modified graphene. RSC Adv. 2014, 4, 12572–12578. [Google Scholar] [CrossRef]
- Panov, G.I.; Sobolev, V.I.; Dubkov, K.A.; Parmon, V.N.; Ovanesyan, N.S.; Shilov, A.E.; Shteinman, A.A. Iron complexes in zeolites as a new model of methane monooxygenase. React. Kinet. Catal. Lett. 1997, 61, 251–258. [Google Scholar] [CrossRef]
- Pantu, P.; Pabchanda, S.; Limtrakul, J. Theoretical investigation of the selective oxidation of methane to methanol on nanostructured Fe-ZSM-5 by the ONIOM method. ChemPhysChem 2004, 5, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, H.; Wu, T. Approaching and bond breaking energies in the C-H activation and their application in catalyst design. J. Phys. Chem. A 2011, 115, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Latimer, A.A.; Aljama, H.; Kakekhani, A.; Yoo, J.S.; Kulkarni, A.; Tsai, C.; Garcia-Melchor, M.; Abild-Pedersen, F.; Nørskov, J.K. Mechanistic insights into heterogeneous methane activation. Phys. Chem. Chem. Phys. 2017, 19, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon nanotubes—The route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Banks, C.E.; Compton, R.G. Metal nanoparticles and related materials supported on Carbon nanotubes: Methods and applications. Small 2006, 2, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, J.; Misewich, J.A.; Wong, S.S. Carbon nanotube-nanocrystal heterostructures. Chem. Soc. Rev. 2009, 38, 1076–1098. [Google Scholar] [CrossRef] [PubMed]
- Planeix, J.M.; Coustel, N.; Coq, B.; Brotons, V.; Kumbhar, P.S.; Dutartre, R.; Geneste, P.; Bernier, P.; Ajayan, P.M. Application of Carbon Nanotubes as Supports in Heterogeneous Catalysis. J. Am. Chem. Soc. 1994, 116, 7935–7936. [Google Scholar] [CrossRef]
- Ye, X.R.; Lin, Y.; Wang, C.; Engelhard, M.H.; Wang, Y.; Wai, C.M. Supercritical fluid synthesis and characterization of catalytic metal nanoparticles on carbon nanotubes. J. Mater. Chem. 2004, 14, 908–913. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, H.; Liew, K.M. The structures and electrical transport properties of germanium nanowires encapsulated in carbon nanotubes. J. Appl. Phys. 2007, 102, 073709. [Google Scholar] [CrossRef]
- Leghrib, R.; Dufour, T.; Demoisson, F.; Claessens, N.; Reniers, F.; Llobet, E. Gas sensing properties of multiwall carbon nanotubes decorated with rhodium nanoparticles. Sens. Actuator B-Chem. 2011, 160, 974–980. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Chen, Y.; Yang, M. A multi-walled carbon nanotube/palladium nanocomposite prepared by a facile method for the detection of methane at room temperature. Sens. Actuator B-Chem. 2008, 132, 155–158. [Google Scholar] [CrossRef]
- Star, A.; Joshi, V.; Skarupo, S.; Thomas, D.; Gabriel, J.C.P. Gas sensor array based on metal-decorated carbon nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [Google Scholar] [CrossRef] [PubMed]
- Vermisoglou, E.C.; Romanos, G.E.; Karanikolos, G.N.; Kanellopoulos, N.K. Catalytic NOx removal by single-wall carbon nanotube-supported Rh nanoparticles. J. Hazard. Mater. 2011, 194, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Q.; Li, J.; Ding, F. The transition metal surface dependent methane decomposition in graphene chemical vapor deposition growth. Nanoscale 2017, 9, 11584–11589. [Google Scholar] [CrossRef] [PubMed]
- Bunnik, B.S.; Kramer, G.J. Energetics of methane dissociative adsorption on Rh{111} from DFT calculations. J. Catal. 2006, 242, 309–318. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Li, Y.; Zhang, X. Oxygen evolution reaction in nanoconfined carbon nanotubes. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 99, 1–5. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, H. A first-principles study on the interaction of biogas with noble metal (Rh, Pt, Pd) decorated nitrogen doped graphene as a gas sensor: A DFT study. Appl. Surf. Sci. 2018, 435, 1199–1212. [Google Scholar] [CrossRef]
- Luna, C.R.; Verdinelli, V.; Germán, E.; Seitz, H.; Volpe, M.A.; Pistonesi, C.; Jasen, P.V. Hydrogen adsorption and associated electronic and magnetic properties of Rh-decorated (8,0) carbon nanotubes using Density Functional Theory. J. Phys. Chem. C 2015, 119, 13238–13247. [Google Scholar] [CrossRef]
- Chopra, N.G.; Luyken, R.J.; Cherrey, K.; Crespi, V.H.; Cohen, M.L.; Louie, S.G.; Zettl, A. Boron nitride nanotubes. Science 1995, 269, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Ritleng, V.; Sirlin, C.; Pfeffer, M. Ru-, Rh-, and Pd-catalyzed C-C bond formation involving C-H activation and addition on unsaturated substrates: Reactions and mechanistic aspects. Chem. Rev. 2002, 102, 1731–1769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Robertson, D.H.; Brenner, D.W.; Mintmire, J.W. Energetics of nanoscale graphitic tubules. Phys. Rev. B 1992, 45, 12592–12595. [Google Scholar] [CrossRef]
- Carstens, J.N.; Bell, A.T. Methane activation and conversion to higher hydrocarbons on supported ruthenium. J. Catal. 1996, 161, 423–429. [Google Scholar] [CrossRef]
- Stewart, C.N.; Ehrlich, G. Dynamics of activated chemisorption: Methane on rhodium. J. Chem. Phys. 1975, 62, 4672–4682. [Google Scholar] [CrossRef]
- Boekfa, B.; Pahl, E.; Gaston, N.; Sakurai, H.; Limtrakul, J.; Ehara, M. C-Cl bond activation on Au/Pd bimetallic nanocatalysts studied by density functional theory and genetic algorithm calculations. J. Phys. Chem. C 2014, 118, 22188–22196. [Google Scholar] [CrossRef]
- Boekfa, B.; Pantu, P.; Probst, M.; Limtrakul, J. Adsorption and tautomerization reaction of acetone on acidic zeolites: The confinement effect in different types of zeolites. J. Phys. Chem. C 2010, 114, 15061–15067. [Google Scholar] [CrossRef]
- Pornsatitworakul, S.; Boekfa, B.; Maihom, T.; Treesukol, P.; Namuangruk, S.; Jarussophon, S.; Jarussophon, N.; Limtrakul, J. The coumarin synthesis: A combined experimental and theoretical study. Monatsh. Chem. 2017, 148, 1245–1250. [Google Scholar] [CrossRef]
- Maitarad, P.; Namuangruk, S.; Zhang, D.; Shi, L.; Li, H.; Huang, L.; Boekfa, B.; Ehara, M. Metal-porphyrin: A potential catalyst for direct decomposition of N2O by theoretical reaction mechanism investigation. Environ. Sci. Technol. 2014, 48, 7101–7110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Size-selective supramolecular chemistry in a hydrocarbon nanoring. J. Am. Chem. Soc. 2007, 129, 8440–8442. [Google Scholar] [CrossRef] [PubMed]
- Bergner, A.; Dolg, M.; Küchle, W.; Stoll, H.; Preuß, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Gonzalez, C.; Bernhard Schlegel, H. An improved algorithm for reaction path following. J. Chem Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boekfa, B.; Treesukol, P.; Injongkol, Y.; Maihom, T.; Maitarad, P.; Limtrakul, J. The Activation of Methane on Ru, Rh, and Pd Decorated Carbon Nanotube and Boron Nitride Nanotube: A DFT Study. Catalysts 2018, 8, 190. https://doi.org/10.3390/catal8050190
Boekfa B, Treesukol P, Injongkol Y, Maihom T, Maitarad P, Limtrakul J. The Activation of Methane on Ru, Rh, and Pd Decorated Carbon Nanotube and Boron Nitride Nanotube: A DFT Study. Catalysts. 2018; 8(5):190. https://doi.org/10.3390/catal8050190
Chicago/Turabian StyleBoekfa, Bundet, Piti Treesukol, Yuwanda Injongkol, Thana Maihom, Phornphimon Maitarad, and Jumras Limtrakul. 2018. "The Activation of Methane on Ru, Rh, and Pd Decorated Carbon Nanotube and Boron Nitride Nanotube: A DFT Study" Catalysts 8, no. 5: 190. https://doi.org/10.3390/catal8050190
APA StyleBoekfa, B., Treesukol, P., Injongkol, Y., Maihom, T., Maitarad, P., & Limtrakul, J. (2018). The Activation of Methane on Ru, Rh, and Pd Decorated Carbon Nanotube and Boron Nitride Nanotube: A DFT Study. Catalysts, 8(5), 190. https://doi.org/10.3390/catal8050190




