Mobility of NH3-Solvated CuII Ions in Cu-SSZ-13 and Cu-ZSM-5 NH3-SCR Catalysts: A Comparative Impedance Spectroscopy Study
Abstract
1. Introduction
2. Results and Discussion
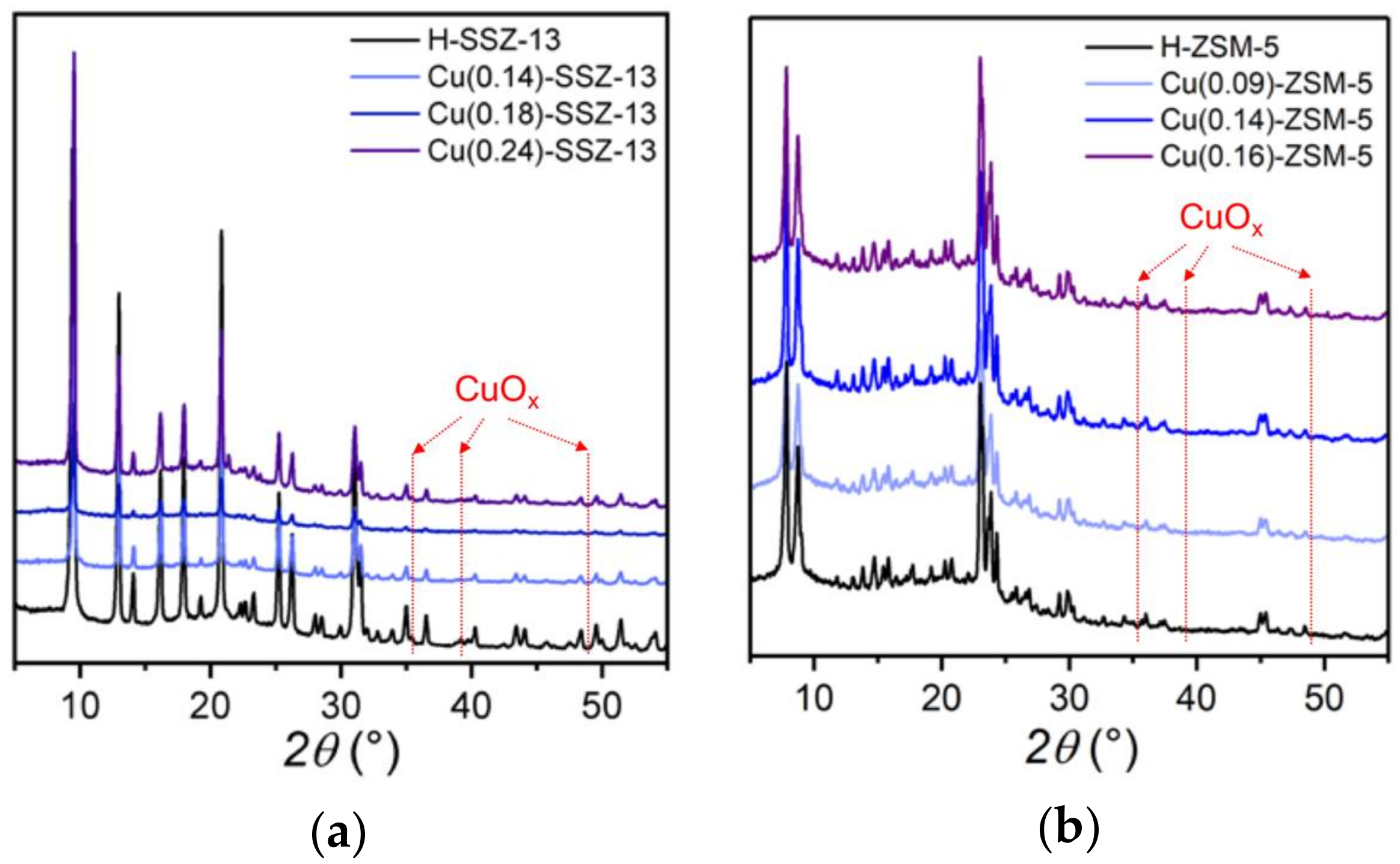
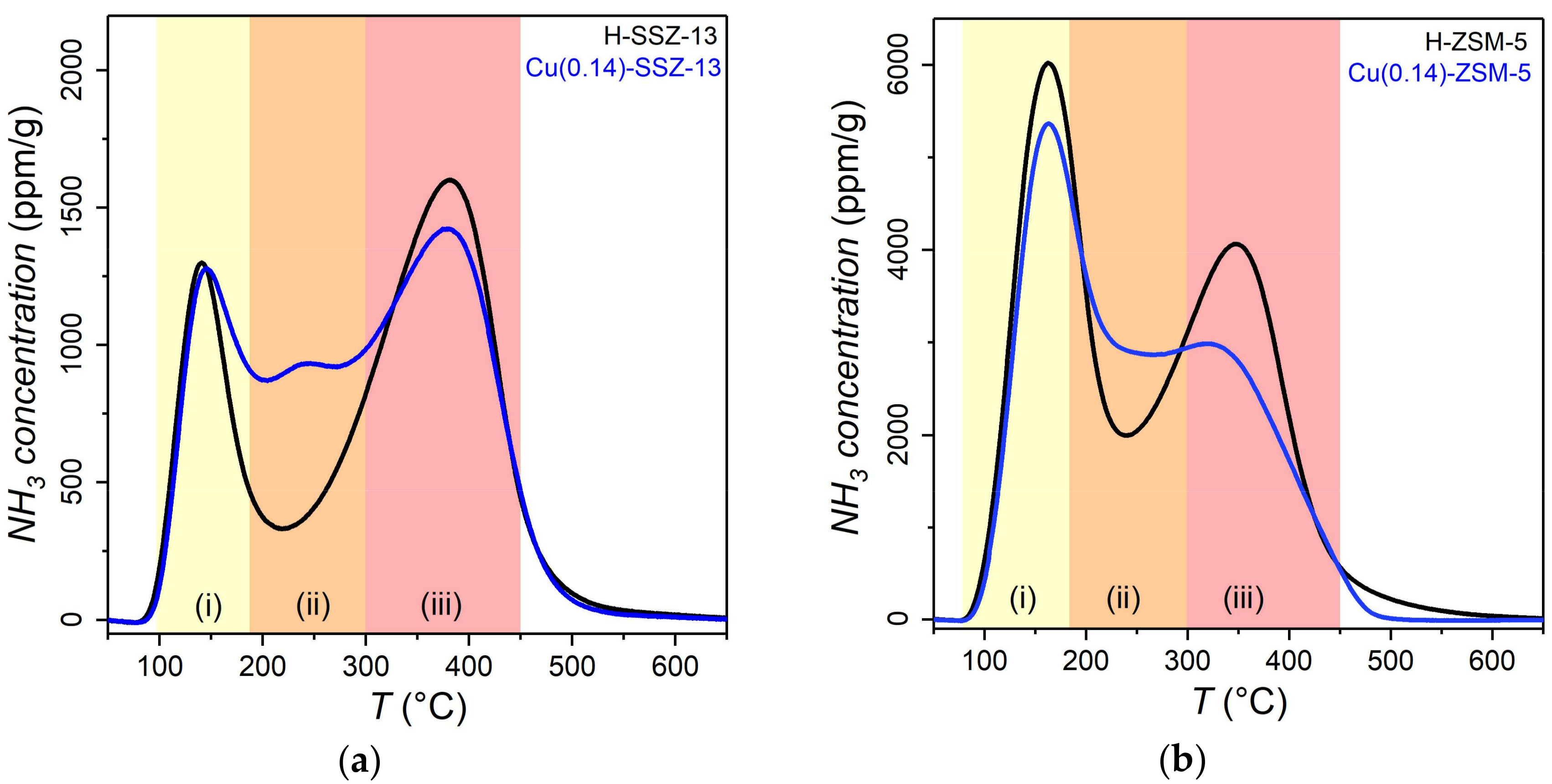
2.1. Catalyst Characterization
2.2. In Situ IS: Modulus Spectra
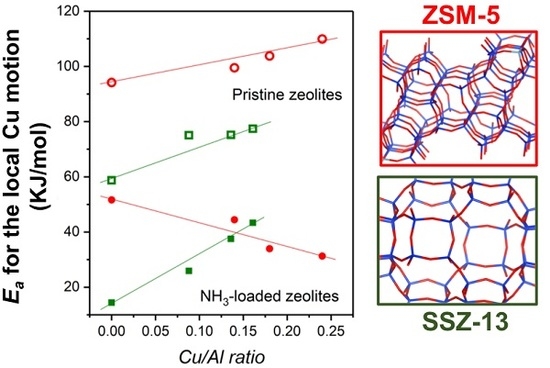
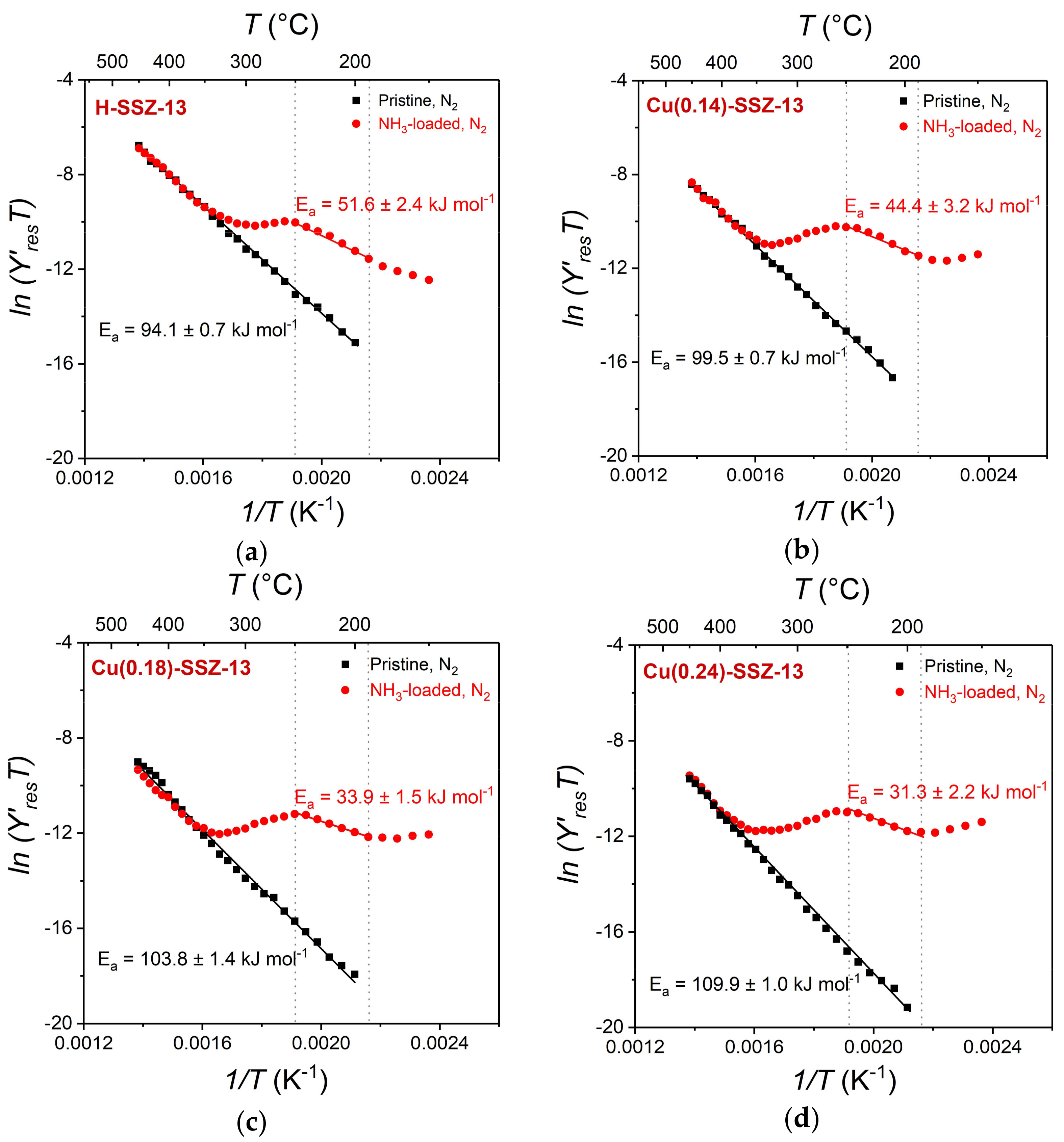
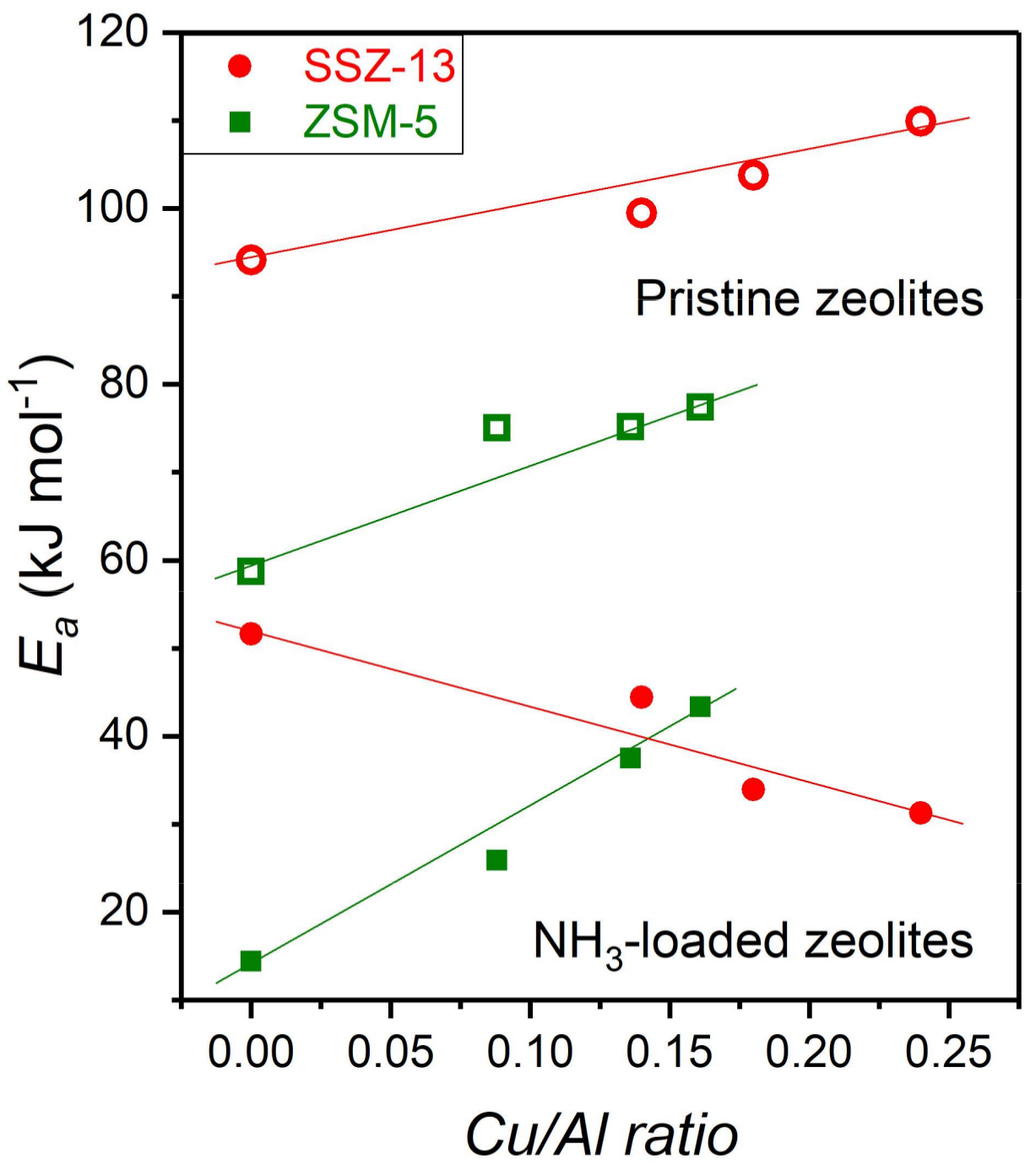
2.3. In Situ IS: HF Arrhenius Plots
3. Materials and Methods
3.1. Zeolite Synthesis
3.2. Zeolites Characterization
3.3. In Situ IS Measurements
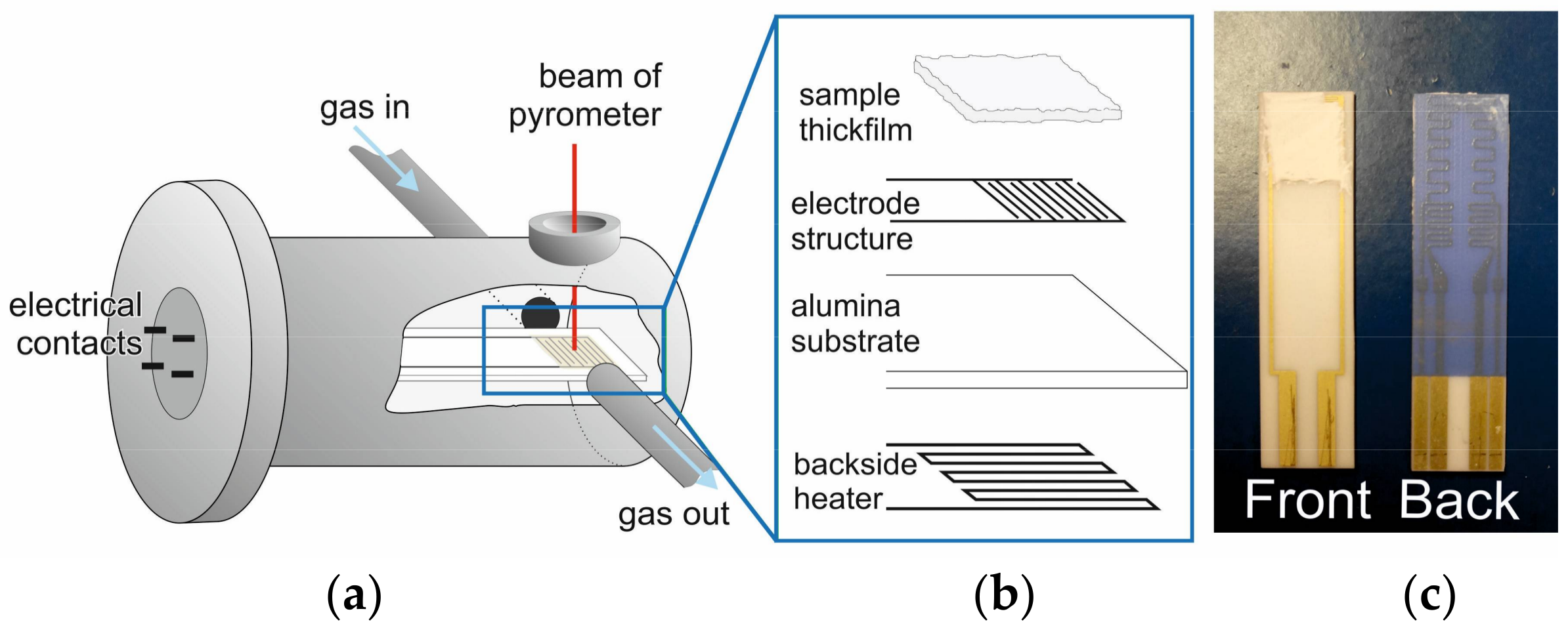
3.3.1. IS Set-Up
3.3.2. Multi-Frequency In Situ IS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Environment Agency. Air Quality in Europe—2016 Report; EEA report, No 28/2016; Publication Office of the European Union: Luxembourg, 2016; ISBN 978-92-9213-847-9. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, N.; Lei, Z.; Chen, B. Selective transformation of various nitrogen-containing exhaust gases toward N2 over zeolites catalysts. Chem. Rev. 2016, 116, 3658–3721. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Joshi, A. Review of Vehicle Engine Efficiency and Emissions; SAE Technical Paper 2017-01-0907; SAE International: Warrendale, PA, USA, 2017. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. Urea-SCR Technology for DeNOx after Treatment of Diesel Exhausts, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-1-4899-8071-7. [Google Scholar]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyl, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The state of the art in selective catalytic reduction of NOx by ammonia using metal-exchanged zeolite catalysts. Catal. Rev. Sci. Eng. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, G.R.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Paolucci, C.; Di Iorio, J.R.; Ribeiro, F.H.; Gounder, R.; Schneider, W.F. Catalysis science of NOx selective catalytic reduction with ammonia over Cu-SSZ-13 and Cu-SAPO-34. Adv. Catal. 2016, 59, 1–107. [Google Scholar] [CrossRef]
- Simon, U.; Franke, M.E. Electrical properties of nanoscaled host/guest compounds. Microporous Mesoporous Mater. 2000, 41, 1–36. [Google Scholar] [CrossRef]
- Paolucci, C.; Parekh, A.A.; Khurana, I.; Di Iorio, J.R.; Li, H.; Albarracin-Caballero, J.D.; Shih, A.J.; Angarra, T.; Delgass, W.N.; Miller, J.T.; et al. Catalysis in a cage: Condition-dependent Speciation and dynamics of exchanged Cu cations in SSZ-13 zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; Di Iorio, J.R.; Albarracin Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by moblized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Mei, D.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Selective catalytic reduction over Cu/SSZ-13: Linking homo- and heterogeneous catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Franke, M.E.; Simon, U. Proton mobility in H-ZSM5 studied by impedance spectroscopy. Solid State Ion. 1999, 118, 311–316. [Google Scholar] [CrossRef]
- Franke, M.E.; Simon, U. Solvate supported proton transport in zeolites. Chem. Phys. Chem. 2004, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Simons, T.; Simon, U. Zeolites as nanoporous, gas-sensitive materials for in situ monitoring of DeNOx-SCR. Beilstein J. Nanotechnol. 2012, 3, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Schönebaum, S.; Simons, T.; Rauch, D.; Dietrich, M.; Moos, R.; Simon, U. Correlating the integral sensing properties of zeolites with molecular processes by combining broadband impedance and DRIFT spectroscopy—A new approach for bridging the scales. Sensors 2015, 15, 28915–28941. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Simon, U. In Situ spectroscopic studies of proton transport in zeolite catalysts for NH3-SCR. Catalysts 2016, 6, 204. [Google Scholar] [CrossRef]
- Chen, P.; Rauch, D.; Weide, P.; Schönebaum, S.; Simons, T.; Muhler, M.; Moos, R.; Simon, U. The effect of Cu and Fe cations on NH3-supported proton transport in DeNOx-SCR zeolite catalysts. Catal. Sci. Technol. 2016, 6, 3362–3366. [Google Scholar] [CrossRef]
- Chen, P.; Simböck, J.; Schönebaum, S.; Rauch, D.; Simons, T.; Palkovits, R.; Moos, R.; Simon, U. Monitoring NH3 storage and conversion in Cu-ZSM-5 and Cu-SAPO-34 catalysts for NH3-SCR by simultaneous impedance and DRIFTS spectroscopy. Sens. Actuators B 2016, 236, 1075–1082. [Google Scholar] [CrossRef]
- Chen, P.; Jabłońska, M.; Weide, P.; Caumanns, T.; Weirich, T.; Muhler, M.; Moos, R.; Palkovits, R.; Simon, U. Formation and effect of NH4+ intermediates in NH3-SCR over Fe-ZSM-5 zeolite catalysts. ACS Catal. 2016, 6, 7696–7700. [Google Scholar] [CrossRef]
- Simons, T.; Chen, P.; Rauch, D.; Moos, R.; Simon, U. Sensing catalytic conversion: Simultaneous DRIFT and impedance spectroscopy for in situ monitoring of DeNOx-SCR on zeolites. Sens. Actuators B Chem. 2016, 224, 492–499. [Google Scholar] [CrossRef]
- Chen, P.; Khetan, A.; Jabłońska, M.; Simböck, J.; Muhler, M.; Palkovits, R.; Pitsch, H.; Simon, U. Local dynamics of copper active sites in zeolite catalysts for selective catalytic reduction of NOx with NH3. Unpublished manuscript.
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Lezcano-Gonzalez, I.; Deka, U.; Arstad, B.; Van Yperen-De Deyne, A.; Hemelsoet, K.; Waroquier, M.; Van Speybroeck, V.; Weckhuysen, B.M.; Beale, A.M. Determining the storage, availability and reactivity of NH3 within Cu-Chabazite-based ammonia selective catalytic reduction systems. Phys. Chem. Chem. Phys. 2014, 16, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, S.; Kröcher, O.; Wokaun, A.; Tissler, A.; Althoff, R. The role of Brønsted acidity in the selective catalytic reduction of NO with ammonia over Fe-ZSM-5. J. Catal. 2009, 268, 297–306. [Google Scholar] [CrossRef]
- Simon, U.; Flesch, U. Cation-Cation interaction in dehidrated zeolites X and Y monitored by modulus spectroscopy. J. Porous Mater. 1999, 6, 33–40. [Google Scholar] [CrossRef]
- Chen, P.; Moos, R.; Simon, U. Metal loading affects the proton transport properties and the reation monitoring performance of Fe-ZSM-5 and Cu-ZSM-5 in NH3-SCR. J. Phys. Chem. C 2016, 120, 25361–25370. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, L.; Rodriguez-Castellon, E.; Jimenez-Lopez, A.; Simon, U. Correlation of TPD and impedance measurements on the desorption of NH3 from zeolites H-ZSM-5. Solid State Ion. 2008, 179, 1968–1973. [Google Scholar] [CrossRef]
- Sierka, M.; Sauer, J. Proton mobility in Chabazite, Faujasite, and ZSM-5 zeolite catalysts. Comparison bases on ab initio calculations. J. Phys. Chem. B 2001, 105, 1603–1613. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.M.; Beale, A.M. Local environment and nature of Cu active sites in zeolite-based catalysts for the selective catalytic reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Shishkin, A.; Kannisto, H.; Carlsson, P.; Hårelind, H.; Skoglundh, M. Synthesis and functionalization of SSZ-13 as an NH3-SCR catalyst. Catal. Sci. Technol. 2014, 4, 3917–3926. [Google Scholar] [CrossRef]


| Sample | Cu (wt. %) | Cu/Al |
|---|---|---|
| H-SSZ-13 | - | - |
| Cu(0.14)-SSZ-13 | 0.58 | 0.14 |
| Cu(0.18)-SSZ-13 | 0.76 | 0.18 |
| Cu(0.24)-SSZ-13 | 1.11 | 0.24 |
| H-ZSM-5 | - | - |
| Cu(0.09)-ZSM-5 | 0.64 | 0.09 |
| Cu(0.14)-ZSM-5 | 0.99 | 0.14 |
| Cu(0.16)-ZSM-5 | 1.17 | 0.16 |
| Sample | HF νres | log νres | HF τ |
|---|---|---|---|
| H-SSZ-13 | 1.36 104 | 4.13 | 7.36 10−5 |
| Cu(0.14)-SSZ-13 | 7.36 104 | 4.87 | 1.36 10−5 |
| Cu(0.19)-SSZ-13 | 1.58 105 | 5.20 | 6.31 10−6 |
| Cu(0.24)-SSZ-13 | 2.15 105 | 5.33 | 5.41 10−6 |
| H-ZSM-5 | 1.84 105 | 5.27 | 5.41 10−6 |
| Cu(0.09)-ZSM-5 | 1.17 105 | 5.07 | 8.58 10−6 |
| Cu(0.14)-ZSM-5 | 1.00 105 | 5.00 | 1.00 10−5 |
| Cu(0.16)-ZSM-5 | 8.58 104 | 4.93 | 1.17 10−5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzotto, V.; Chen, P.; Simon, U. Mobility of NH3-Solvated CuII Ions in Cu-SSZ-13 and Cu-ZSM-5 NH3-SCR Catalysts: A Comparative Impedance Spectroscopy Study. Catalysts 2018, 8, 162. https://doi.org/10.3390/catal8040162
Rizzotto V, Chen P, Simon U. Mobility of NH3-Solvated CuII Ions in Cu-SSZ-13 and Cu-ZSM-5 NH3-SCR Catalysts: A Comparative Impedance Spectroscopy Study. Catalysts. 2018; 8(4):162. https://doi.org/10.3390/catal8040162
Chicago/Turabian StyleRizzotto, Valentina, Peirong Chen, and Ulrich Simon. 2018. "Mobility of NH3-Solvated CuII Ions in Cu-SSZ-13 and Cu-ZSM-5 NH3-SCR Catalysts: A Comparative Impedance Spectroscopy Study" Catalysts 8, no. 4: 162. https://doi.org/10.3390/catal8040162
APA StyleRizzotto, V., Chen, P., & Simon, U. (2018). Mobility of NH3-Solvated CuII Ions in Cu-SSZ-13 and Cu-ZSM-5 NH3-SCR Catalysts: A Comparative Impedance Spectroscopy Study. Catalysts, 8(4), 162. https://doi.org/10.3390/catal8040162