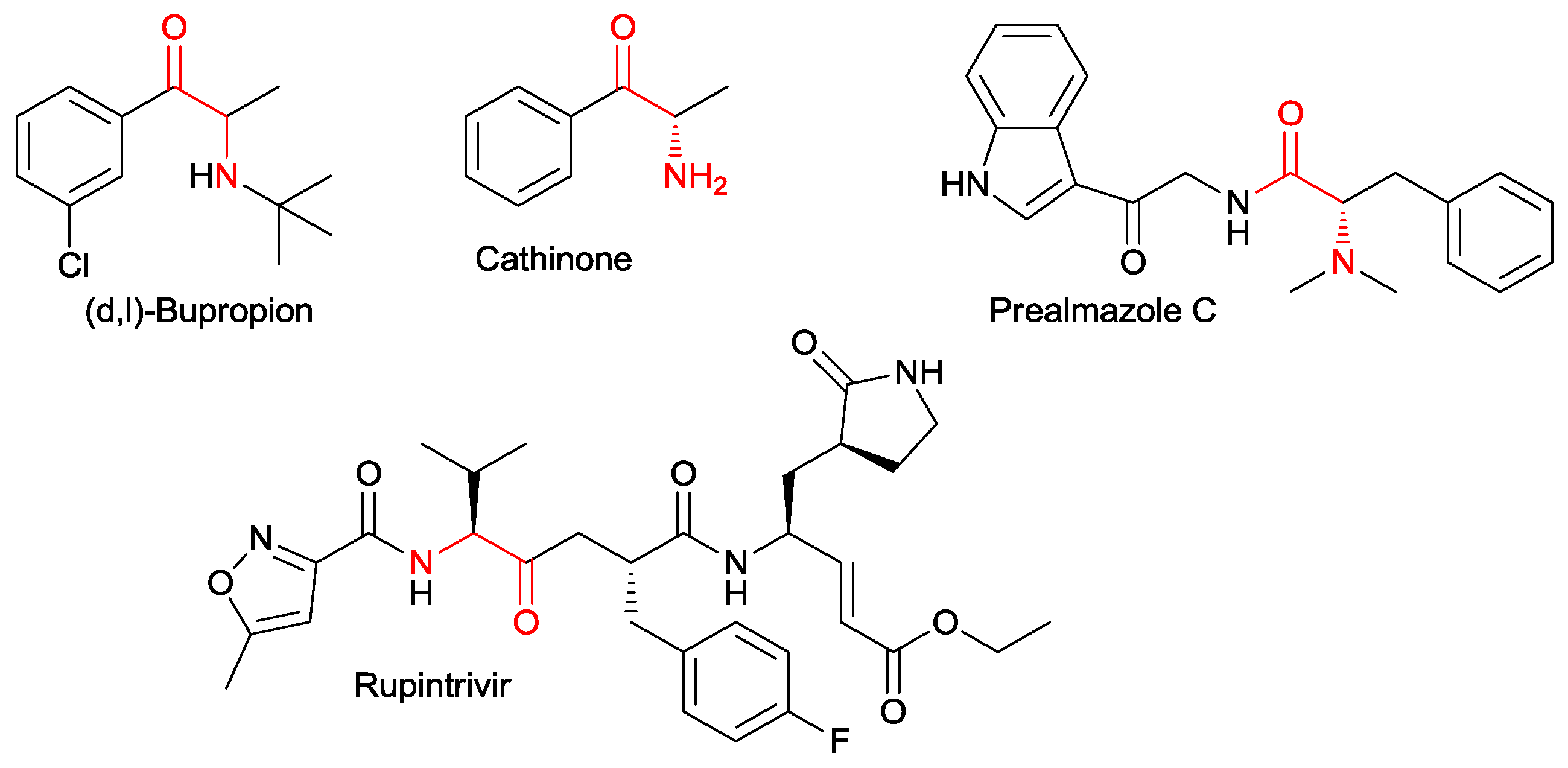
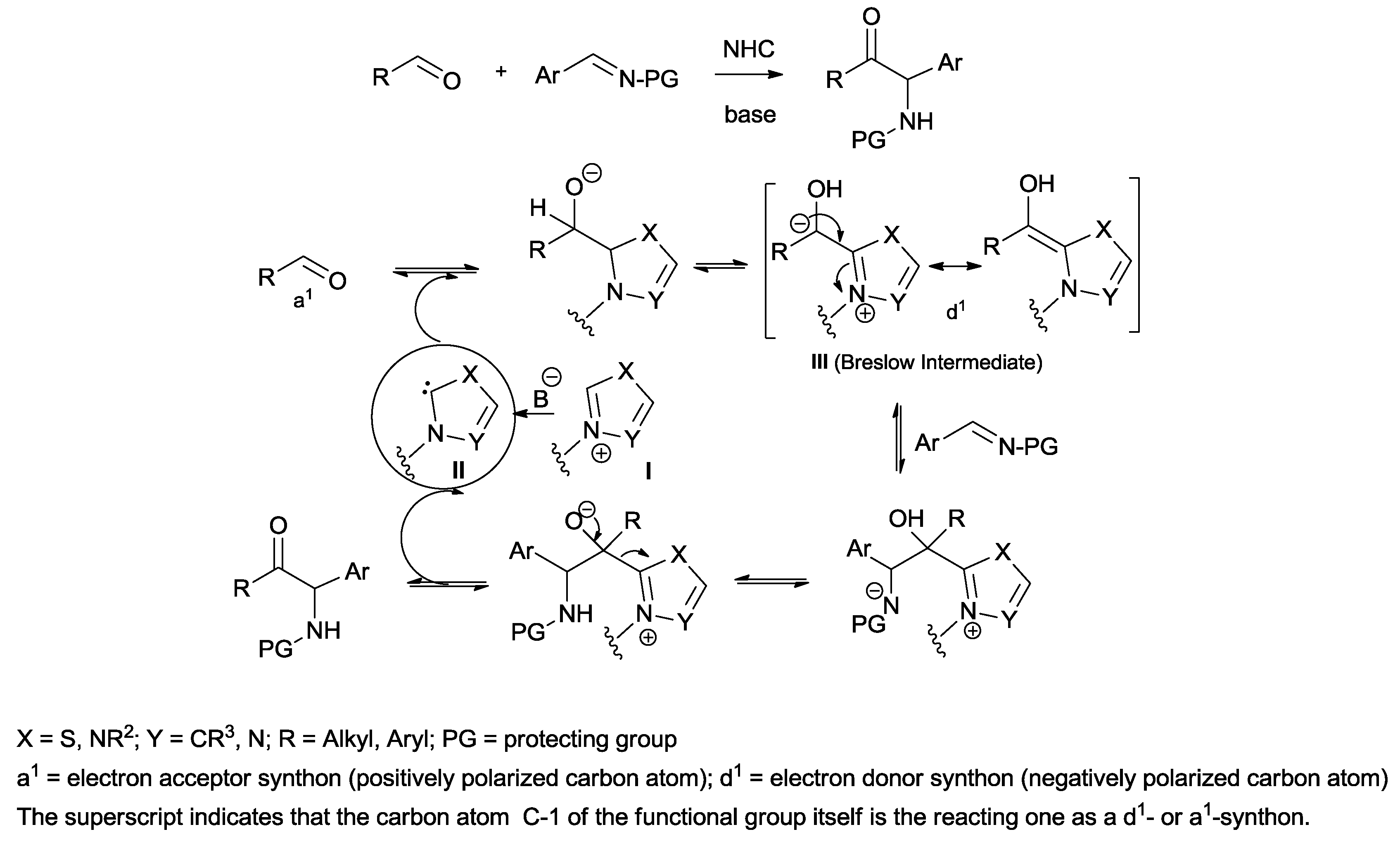
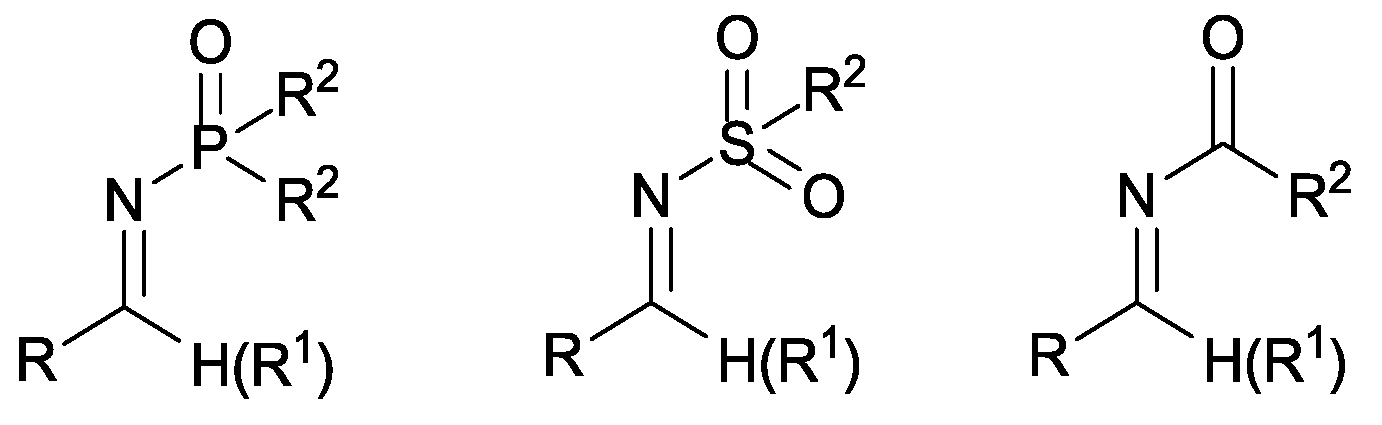
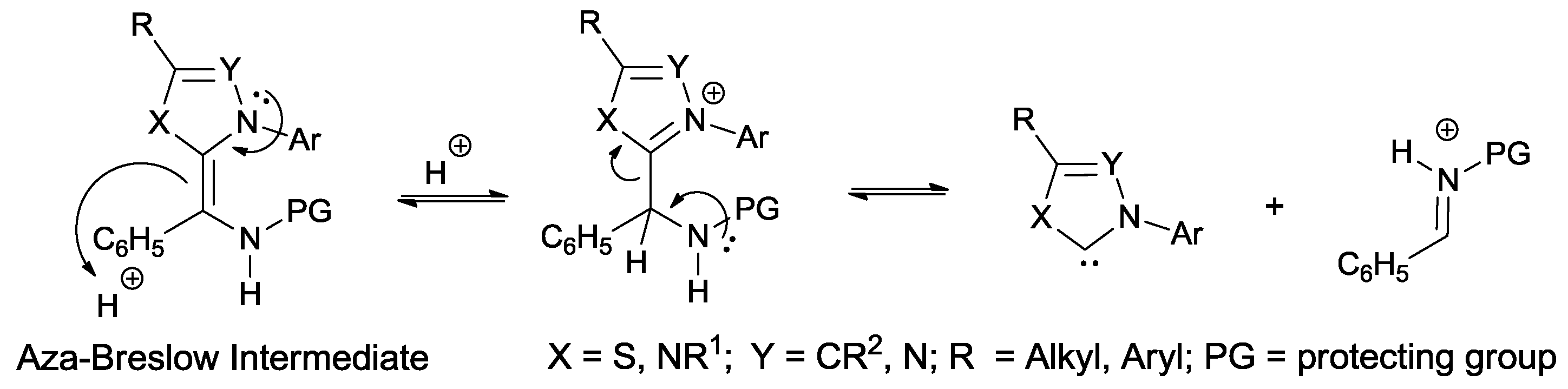
1. Introduction
α-Amino ketones are widespread structural moieties, common to both natural and synthetic significant compounds in medicinal chemistry (
Figure 1) [
1,
2,
3,
4].
They are largely employed as building blocks in the preparation of a large number of molecules, in particular, 1,2-aminoalcohols, and vicinal diamines (
Scheme 1). They are important motifs in many pharmaceutical compounds and are widely applied as chiral auxiliaries and ligands in the field of asymmetric synthesis [
5,
6]. Moreover, they are precursors in the preparation of many heterocycles [
7,
8,
9,
10,
11] and smoothly undergo nucleophilic addition reactions to give a variety of derivatives [
12].
Numerous synthetic routes to α-amino ketones have been reported in the literature. However, these methods involve multistep transformations starting from functionalized reactants such as α-azido ketones [
13], α-nitro ketones [
14] or α-amino acids [
12,
15].
The aza-benzoin condensation reaction, strictly related to the well-known benzoin condensation reaction, represents the more straightforward approach to α-amino ketones and occurs with an atom economy of 100%.
In its most general form, the aza-benzoin condensation reaction, first reported in 1988 [
16], is a
N-heterocyclic carbene (NHC)-catalyzed coupling between an aldehyde and an activated imine (
Scheme 2). The mechanism of this process, in analogy with the benzoin condensation, envisages the formation of a nucleophilic NHC
II from azolium salt
I, under basic conditions. Its addition to aldehyde followed by proton transfer generates an acyl anion equivalent
III known as a Breslow-Intermediate, thus causing a reversal of the original electrophilic carbonyl reactivity, universally known as umpolung (dipole inversion). The acyl anion equivalent can be stabilized by π-back-donation of the carbanion onto the empty p
z orbital of the carbene atom, giving rise to a hydroxy-enamine-type Breslow Intermediate (
Scheme 2).
The nucleophilic attack of a Breslow Intermediate on the electrophilic imine, a second proton transfer step and subsequent elimination furnish the condensation product, at the same time regenerating the catalyst. The wide choice of chiral azolium salts reported in the literature allows access to the asymmetric version of the reaction, affording enantio-enriched α-amino ketones [
17]. In addition to NHCs, bis(amino)-cyclopropenylidenes (BACs) have also been successfully applied as umpolung-promoting species [
18].
Recently, increased attention has been given to the nature of nucleophilic partners, including acylsilanes as acyl donors.
The scope of this review article is to provide an overview of the advances in chemoselective aza-benzoin condensation reactions, covering methods involving both racemic and enantiomerically-enriched α-amino ketones. The synthesis of more complex molecules via tandem reactions which involve an aza-benzoin coupling step is also described [
19].
Finally, the synthesis of a selected pharmaceutical candidate which employs aza-benzoin condensation as the key reaction of the process is considered.
2. Chemoselective Aza-Benzoin Condensation Reactions
The major issue regarding successful execution of the aza-benzoin condensation reaction is the requirement that the whole process should evolve under kinetical control, in which imines are more reactive towards Breslow Intermediates than to a second molecule of aldehyde, but less reactive with the NHC catalyst compared to aldehyde.
Activated imines (
Figure 2) are often employed in organic chemistry as equivalents of carbonyl compounds in reactions with a wide array of nucleophilic reagents [
20,
21,
22].
Compared to the controlled cross-acyloin reaction between two different aldehydes [
23], the use of imines as acyl anion acceptors is advantageous due to the difference in electrophilicity between aldehydes and imines and to the possibility of further fine adjustment to the reactivity of imines because of the trivalency of nitrogen. The choice of the imine-protecting group is critical. In fact, the addition of carbene to activated
N-tosyl and
N-phosphinoyl imines gives stable nitrogen analogues of Breslow Intermediate that can stop the catalytic cycle [
24]. However, recent studies have shown that the carbene/iminium ion pair undergoes a fast dissociation-recombination process in the presence of an acid catalyst (
Scheme 3) [
25,
26].
Arylsulfonylamides and tert-butyl- or benzyl aryl(tosyl) carbamates have been widely employed as imine precursors thanks to their simple preparation, high stability and the aptitude to provide, in situ, reactive imines under mild conditions. Moreover, the introduction of alkoxycarbonyl functionalities (e.g., tert-butoxycarbonyl (Boc) or carboxybenzyl (Cbz)) as activating groups allows the final amino derivatives which can be easily deprotected to be obtained.
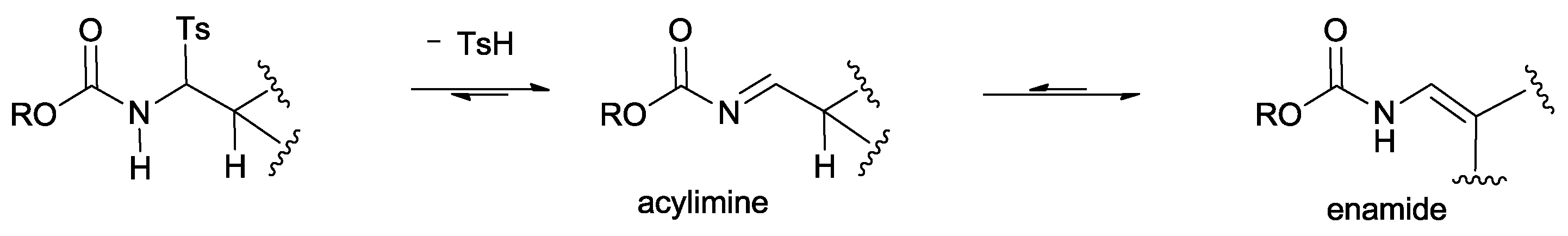
The substrate scope is presently limited to aldimines derived from aryl or heteroaryl aldehydes. Conversely, imines obtained from aliphatic aldehydes undergo decomposition or tautomerization to the more stable enamide derivatives, and their use has not yet been realized (
Scheme 4).
On the other hand, ketimines are of particular interest because they serve as precursors of tetrasubstituted carbon atoms. However, their utilization has proved to be more challenging due to their poor reactivity.
3. Methods to Produce Racemic α-Amino Ketones
3.1. Use of N-heterocyclic Carbenes
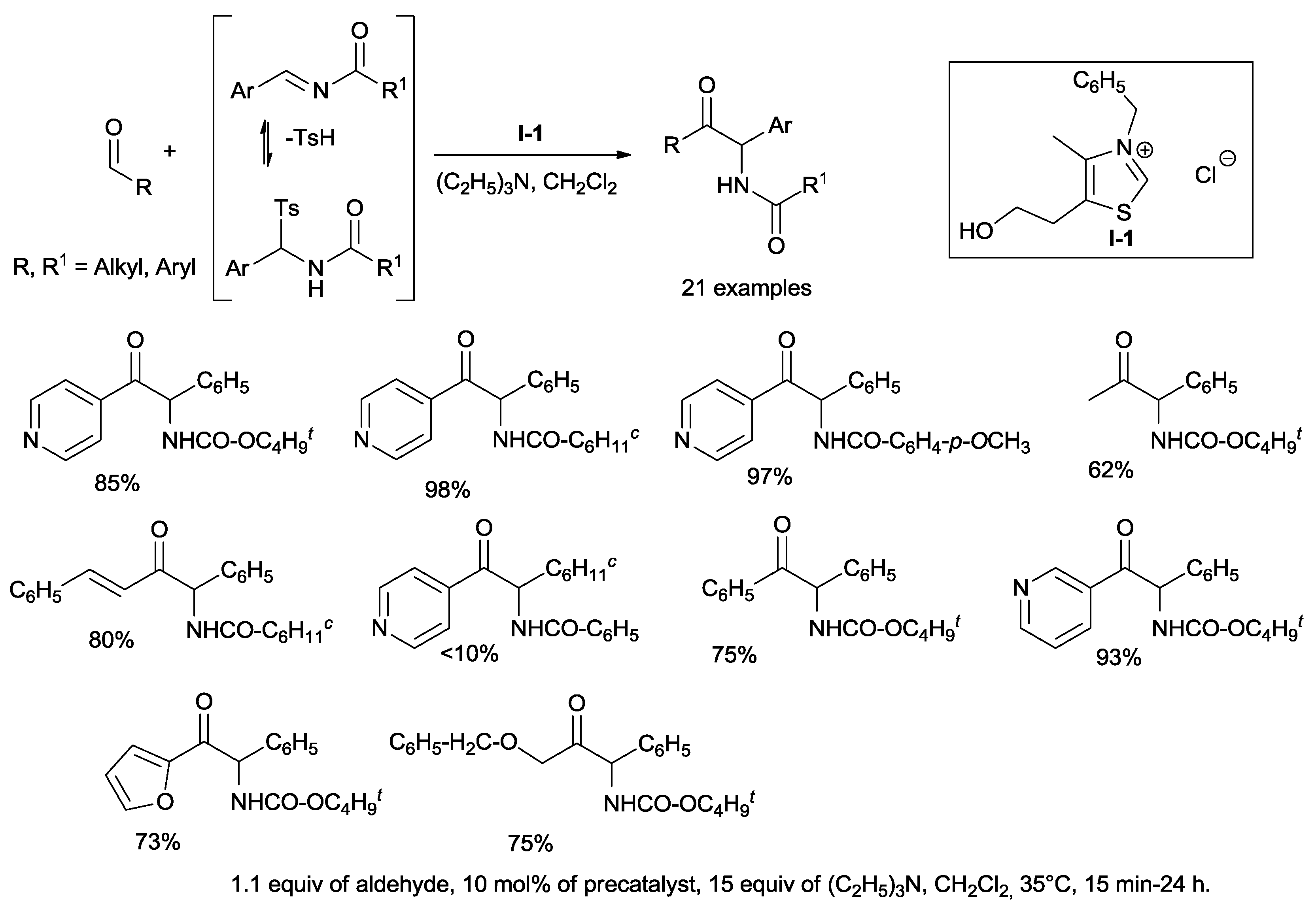
Murry and co-workers envisioned the coupling of
N-benzylidene cyclohexanecarboxamide, slowly generated in situ by elimination of sulfinic acid from the parent α-amido sulfone, with 4-pyridinecarboxaldehyde in the presence of commercially available thiazolium salt
I-1 and triethylamine (
Scheme 5). Under these conditions, the corresponding amino ketone was obtained with a yield of 98% [
27].
The reaction displays a wide scope with respect to the aldehyde. It is noteworthy that α,β-unsaturated cinnamaldehyde, under these reaction conditions, does not undergo 1,4-addition and also aliphatic acetaldehyde reacts although with moderate yield (
Scheme 5).
The process is also tolerant in regard to the amide portion of the tosylamide. However, tosylamides derived from aliphatic aldehydes bearing an α-proton fail to generate the corresponding acylimines, likely due to the aptitude of these compounds to isomerize to enamides.
Crossover experiments have highlighted that this reaction is under kinetic control and that the corresponding benzoins are not observed and do not serve as substrates.
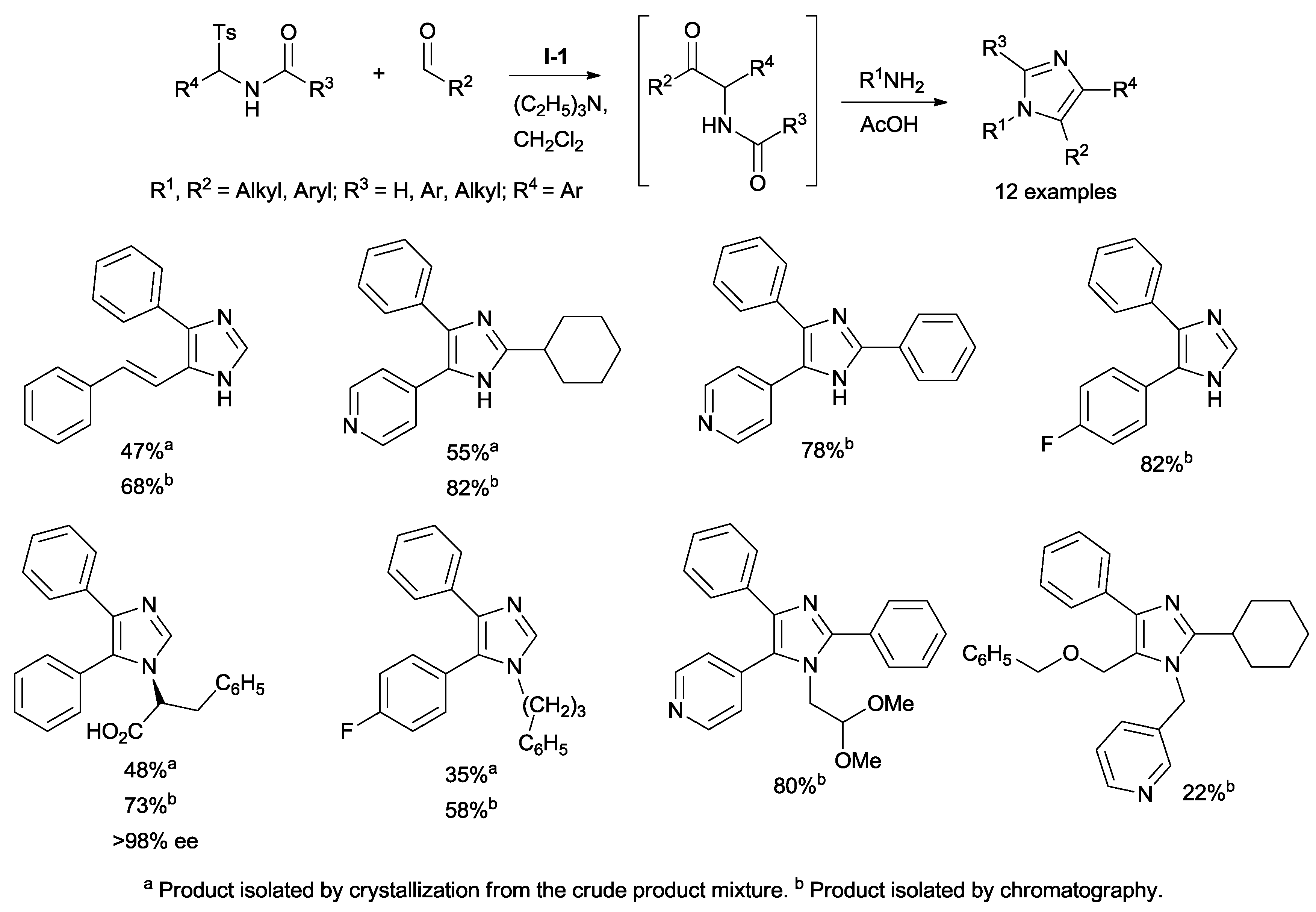
Subsequently, Murry disclosed the application of his methodology to a novel one-pot synthesis of highly-functionalized imidazoles, an important class of heterocycles widespread in natural products and in medicinal chemistry (
Scheme 6).
The addition of an appropriate amine and acetic acid to the reaction mixture of the α-amino ketone intermediate followed by heating to reflux allowed the ring to close, forming imidazole. Moreover, chiral imidazoles can be prepared starting from chiral amines or amino acids. It is noteworthy that tetra-substituted imidazoles, difficult to obtain by other routes, can be synthesized with moderate to good yields using this methodology. This approach also allows the production of substituted oxazoles and thiazoles with good yields by replacing the amine with triphenylphosphine/iodine or the Lawesson’s reagent, respectively [
28].
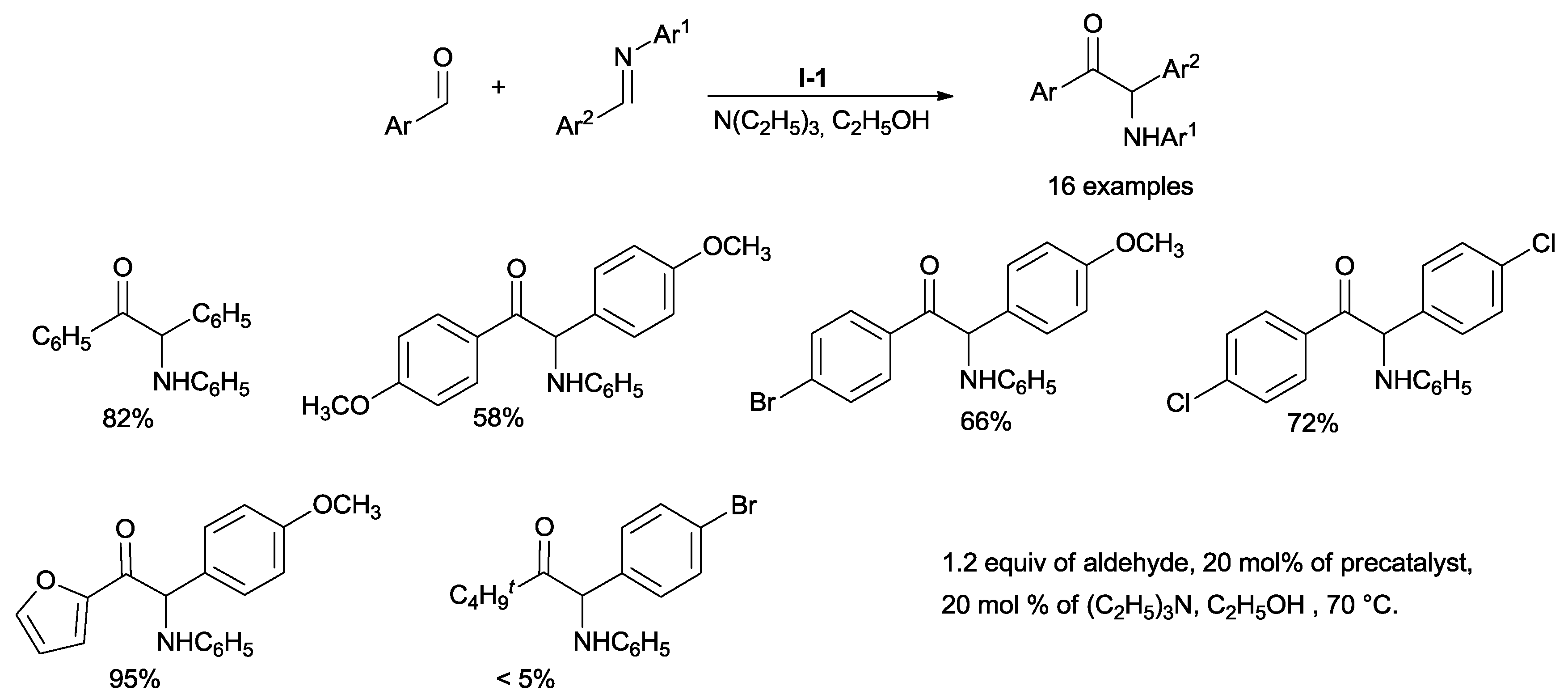
Pseudo-homo-couplings (defined as an aldehyde reacting with an imine derived from the same aldehyde, Ar = Ar
2) and cross-couplings (Ar ≠ Ar
2) under thermodynamic control were developed some years later using unactivated aryl imines, aryl aldehydes, thiazolium salt
I-1 as the precatalyst and triethylamine as the base, during the reflux of ethanol for 48 h (
Scheme 7).
Under these conditions, competing benzoins can reversibly form and behave as substrates for α-aminoketone formation.
Cross experiments have highlighted that α-aminoketone formation is reversible. This protocol allows aza-benzoin coupling using less reactive aryl imines [
29].
Acylsilanes, discovered by Brook in 1957, are considered to be sterically-hindered aldehydes by virtue of the removable silyl group, and undergo smoothly nucleophilic addition reactions [
30]. They have been employed as unconventional donor partners in regioselective intermolecular acyloin condensation in a number of procedures catalyzed by cyanides [
23].
In the benzoin-type condensation reaction, after the nucleophilic attack of the cyanide catalyst on the acylsilane, the mechanism involves a [
1,
2] shift of the migrating SiR
3 group (Brook rearrangement), generating the key stabilized acyl anion equivalent, in analogy with the Breslow catalytic cycle.
The subsequent addition of this species to a competent electrophile, followed by catalyst release, leads to the desired condensation product (
Scheme 8).
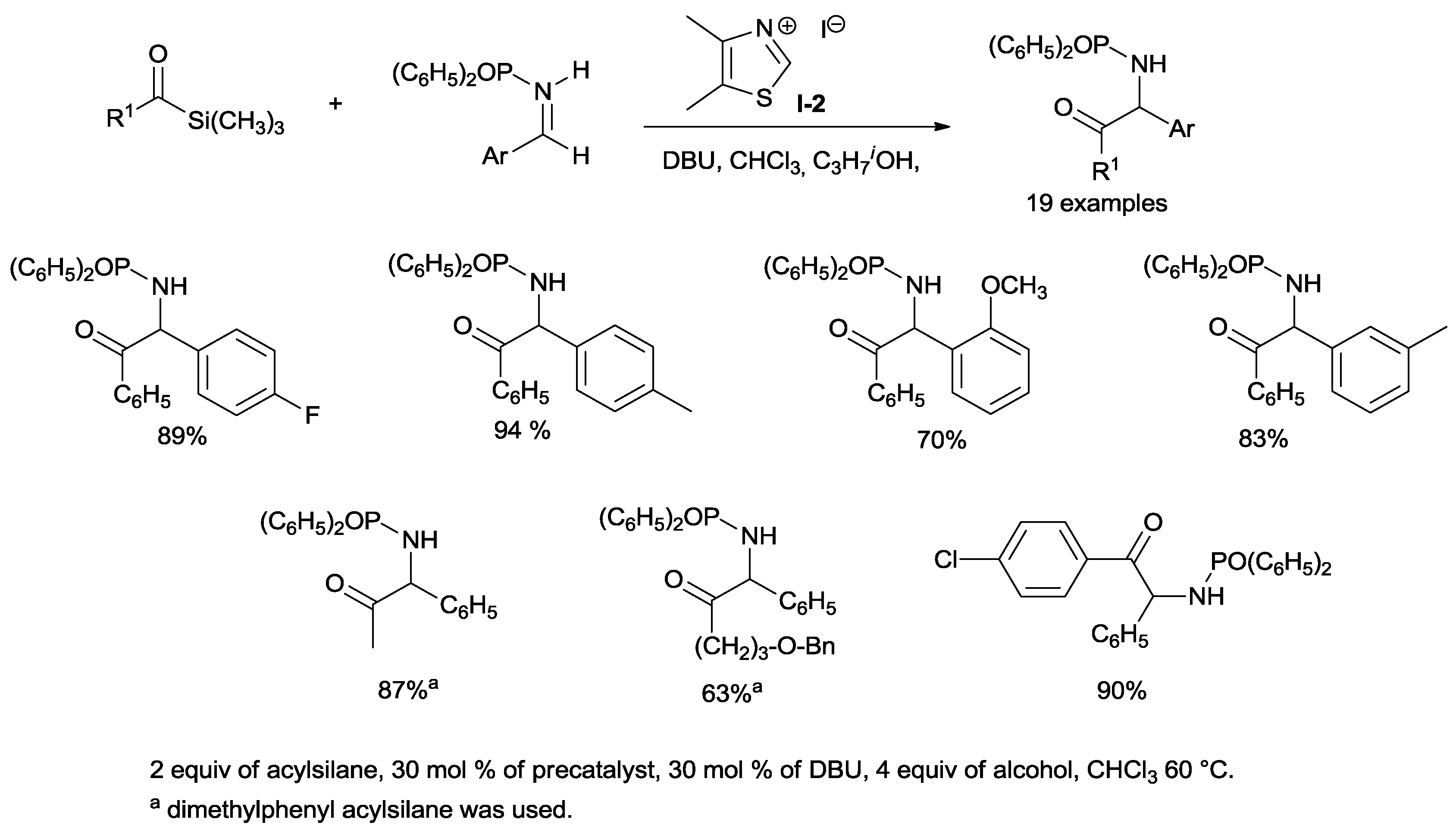
Scheidt disclosed the reaction of alkyl and aryl acylsilanes with aromatic
N-diphenylphosphinoyl imines upon exposure to catalytic
N-methyl 4,5-dimethyl thiazolium salt (
I-2) (30 mol %), using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as the base and a stoichiometric amount of
isopropanol for 48 h. Under these new conditions, the Brook rearrangement occurs smoothly without the need for charged and potentially toxic cyanide, fluoride or phosphite anions [
31].
N-phosphinylated amino ketones, completely devoid of any homo-coupling product contamination have afforded yields of 51–94% (
Scheme 9).
The phosphinoyl group on the nitrogen atom can be removed at the end of the reaction under mild conditions to give α-aminoketones. Alkyl
N-phosphinoyl imines are unsuitable for the reaction since they undergo isomerization to more stable enamides due to the presence of an enolizable proton. On the other hand, the
N-phosphinoyl protecting group is essential for the success of the reaction. In fact, more reactive
N-benzoyl,
N-sulfinyl and
N-sulfonyl imines interact irreversibly with the catalyst, thus stopping the catalytic cycle. NHCs derived from imidazolium or triazolium salts do not allow the desired reaction. The proposed mechanism, illustrated in
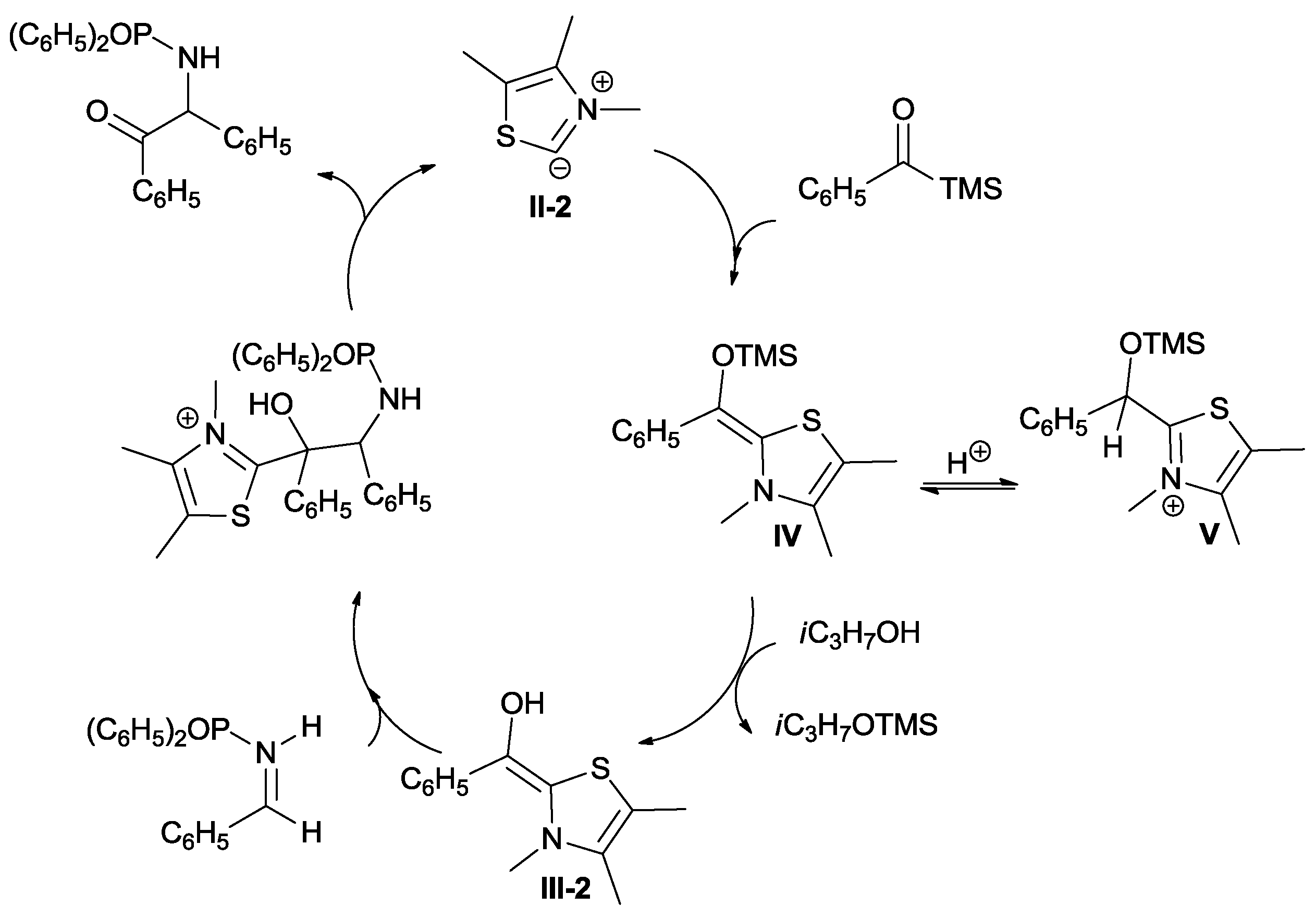
Scheme 10, envisages the addition of carbene
II-2 to acylsilane followed by the formation of intermediate
IV via Brook rearrangement. The reaction of this intermediate with the imine is reversible and thus, unproductive. The subsequent transfer of Si(CH
3)
3 to
isopropanol provides the less congested intermediate
III-2 (Breslow Intermediate) which, after imine addition, allows the production of the protected α-amino ketone and regenerates the catalyst.
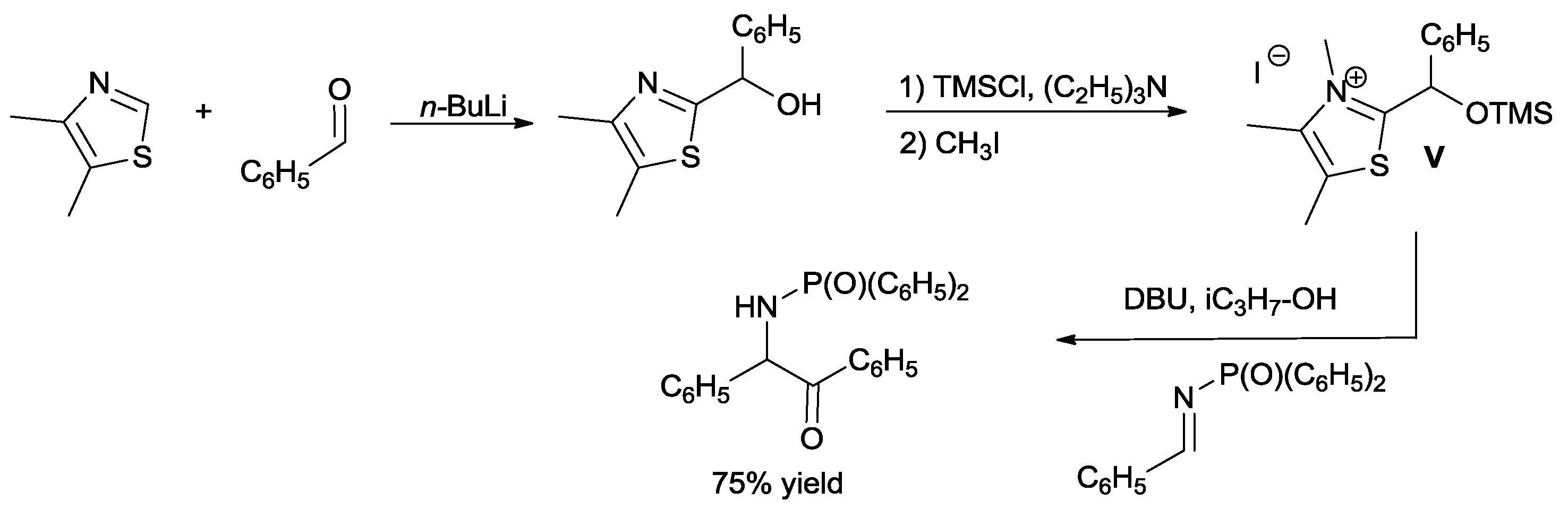
An independent synthesis of intermediate
V and its reaction with
N-(diphenylphosphinyl) benzaldimine in the presence of DBU and
isopropanol to give the desired product provided substantial evidence for the proposed catalytic cycle (
Scheme 11) [
31]. This strategy opened a new route of access, induced by neutral carbenes, for the Brook rearrangement.
Non-enolizable
N-protected aryl trifluoromethyl ketimines have been used as acceptor partners in coupling with a series of highly-reactive furan-2-carbaldehydes to produce the corresponding α-amino-α-trifluoromethyl ketones, bearing a valuable quaternary stereocenter, in moderate to good yields (32–87%) in the presence of triazolium salt
I-3 (
Scheme 12) [
32].
3.2. Use of Bis(Amino)-Cyclopropenylidenes (BACs)
N-heterocyclic carbenes have emerged as powerful, efficient and versatile organocatalysts, which still allow access to new and unexpected organic trasformations. Efforts to develop non five-membered nitrogen-containing heterocyclic carbenes have been rather limited as a consequence of the success of NHCs. However, bis(amino)-cyclopropenylidenes (BACs), the smallest aromatic rings containing a carbene center, have recently been employed in some intriguing applications [
33,
34]. Easily prepared in a one-pot reaction, BACs, likewise NHCs, catalytically induce acyl anion reactivity in aldehydes. Moreover, a significant amount of aldehyde self-condensation side product is often formed during NHC chemistry whereas it is normally absent in umpolung reactions catalyzed by BACs.
The limited ability of BACs to mediate aldehyde couplings, even under ideal conditions, prompted the exploration of their potential in aza-benzoin reactions. After fruitless attempts with Boc and tosyl imines, P,P-diphenyl
N-[(aryl)(tosyl)methyl] phosphinic amides, the more practical surrogates of the corresponding protected imines, gave productive results in the reaction with aromatic aldehydes in the presence of bis(diethylamino)cyclopropenium salt
VI (
Scheme 13) [
18].
The reaction is effective with heteroaromatic, para or meta-substituted benzaldehydes. In some cases, an excess of aldehyde has been necessary to drive the reaction towards the product.
Both electron-poor and electron-rich groups on the para position of the aromatic ring of the acceptor are compatible with the reaction. Although the acidic deprotection of phosphinic amides can be performed under mild conditions, the product has shown in stability as a free base, therefore, it is necessary to reinsert the nitrogen-protecting group.
Until now, attempts to develop an asymmetric version of the reaction using a chiral BAC have not succeeded.
4. Methods to Produce Enantiomerically-Enriched α-Amino Ketones
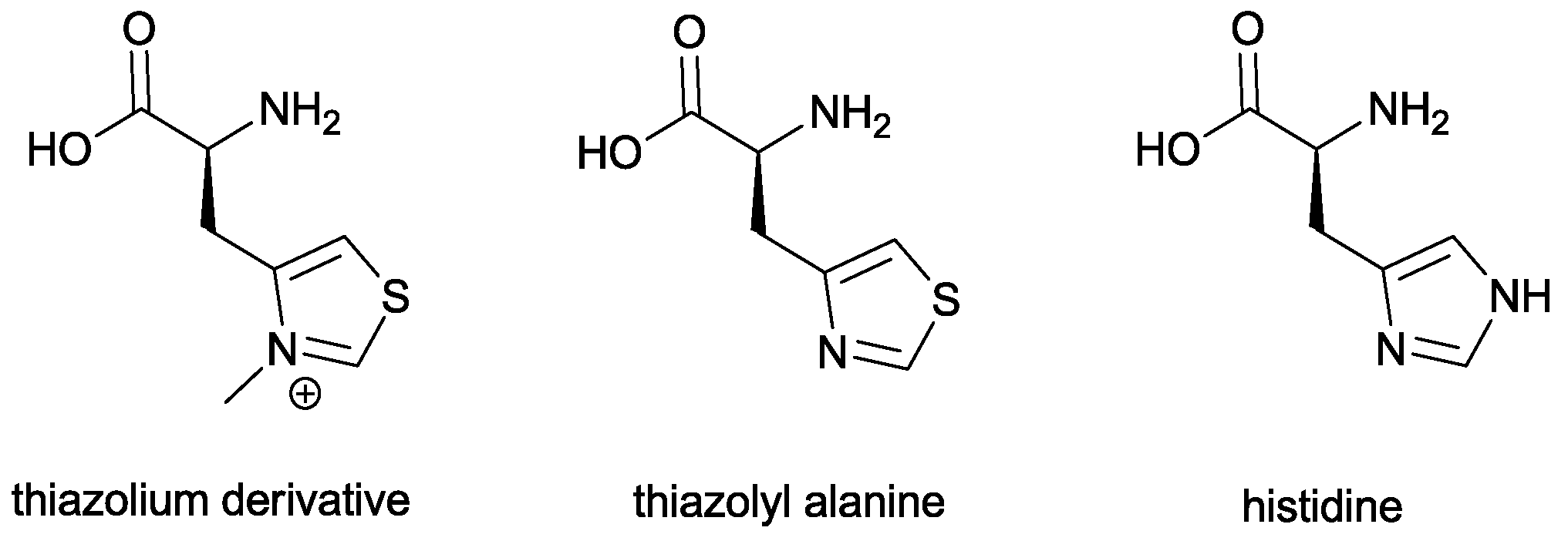
The first example of asymmetric aza-benzoin reaction was given by Miller and co-workers, who used an unconventional chiral thiazolium salt. Ideally, it is derived from histidine by replacing the imidazole ring with the thiazole one (
Figure 3).
In order to ensure the presence of a chiral binding pocket for the reaction partners, thiazolylalanine has been used as the middle amino acid in a tripeptide sequence and is subsequently converted to the corresponding thiazolium salt.
Enantiomerically-enriched α-amino ketones have been obtained by the coupling of aromatic aldehydes with in situ-generated acylimines in the presence of the chiral thiazolylalanine (Taz) containing peptide salt
I-4 (
Scheme 14) [
35].
The reaction product undergoes racemization under basic reaction conditions due to enolization. In order to ensure an excess of enantiomeric is produced, the amount of amine and the reaction time need to be carefully evaluated.
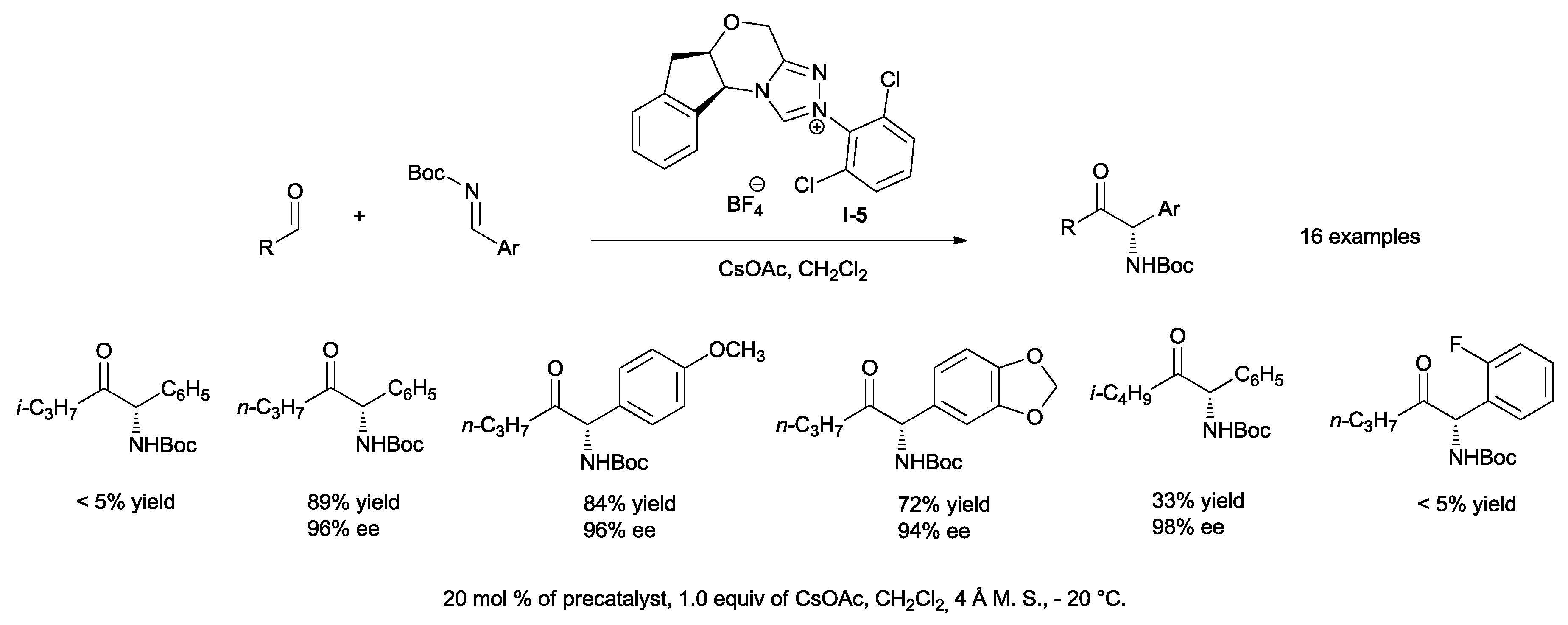
The assumption that a less activated imine would lead to increased stability of the newly-formed stereocenter prompted Rovis to employ
N-Boc-protected imines in the presence of chiral
I-5 and aliphatic aldehydes (
Scheme 15) [
25].
Cesium acetate has been used as a base in order to generate, in situ, the catalytic amount of the acid required for catalyst regeneration. The reactions have been carried out at −20 °C to suppress racemization, and molecular sieves have been added to prevent the hydrolysis of imines due to the igroscopic nature of the salt. Following the optimization of the conditions, the scope of the reaction has been explored. Excellent ees and high yields have been obtained with a variety of straight chain aldehydes. On the other hand, lower yields have been observed when β-branched aliphatic aldehydes such as iso-butyraldehyde have been employed, whereas α-branched aldehydes do not react. Electron-rich and electron-poor Boc-arylimines have been used; however, ortho-fluoro aryl derivatives do not participate in the reaction.
One of the challenges for organic chemists is the ability to highlight different substrate reactivities in a selective manner.
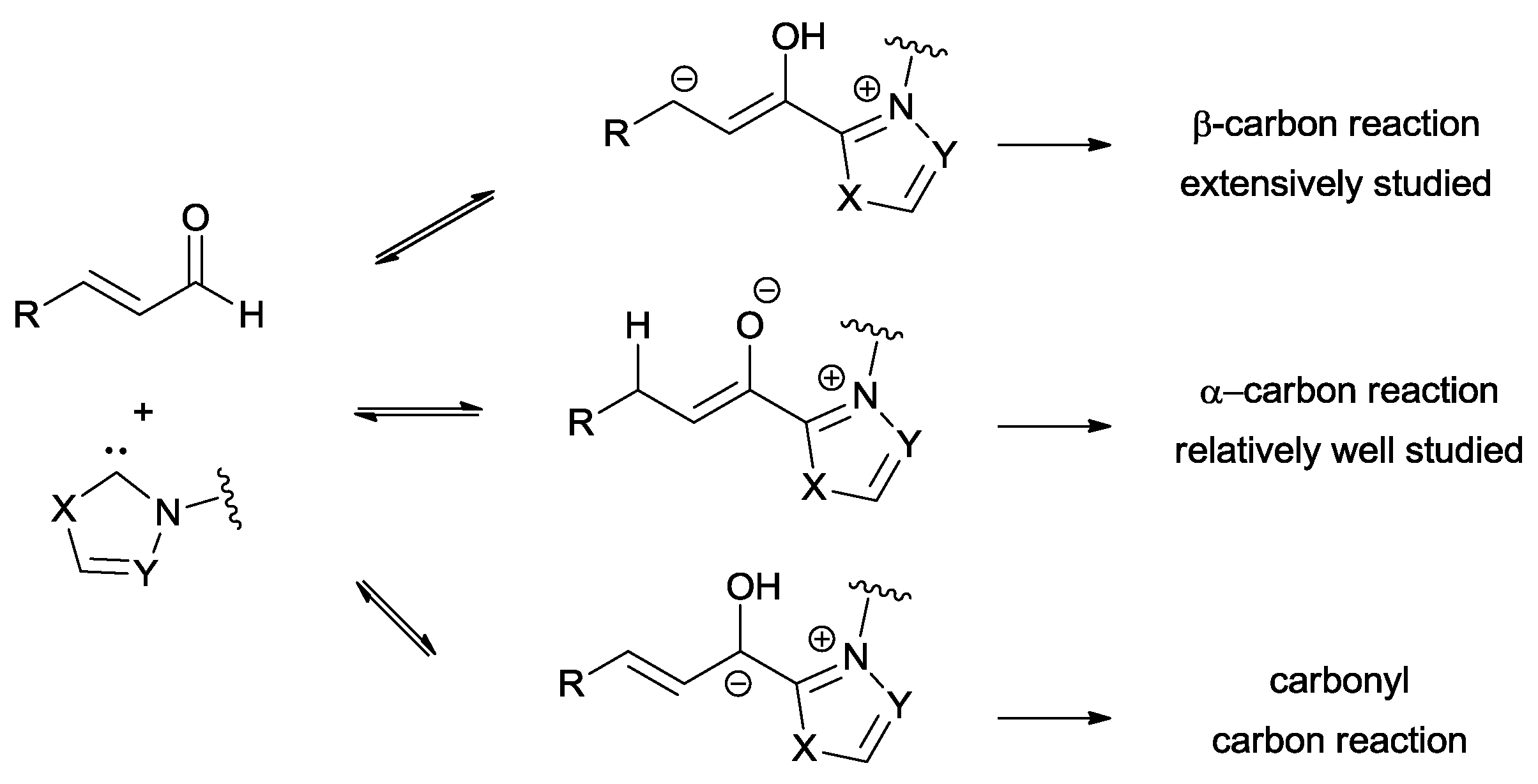
The use of enals in cross-acyloin couplings is an arduous task since homoenolate, enolate and acyl anion equivalent can all be generated by reacting with NHCs through different reaction pathways (
Scheme 16).
The choice of the catalyst is the key factor that controls the chemoselectivity of these three species.
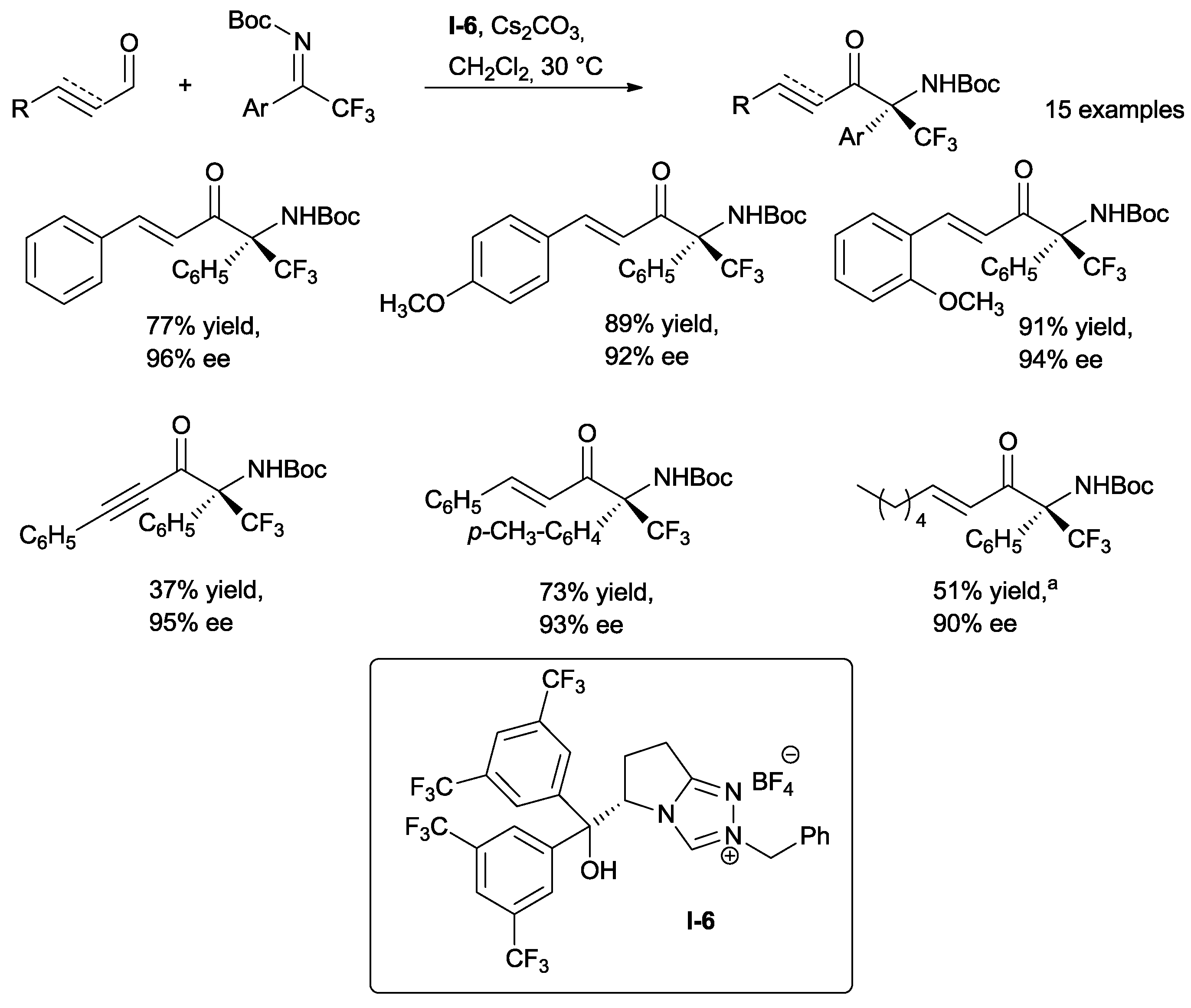
Ye studied the influence of steric and electronic factors of a series of L-pyroglutamic acid-derived triazolium salts on the reactivity of cynnamaldehyde with
N-Boc-protected trifluoromethyl phenyl ketimine (
Scheme 17) [
36].
The free hydroxy group on the catalyst plays a key role not only in the reduction of steric hindrance compared to its silylated analogue, but, more importantly, thanks to the possible hydrogen bond formation with the ketimine. The desired products have been obtained in high yields and enantioselectivities using catalyst
I-6 (
Scheme 17). Electron-withdrawing and electron-donating substituents on the aromatic ring of enals do not change yields and enantioselectivities. β-alkyl enals have been shown to work well in the reaction; however, the use of β-alkyl and β-aryl ynals has resulted in decreased yields, although high ees have still been obtained.
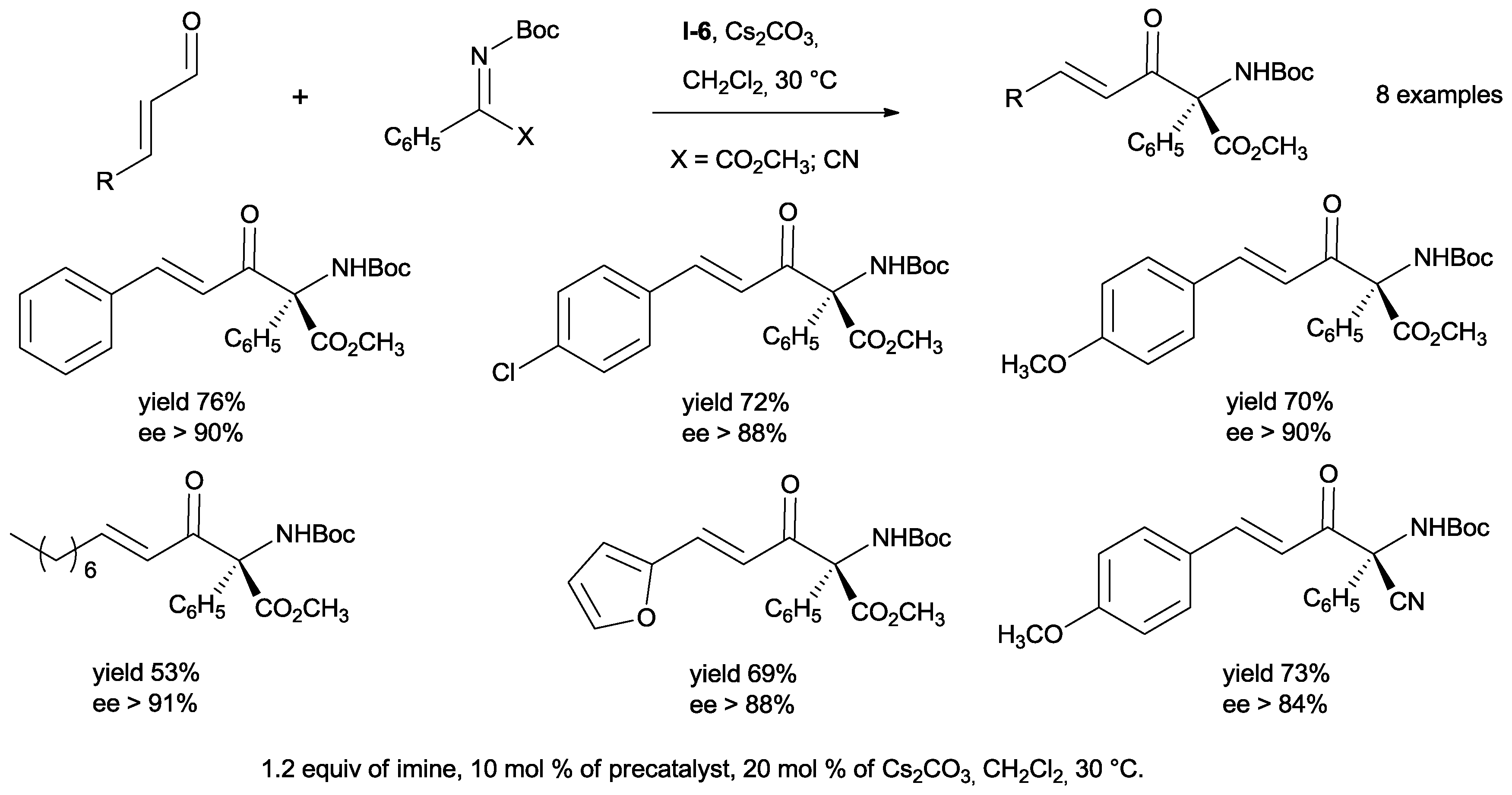
In order to further explore the scope of the reaction, (
Z)-methyl 2-((
tert-butoxycarbonyl) imino)-2-phenylacetates and (
Z)-
tert-butyl(cyano(phenyl))methylene)carbamate were used as acceptors. The aza-benzoin products were obtained with good yields and high enantiomeric excesses (
Scheme 18).
Cyclic N-protected ketimines have attracted significant interest, especially within asymmetric synthesis, due to their easy preparation and handling and their stable E/Z configuration which ensures high enantiofacial differentiation. In particular, the oxindole scaffold is a privileged structural motif common in natural products and in pharmacological active compounds.
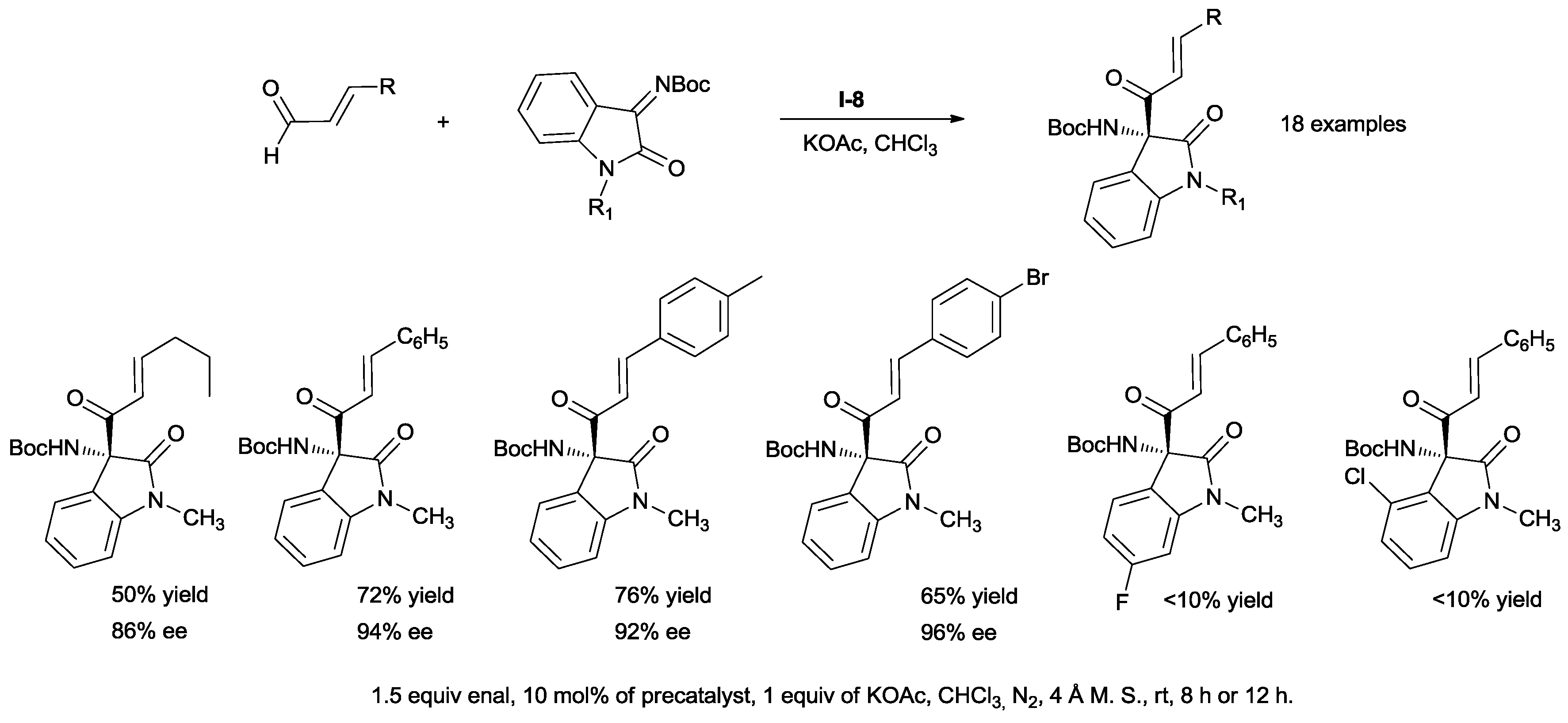
The chemoselectivity of the reaction between 2,3-dioxo-2,3-dihydroindole (isatin)-derived ketimines and enals has been studied by Chi [
37]. When precatalyst
I-7 was used, the reaction produced homoenolate-derived adducts (pathway A). The replacement of the encumbered and electron-rich
N-mesityl substituent with the less hindered and electron-deficient pentafluorophenyl moiety (
I-8) switched the outcome of the reaction towards the aza-coupling product with high chemoselectivity (pathway B) (
Scheme 19).
3-Aminooxindoles bearing a quaternary stereocenter with high ees and good yields have been prepared (
Scheme 20).
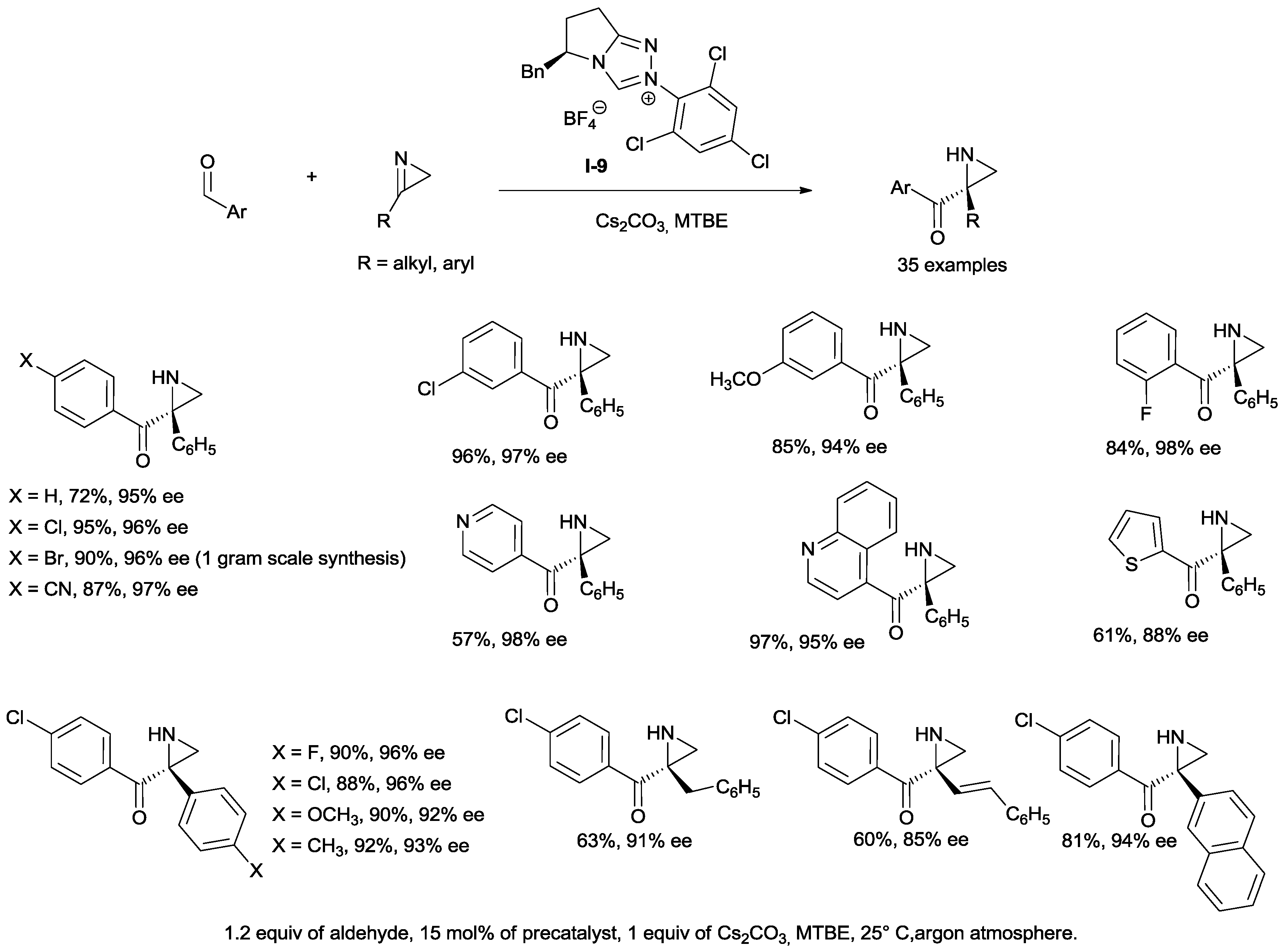
An unprecedented enantioselective aza-benzoin coupling, starting from ring-strained 2
H-azirines and aromatic aldehydes to give chiral aziridines, useful building synthons and valuable pharmaceutical structural motifs has been recently reported [
38]. Functionalized benzaldehydes and heteroaromatic aldehydes are well tolerated (
Scheme 21). Aliphatic aldehydes failed to participate in the reaction (data not shown).
The scope of the reaction has been also tested with respect to the 2H-azirines by systematically varying substituent patterns on the aromatic ring. In all cases, high ees and yields have been obtained
When alkyl or alkenyl groups replaced the aromatic ring, excellent enantiomeric excesses were still achieved, although with lower yields.
5. Tandem Reactions
In 2011, almost simultaneously, two papers dealing with the preparation of functionalized dihydroindenones with divergent diastereoselectivity were published.
Ye and coworkers developed a tandem aza-benzoin/aldol reaction starting from benzene 1,2-dicarboxaldehyde and
N-Boc imines using
I-1 as precatalyst which exclusively produced
cis-2-amino-3-hydroxyindenones with yields of up to 93% (
Scheme 22) [
39].
The achievement of optimized conditions required the use of cesium carbonate to generate the carbene and diisopropylethylamine in order to promote the formation of the imine.
Phenyl imines with electron-withdrawing groups gave the corresponding indenones with higher yields compared to imines with electron-donating substituents. Imines bearing both a m-chlorophenyl or a p-chlorophenyl group showed a similar reactivity. Also, heteroarylimines gave high yields.
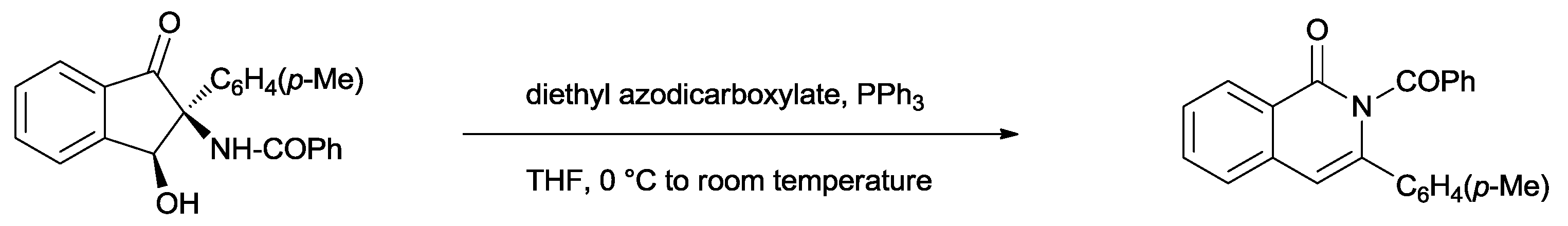
The resulting
cis-1-hydroxy-3-oxo-2-(
p-tolyl)-2,3-dihydro-1
H-inden-2-yl)benzamide was shown to easily convert to the corresponding isoquinolinone under Mitsunobu conditions (
Scheme 23) [
39].
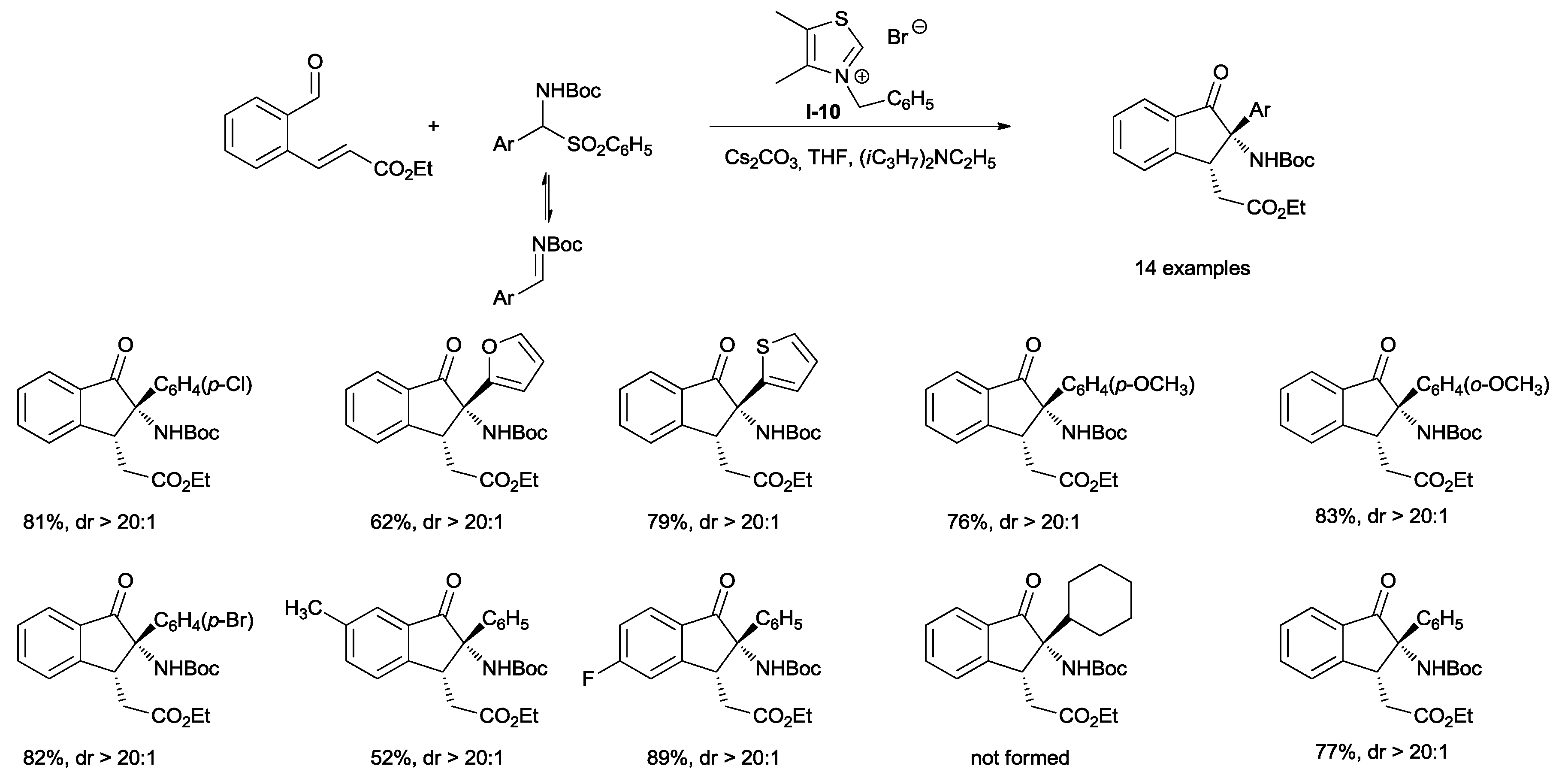
You and coworkers developed a process to substitute
trans dihydroindenones through an NHC-catalyzed tandem aza-benzoin/Michael reaction, starting from
tert-butyl aryl(tosyl) methylcarbamates and (
E)-ethyl 3-(2-formylphenyl)acrylates (
Scheme 24) [
40]. In order to obtain high yields of the desired product, 2.2 equivalents of cesium carbonate were used. A reduced base loading (1.5 equiv.) hampered the complete conversion of the initially formed aza-benzoin product to dihydroindenone. On the other hand, lower yields were obtained with a higher excess (4 equiv.) of Cs
2CO
3. Under the optimized conditions, the tandem reaction tolerated both electron-withdrawing and electron-donating substituents on the phenyl group of the imine and also, heteroarylimines gave good results. Cyclohexyl-substituted carbamate did not react.
Also, it has been shown that functionalized acrylates are suitable substrates. The catalytic cycle is depicted in
Scheme 25.
The Breslow Intermediate, generated from the reaction of the carbene catalyst with acrylate, produces intermediate VII after the addition of imine. A subsequent proton transfer gives intermediate VIII, which releases the catalyst and the α-amino ketone. It is worth noting that the imine carbon acts as an electrophile in the first step of the process when it reacts with the Breslow Intermediate, but as a nucleophile in the following Michael addition step. In fact, the enolizable α-carbon atom in the aza-benzoin product results in a stronger nucleophilic site compared to the contiguous nitrogen atom and reacts in the Michael addition, furnishing exclusively the dihydroindenone derivative.
Bode developed a cascade sequence involving an aza-benzoin/oxy-Cope strategy for the synthesis of bicyclic α-lactams with a diastereoisomeric ratio higher than 10:1 and an enantioselectivity of up to 98%. The starting reactants included both 3-alkyl or 3-arylenals and chalcone-derived sulfonyl imine (
Scheme 26) [
41].
Strictly related to the one-pot processes reviewed in this section is a paper dealing with the addition of homoenolate equivalents to appropriate imines, followed by cyclization steps generating γ-lactams (
Scheme 27) [
42].
Disubstituted γ-lactams with high diastereoselectivity have been obtained from the reaction of a series of cinnamaldehydes with electron-rich N-sulfonyl imines in the presence of precatalyst I-11.
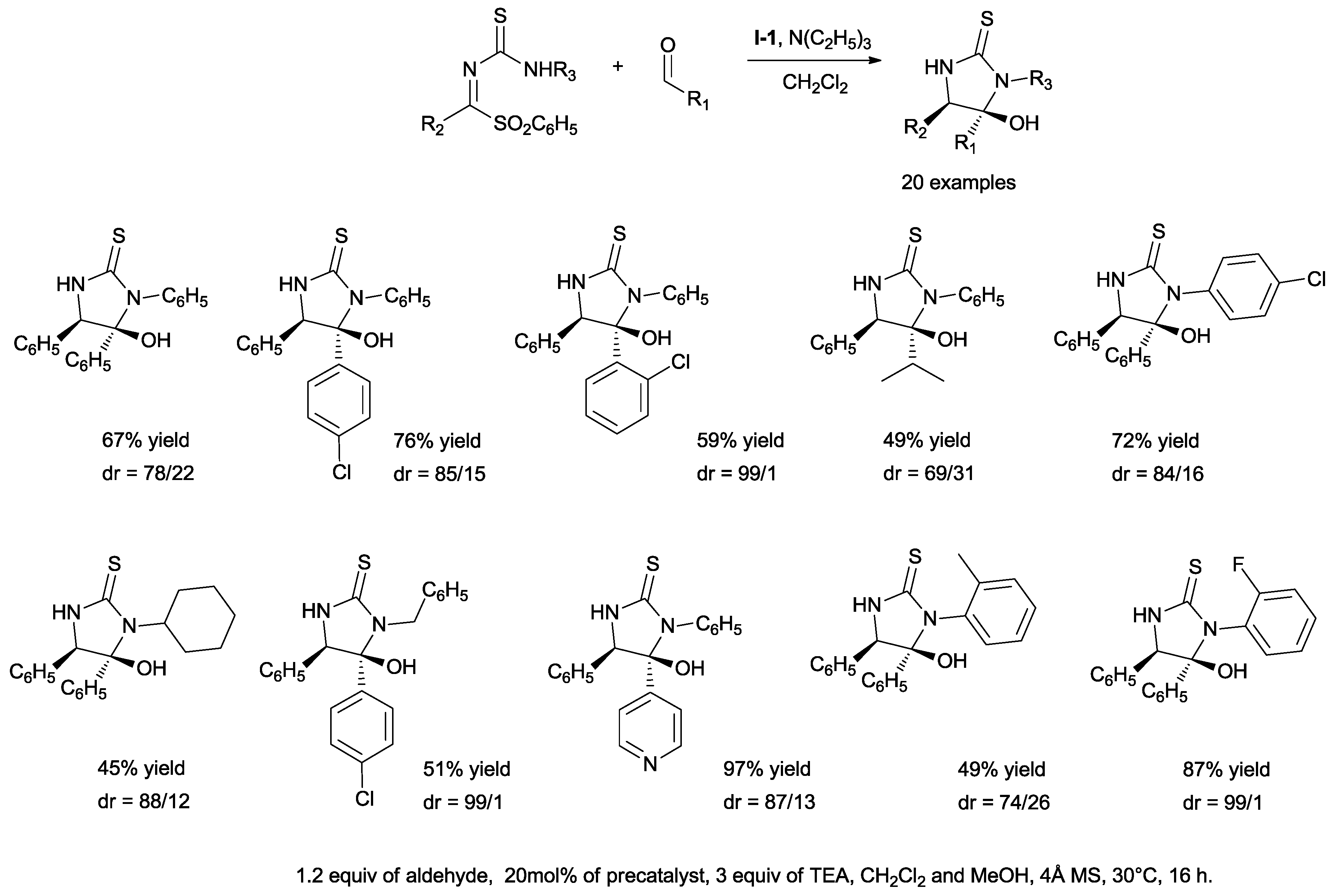
Novel acyl anion acceptors, namely benzylidene thio-ureas, have been used in a domino aza-benzoin/intermolecular aza-acetalization process for the synthesis of 5-hydroxy-imidazolidene-2-thiones, a class of heterocycles displaying relevant biological activities (
Scheme 28) [
42].
The generality of the strategy toward imidazolidine-2-thiones was investigated by considering variations in the structures of both aromatic aldehydes and α-sulfonyl amines (
Scheme 29).
Further studies demonstrated that the novel cyclization reaction could be run under optimized conditions on a large scale without losing reactivity or diastereoselectivity.
6. A Successful Application of Aza-Benzoin Condensation to the Synthesis of a Pharmaceutical Candidate
Metabotropic glutamate receptor 5 (mGluR5) is broadly expressed throughout the central nervous system and is implicated in different cognitive and behavioural processes. The molecule depicted in
Figure 4 has been identified as a potential candidate for the pre-clinical development of mGluR5 modulators [
43].
tert-Butyl and benzyl-((1R,2S)-1-(5-bromo pyridin-3-yl)-2-(2,5-difluorophenyl)-2-hydroxyethyl) carbamate have been selected as key intermediates in the synthesis of the target mGluR5 modulator. Unfortunately, their preparation via aminohydroxylation occurs with low regioselectivity.
The resolutive approach to this issue has been suggested as the asymmetric reduction of a protected α-aminoketone assembled by a regioselective aza-benzoin condensation catalysed by I-1.
The same approach could be applied for the synthesis of other 1,2-amino alcohols, where the traditional methods based on functionalization of alkenes may suffer from selectivity issues.
7. Conclusions
The aza-benzoin condensation reaction represents the useful enrichment of organic chemistry tools complementary to the traditional cross benzoin reaction.
The different levels of electrophilicity of imines with respect to aldehydes and the possibility of further tuning their reactivity by careful choice of the protecting group on the nitrogen atom, offers the possibility of solving the problem of chemoselectivity which represents the weak point of the cross benzoin coupling between two different aldehydes. For this reason, the aza-benzoin condensation allows easy access, in a regioselective manner, to valuable α-amino ketones. The possibility to take advantage of a great number of structurally-different chiral N-heterocyclic carbenes has successful improved stereoselective protocols, which have produced α-amino ketones with high enantiomeric excesses. Moreover, the experimental requirements include aza-benzoin condensation in domino processes for the straightforward synthesis of complex cyclic derivatives. In conclusion, the aza-benzoin reaction is a general, practical and broad scope methodology which forebodes new interesting developments.