Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment
Abstract
1. Introduction
2. Results and Discussion
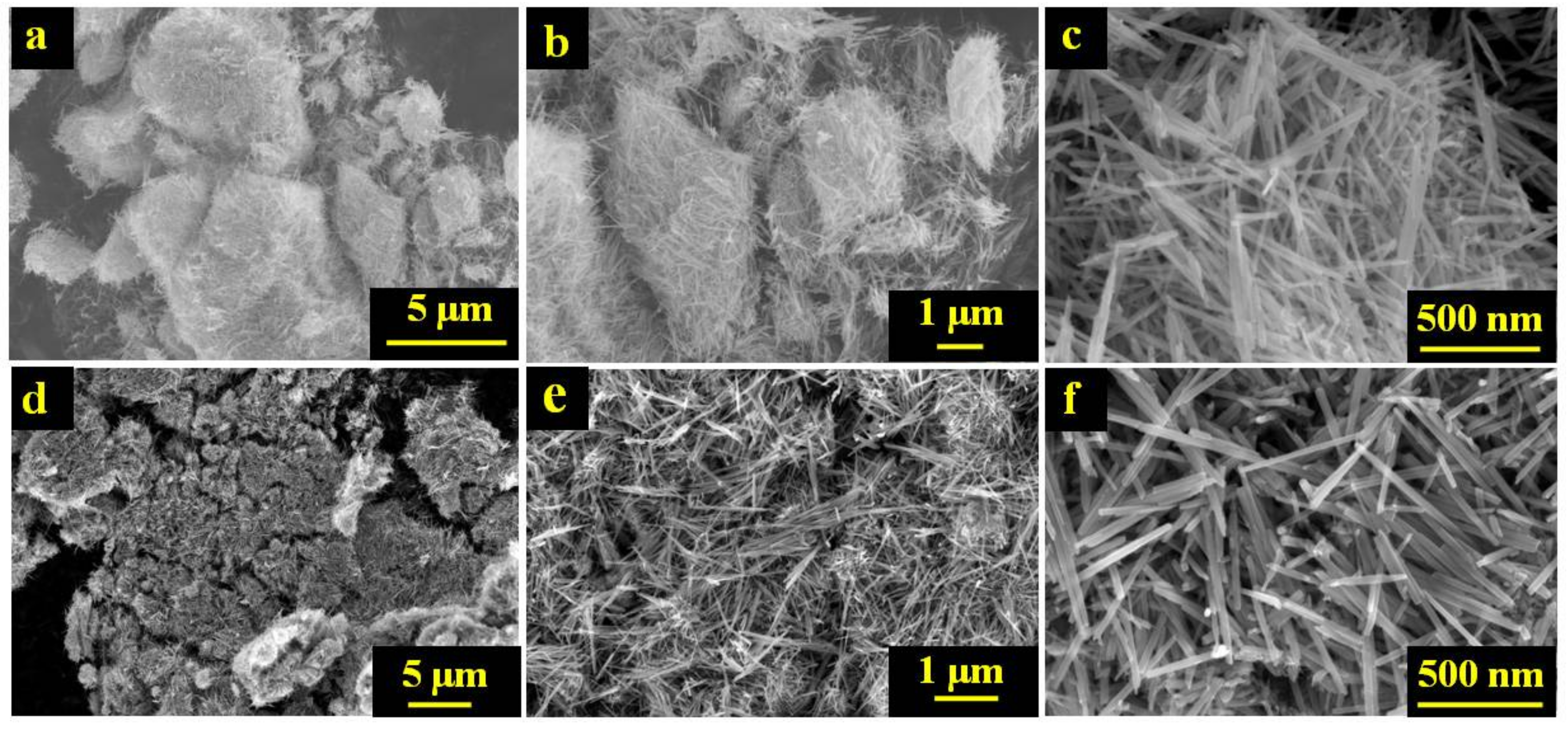
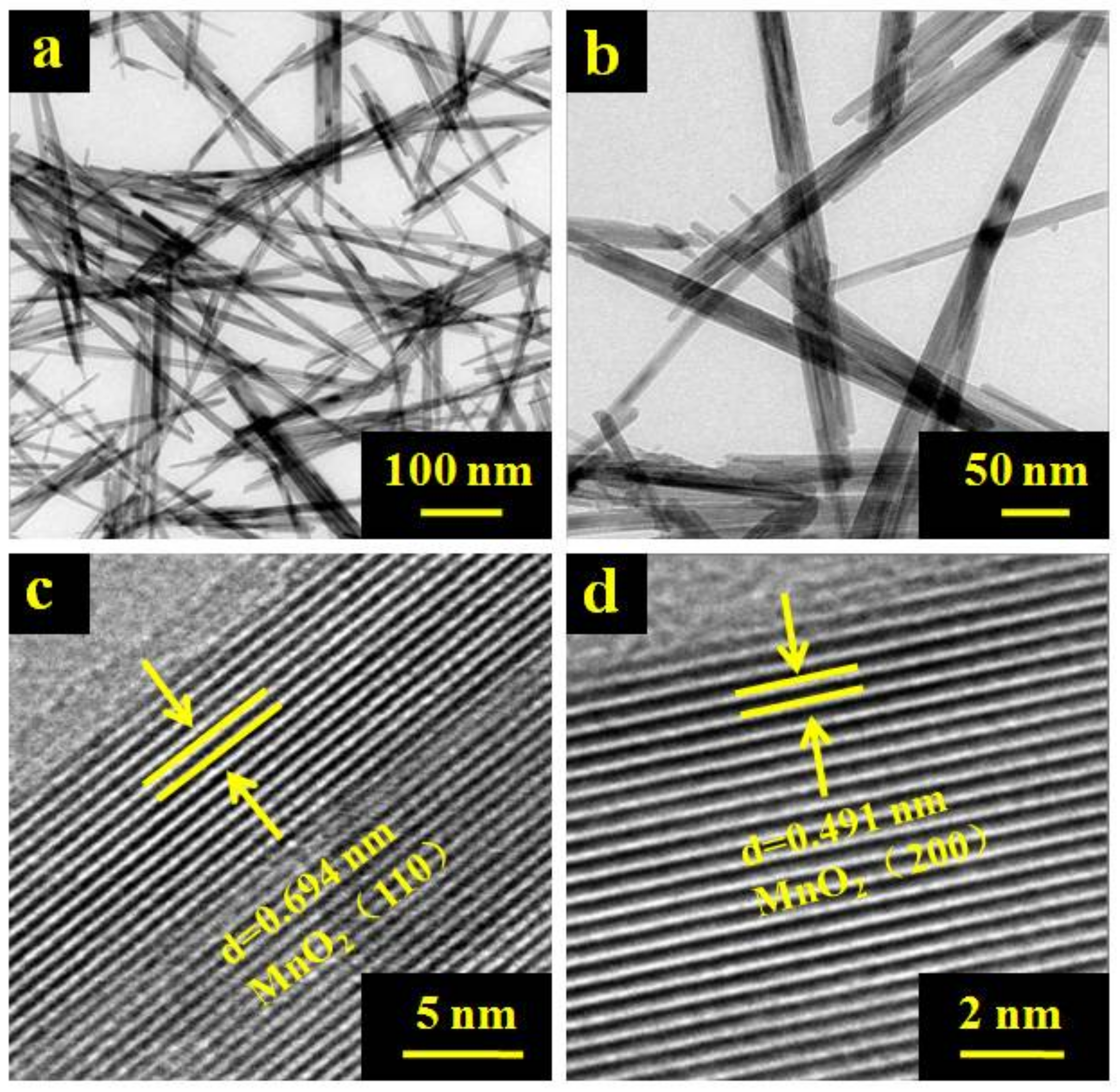
2.1. Morphological Characterization
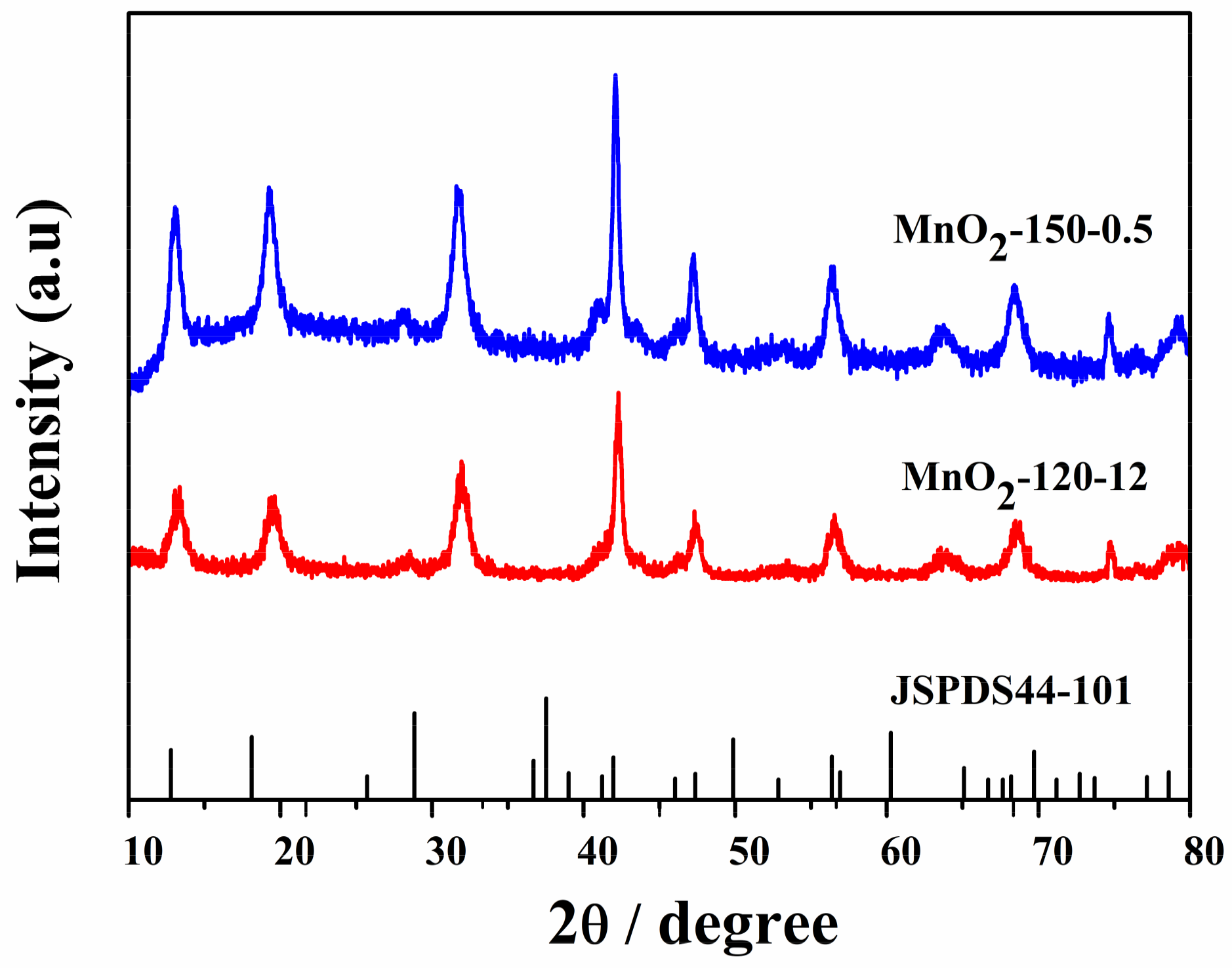
2.2. X-ray Diffraction (XRD) Pattern
2.3. XPS Analysis
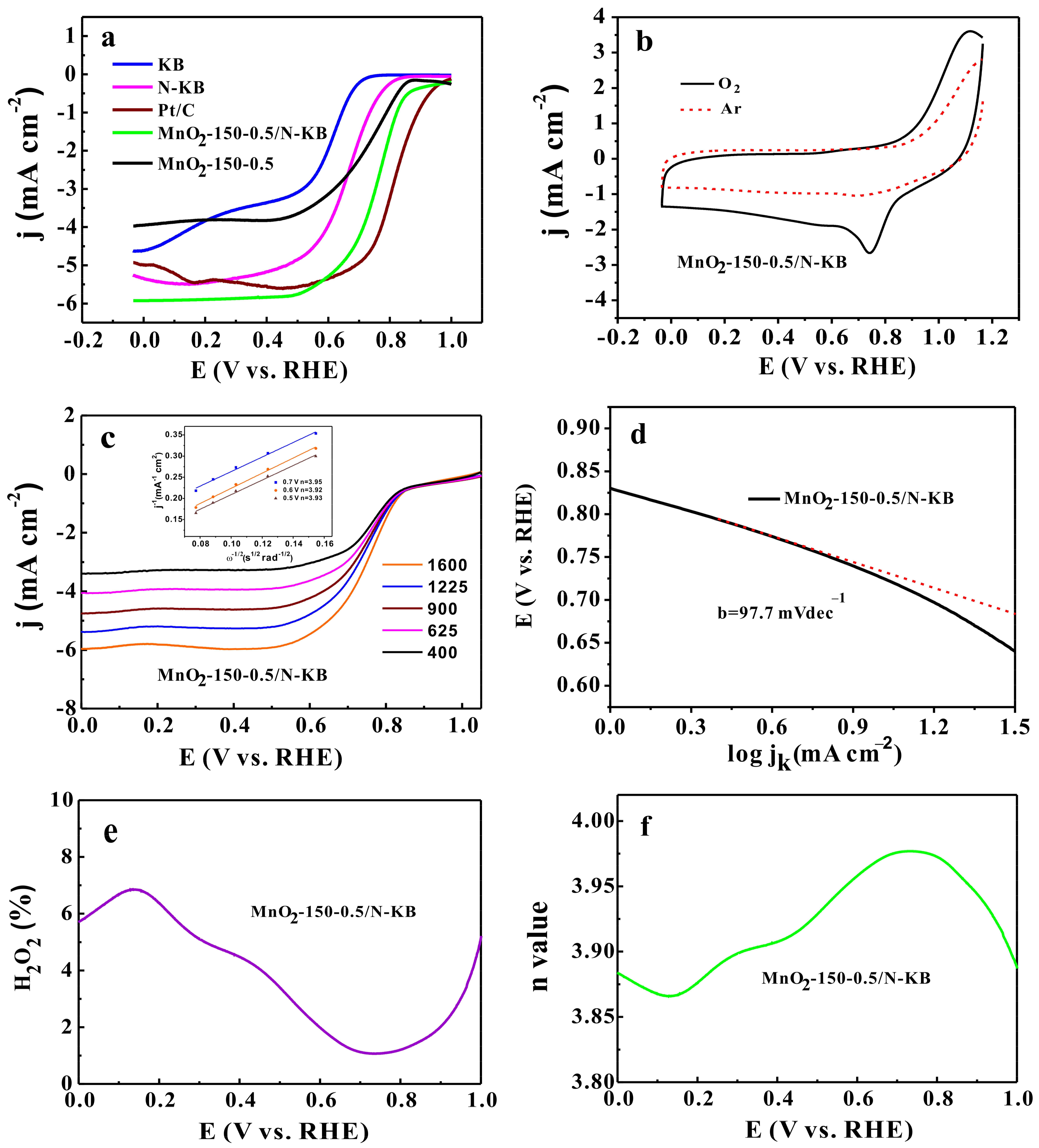
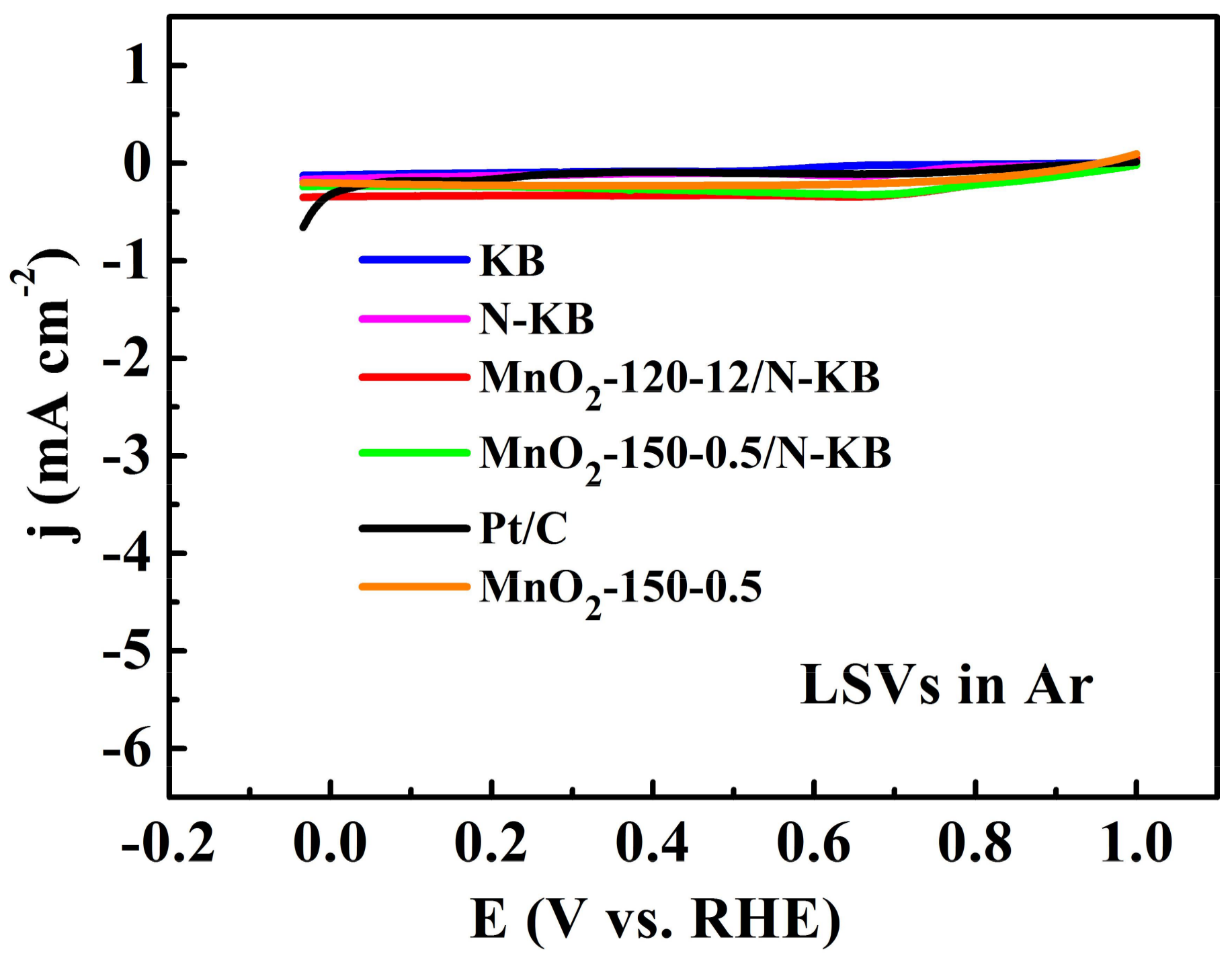
2.4. ORR Catalytic Activities
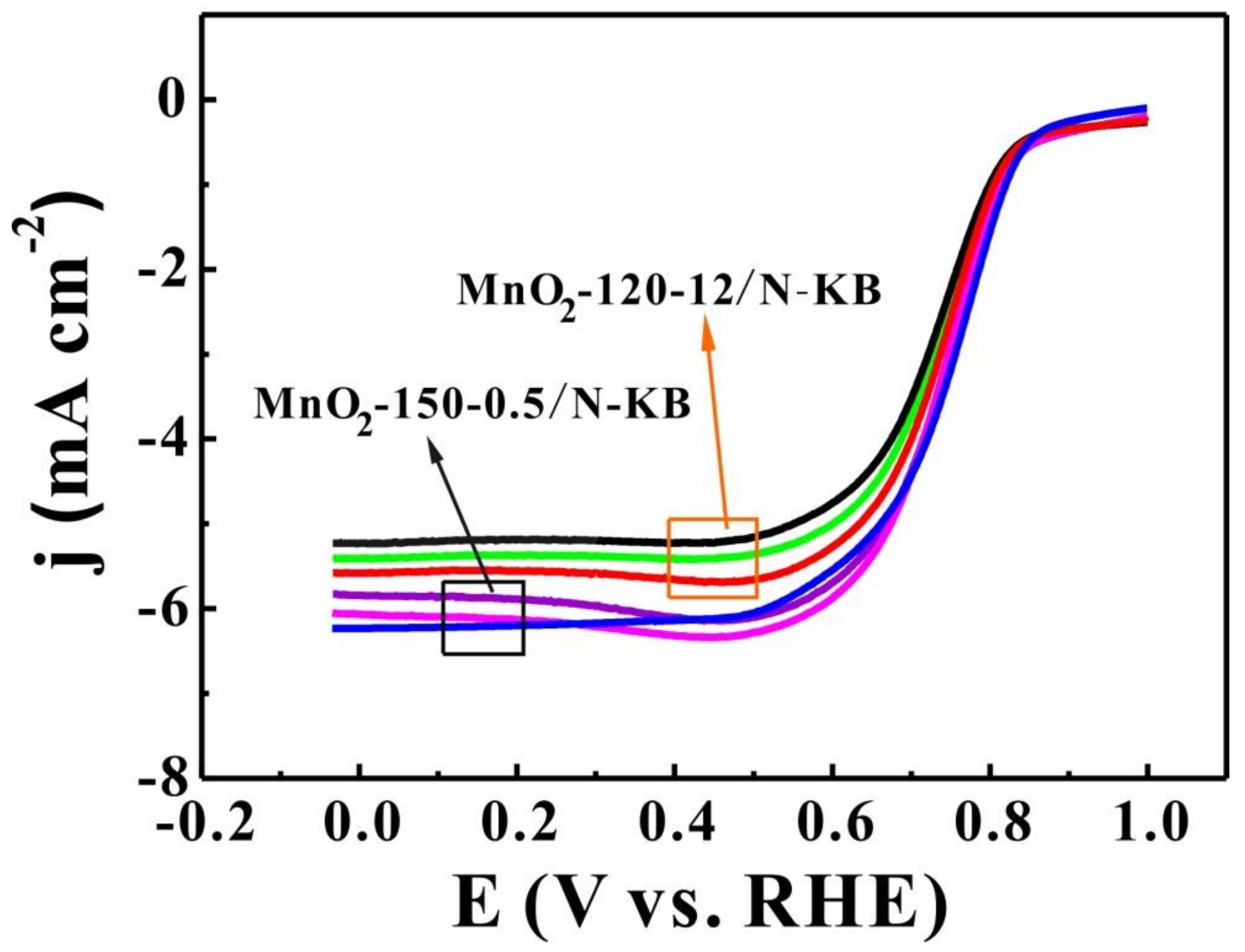
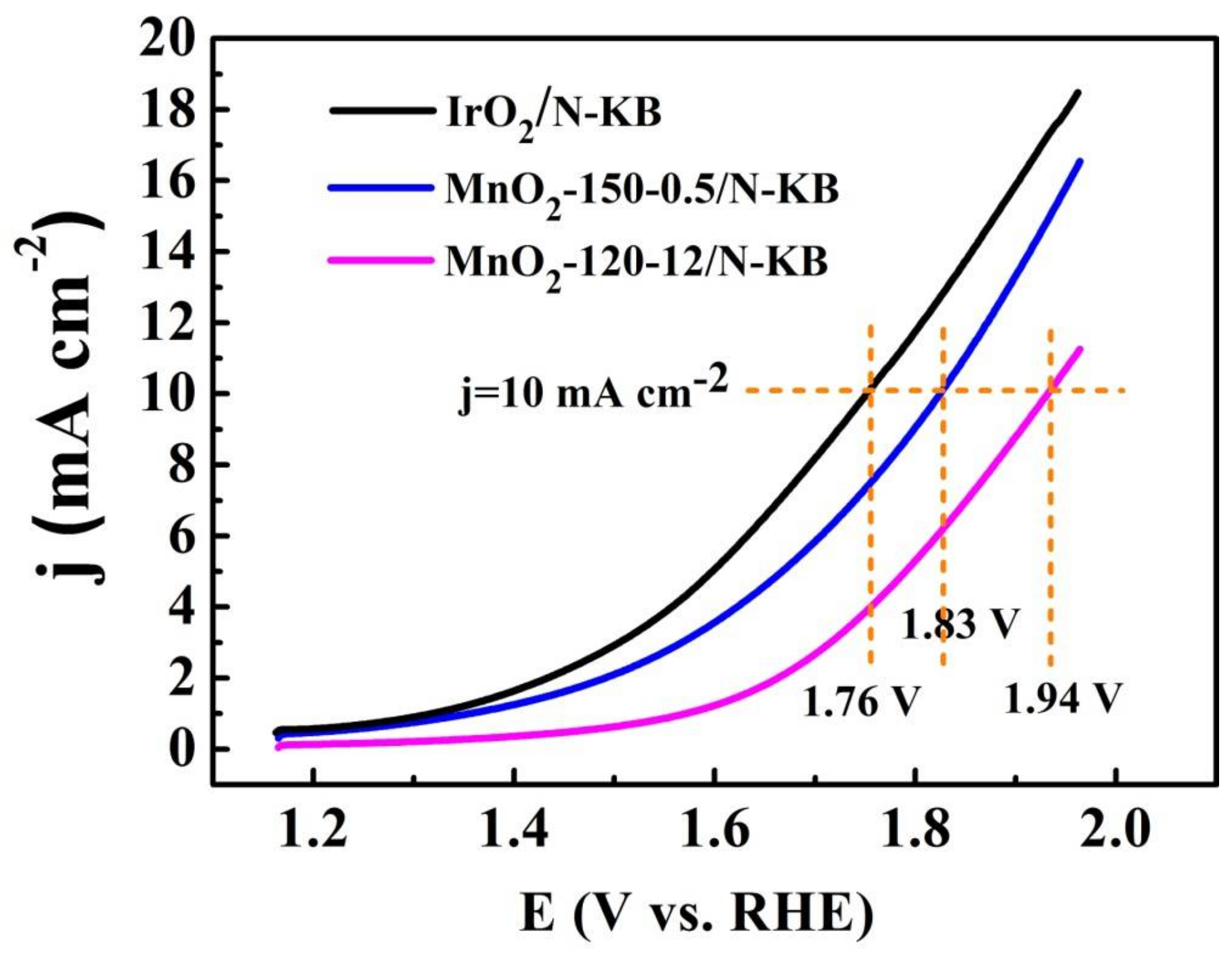
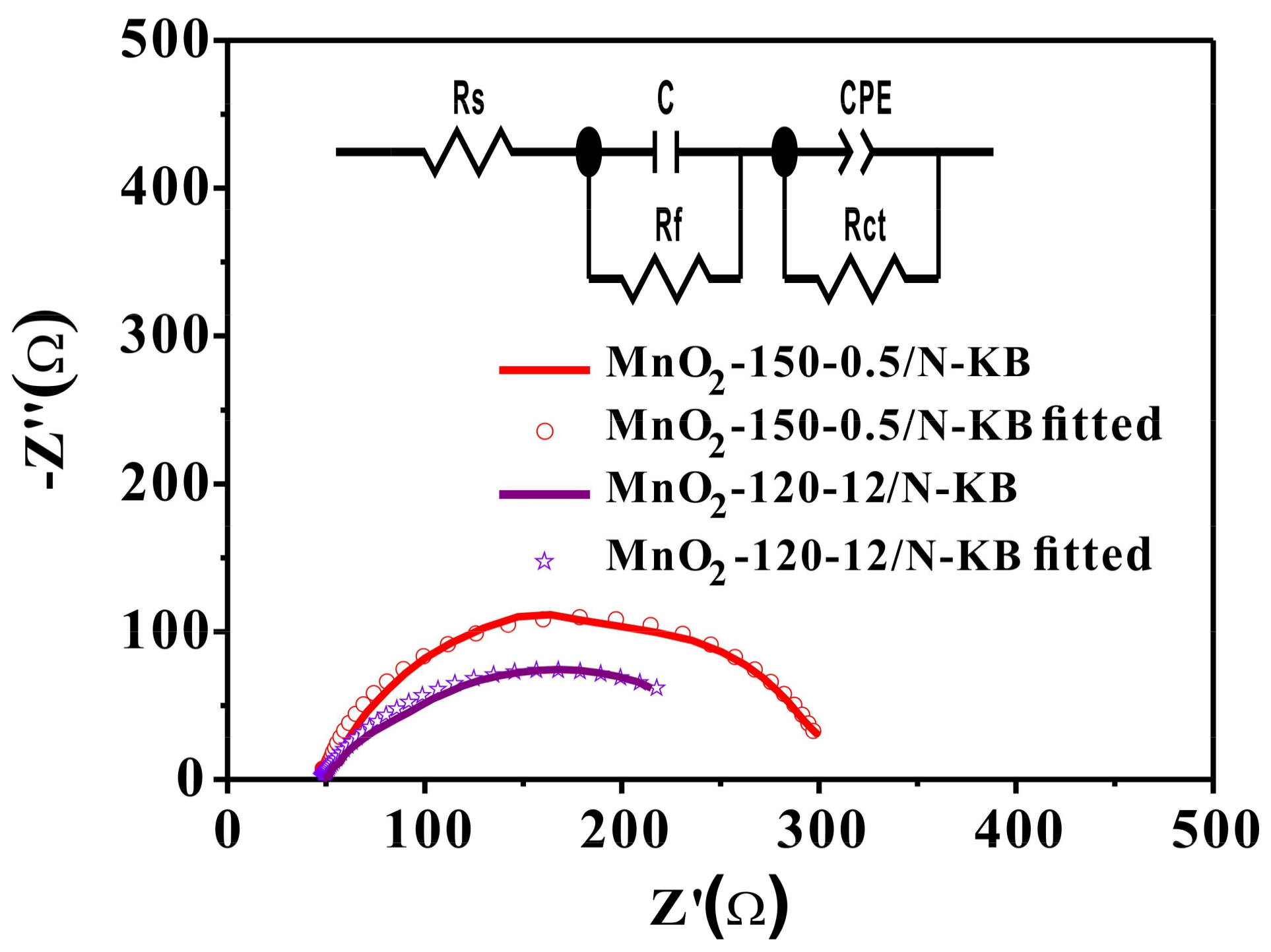
2.5. OER Activities
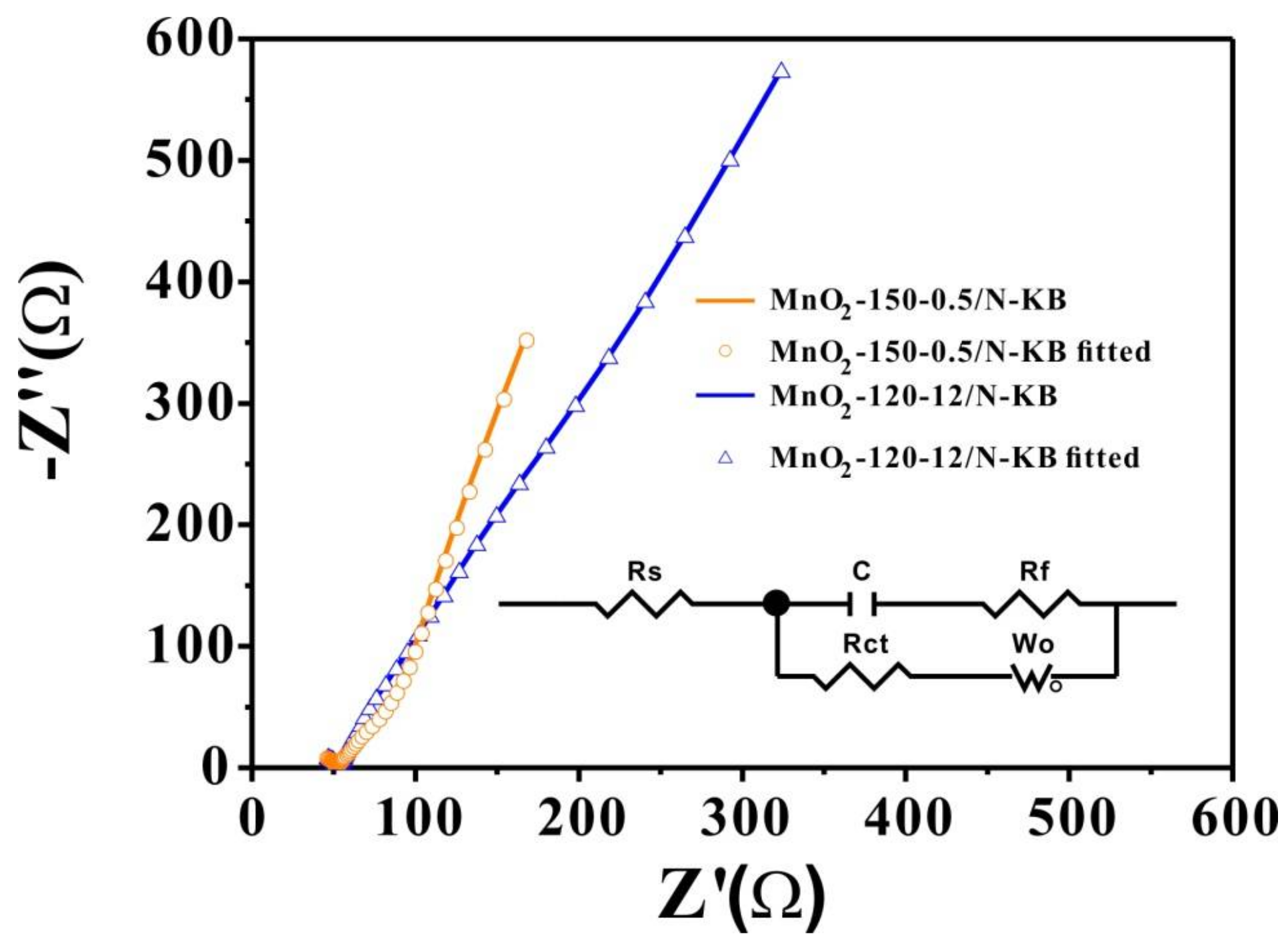
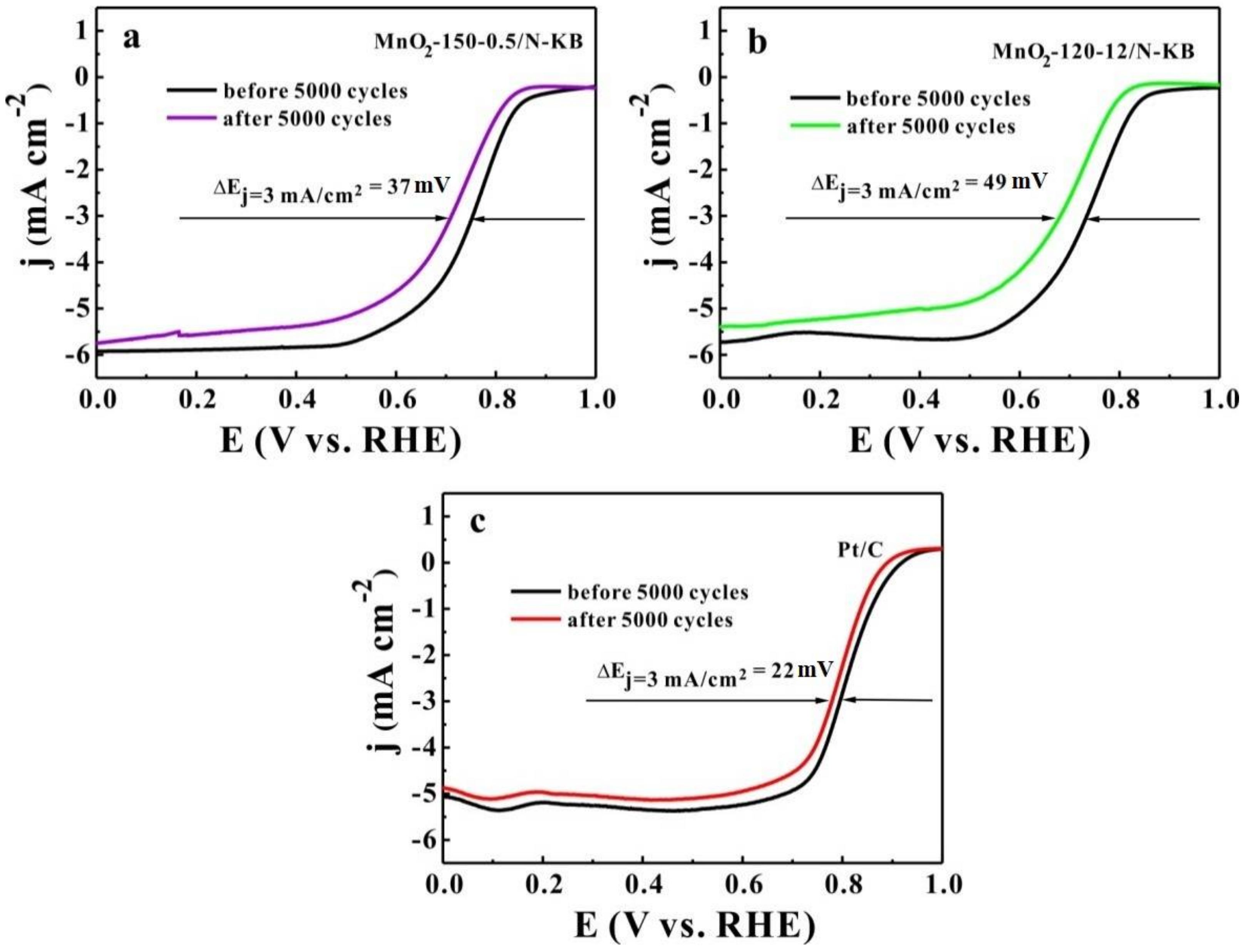
2.6. Durability of Electrocatalysts
3. Experiment
3.1. Chemical and Solutions
3.2. Preparation of α-MnO2 Nanorods
3.3. Preparation of N-Doped Ketjenblack Carbon
3.4. Physical Characterization of Catalysts
3.5. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, S.; Wang, Z.; Cao, Z.; Mao, X.; Shi, M.; Li, Y.; Zhang, R.; Yin, Y. Facile synthesis of well dispersed spinel cobalt manganese oxides microsphere as efficient bi-functional electrocatalysts for oxygen reduction reaction and oxygen evolution reaction. J. Alloys Compd. 2017, 721, 482–491. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, H.; Sun, S.; Wang, Q.; Li, S.; Liu, Z. (La1−x Srx)0.98MnO3 perovskite with a-site deficiencies toward oxygen reduction reaction in aluminum-air batteries. J. Power Sources 2017, 342, 192–201. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Wang, Y.; Wang, M.; Qiu, R.; Shu, L.; Hua, Z.; Cui, F.; Wei, C.; Shi, J. One-step synthesis of sulfur doped graphene foam for oxygen reduction reactions. Dalton Trans. 2014, 43, 3420–3423. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.P.; Datta, M.K.; Velikokhatnyi, O.I.; Kuruba, R.; Damodaran, K.; Jampani, P.; Gattu, B.; Shanthi, P.M.; Damle, S.S.; Kumta, P.N. Noble metal-free bifunctional oxygen evolution and oxygen reduction acidic media electro-catalysts. Sci. Rep. 2016, 6, 28367. [Google Scholar] [CrossRef] [PubMed]
- Sakaushi, K.; Fellinger, T.P.; Antonietti, M. Bifunctional metal-free catalysis of mesoporous noble carbons for oxygen reduction and evolution reactions. Chemsuschem 2015, 8, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.J.; Kwon, K.; Cheon, J.Y.; Kleitz, F.; Sang, H.J. Ordered mesoporous Co3O4 spinels as stable, bifunctional, noble metal-free oxygen electrocatalysts. J. Mater. Chem. 2013, 1, 9992–10001. [Google Scholar] [CrossRef]
- Lübke, M.; Sumboja, A.; Mccafferty, L.; Armer, C.F.; Handoko, A.D.; Du, Y.; Mccoll, K.; Cora, F.; Brett, D.; Liu, Z. Transition-metal-doped α-MnO2 nanorods as bifunctional catalysts for efficient oxygen reduction and evolution reactions. Chemistryselect 2018, 3, 2613–2622. [Google Scholar] [CrossRef]
- Gu, W.; Hu, L.; Li, J.; Wang, E. Recent advancements in transition metal-nitrogen-carbon catalysts for oxygen reduction reaction. Electroanalysis 2018, 30, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Hu, T.; Zhang, L.; Deng, Y. Carbon supported MnO2-CoFe2O4 with enhanced electrocatalytic activity for oxygen reduction and oxygen evolution. Appl. Surf. Sci. 2017, 403, 51–56. [Google Scholar] [CrossRef]
- Ghosh, S.; Kar, P.; Bhandary, N.; Basu, S.; Maiyalagan, T.; Sardar, S.; Pal, S.K. Reduced graphene oxide supported hierarchical flower like manganese oxide as efficient electrocatalysts toward reduction and evolution of oxygen. Int. J. Hydrogen Energy 2017, 42, 4111–4122. [Google Scholar] [CrossRef]
- Wu, X.; Gao, X.; Xu, L.; Huang, T.; Yu, J.; Wen, C.; Chen, Z.; Han, J. Mn2O3 doping induced the improvement of catalytic performance for oxygen reduction of MnO. Int. J. Hydrogen Energy 2016, 41, 16087–16093. [Google Scholar] [CrossRef]
- Wei, Z.H.; Zhao, T.S.; Zhu, X.B.; Tan, P. MnO2−x nanosheets on stainless steel felt as a carbon- and binder-free cathode for non-aqueous lithium-oxygen batteries. J. Power Sources 2016, 306, 724–732. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Zheng, G.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Liu, Z. Durable rechargeable zinc-air batteries with neutral electrolyte and manganese oxide catalyst. J. Power Sources 2016, 332, 330–336. [Google Scholar] [CrossRef]
- Pargoletti, E.; Cappelletti, G.; Minguzzi, A.; Rondinini, S.; Leoni, M.; Marelli, M.; Vertova, A. High-performance of bare and Ti-doped α-MnO2 nanoparticles in catalyzing the oxygen reduction reaction. J. Power Sources 2016, 325, 116–128. [Google Scholar] [CrossRef]
- Cheng, F.; Su, Y.; Liang, J.; Tao, Z.; Chen, J. MnO2-based nanostructures as catalysts for electrochemical oxygen reduction in alkaline media. Chem. Mater. 2010, 22, 898–905. [Google Scholar] [CrossRef]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.Y.; Suib, S.L. Structure-property relationship of bifunctional MnO2 nanostructures: Highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef] [PubMed]
- Stoerzinger, K.A.; Risch, M.; Han, B.; Yang, S.H. Recent insights into manganese oxides in catalyzing oxygen reduction kinetics. ACS Catal. 2015, 5, 446–449. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Renger, G.; Hołyńska, M.; Moghaddam, A.N.; Aro, E.M.; Carpentier, R.; Nishihara, H.; Eatonrye, J.J.; Shen, J.R.; Allakhverdiev, S.I. Manganese compounds as water-oxidizing catalysts: From the natural water-oxidizing complex to nanosized manganese oxide structures. Chem. Rev. 2016, 116, 2886. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Q.; Liu, Y.R.; Hu, W.H.; Dong, B.; Li, X.; Shang, X.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. Crystallographic structure and morphology transformation of MnO2 nanorods as efficient electrocatalysts for oxygen evolution reaction. J. Electrochem. Soc. 2016, 163, H67–H73. [Google Scholar] [CrossRef]
- Pokhrel, R.; Goetz, M.K.; Shaner, S.E.; Wu, X.; Stahl, S.S. The “best catalyst” for water oxidation depends on the oxidation method employed: A case study of manganese oxides. J. Am. Chem. Soc. 2015, 137, 8384–8387. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Teng, F.; Bulgan, G.; Ruilong Zong, A.; Zhu, Y. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J. Phys. Chem. C 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Cao, Y.L.; Yang, H.X.; Ai, X.P.; Xiao, L.F. The mechanism of oxygen reduction on MnO2 -catalyzed air cathode in alkaline solution. J. Electroanal. Chem. 2003, 557, 127–134. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, R.; Wang, H.; Key, J.; Ji, S. Control of MnO2 nanocrystal shape from tremella to nanobelt for ehancement of the oxygen reduction reaction activity. J. Power Sources 2015, 280, 526–532. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, D.; Lou, X.W. Shape-controlled synthesis of MnO2 nanostructures with enhanced electrocatalytic activity for oxygen reduction. J. Phys. Chem. C 2010, 114, 1430–1434. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 647. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shaohorn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2012, 43, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhang, T.; Zhang, Y.; Du, J.; Han, X.; Chen, J. Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies. Angew. Chem. Int. Ed. 2013, 52, 2474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cheng, F.; Du, J.; Hu, Y.; Chen, J. Efficiently enhancing oxygen reduction electrocatalytic activity of MnO2 using facile hydrogenation. Adv. Energy Mater. 2015, 5, 1400654. [Google Scholar] [CrossRef]
- Davis, D.J.; Lambert, T.N.; Vigil, J.A.; Rodriguez, M.A.; Brumbach, M.T.; Coker, E.N.; Limmer, S.J. Role of Cu-ion doping in Cu-α-MnO2 nanowire electrocatalysts for the oxygen reduction reaction. J. Phys. Chem. C 2014, 118, 17342–17350. [Google Scholar] [CrossRef]
- King’Ondu, C.K.; Opembe, N.; Chen, C.H.; Ngala, K.; Huang, H.; Iyer, A.; Garcés, H.F.; Suib, S.L. Manganese oxide octahedral molecular sieves (OMS-2) multiple framework substitutions: A new route to OMS-2 particle size and morphology control. Adv. Funct. Mater. 2011, 21, 312–323. [Google Scholar] [CrossRef]
- Chen, S.Y.; Song, W.; Lin, H.J.; Wang, S.; Biswas, S.; Mollahosseini, M.; Kuo, C.H.; Gao, P.X.; Suib, S.L. Manganese oxide nanoarray-based monolithic catalysts: Tunable morphology and high efficiency for CO oxidation. ACS Appl. Mater. Interfaces 2016, 8, 7834. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yu, L.; Lan, B.; Cheng, G.; Lin, T.; He, B.; Ye, W.; Sun, M.; Ye, F. Three-dimensional radial α-MnO2 synthesized from different redox potential for bifunctional oxygen electrocatalytic activities. J. Power Sources 2017, 362, 332–341. [Google Scholar] [CrossRef]
- Ozcan, S.; Tokur, M.; Cetinkaya, T.; Guler, A.; Uysal, M.; Guler, M.O.; Akbulut, H. Free standing flexible graphene oxide + α-MnO2 composite cathodes for Li–air batteries. Solid State Ion. 2016, 286, 34–39. [Google Scholar] [CrossRef]
- Li, F.; Fu, L.; Li, J.; Yan, J.; Tang, Y.; Pan, Y.; Wangz, H. Ag/Fe3O4-N-doped ketjenblack carbon composite as highly efficient oxygen reduction catalyst in Al-air batteries. J. Electrochem. Soc. 2017, 164, A3595–A3601. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Wang, S.; Ren, Z.; Yu, J. Transition metal doped MnO2 nanosheets grown on internal surface of macroporous carbon for supercapacitors and oxygen reduction reaction electrocatalysts. Appl. Mater. Today 2016, 3, 63–72. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, X.; Feng, X.; Qiu, G.; Tan, W.; Liu, F. Large-scale size-controlled synthesis of cryptomelane-type manganese oxide OMS-2 in lateral and longitudinal directions. J. Mater. Chem. A 2011, 21, 462–465. [Google Scholar] [CrossRef]
- Lee, S.; Nam, G.; Sun, J.; Lee, J.S.; Lee, H.W.; Chen, W.; Cho, J.; Cui, Y. Enhanced intrinsic catalytic activity of λ-MnO2 by electrochemical tuning and oxygen vacancy generation. Angew. Chem. Int. Ed. 2016, 128, 8599–8604. [Google Scholar] [CrossRef] [PubMed]
- Brenet, J.P. Electrochemical behaviour of metallic oxides. J. Power Sources 1979, 4, 183–190. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Z.; Liu, K.; Li, F.; Peng, Z.; Tang, Y.; Wang, H. Co3O4/Co-N-C modified ketjenblack carbon as an advanced electrocatalyst for Al-air batteries. J. Power Sources 2017, 343, 30–38. [Google Scholar] [CrossRef]
- Liu, K.; Huang, X.; Wang, H.; Li, F.; Tang, Y.; Li, J.; Shao, M. Co3O4-CeO2/C as a highly active electrocatalyst for oxygen reduction reaction in Al-air batteries. ACS Appl. Mater. Int. 2016, 8, 34422–34430. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Peng, Z.; Wang, H.; Ren, Y.; Liu, D.; Li, J.; Tang, Y.; Zhang, N. Fe3C@Fe/N doped graphene-like carbon sheets as a highly efficient catalyst in Al-air batteries. J. Electrochem. Soc. 2017, 164, F475–F483. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Wang, H.; Ren, Y.; Liu, K.; Tang, Y.; Shao, M. Fe/N co-doped carbon materials with controllable structure as highly efficient electrocatalysts for oxygen reduction reaction in Al-air batteries. Energy Storage Mater. 2017, 8, 49–58. [Google Scholar] [CrossRef]
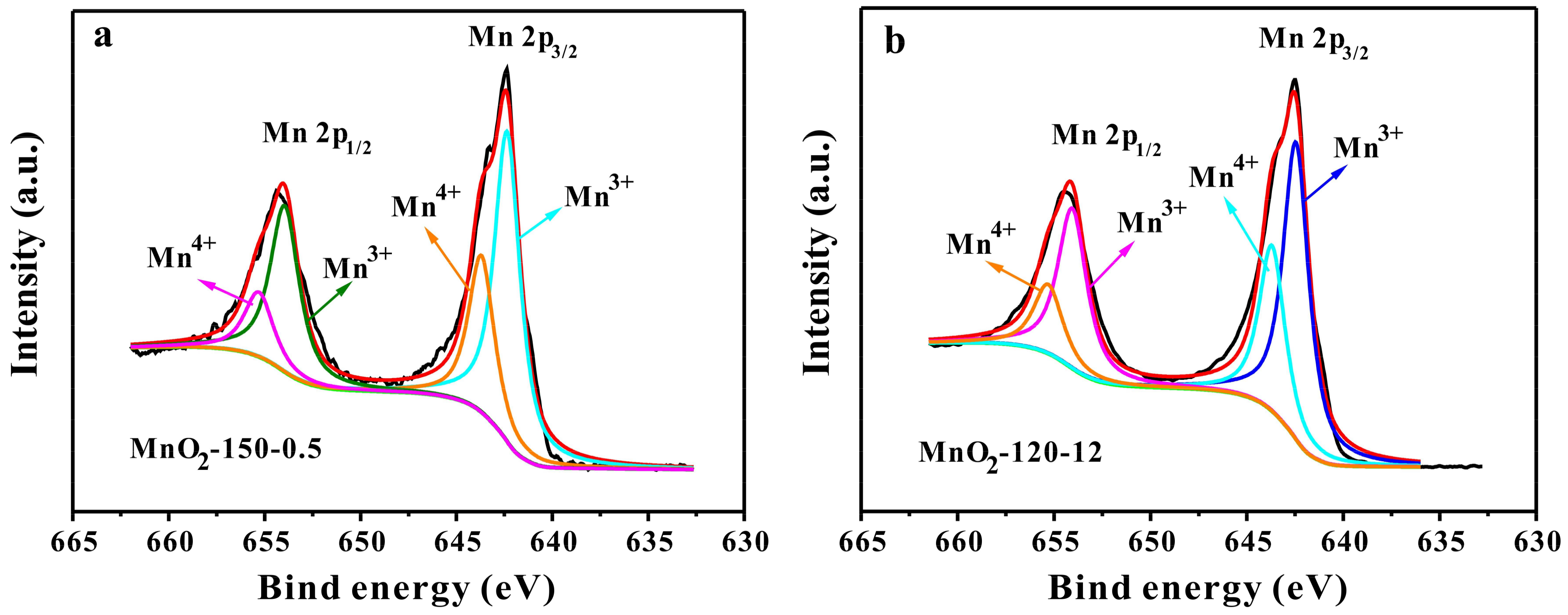
| Samples | Peaks | Ion Species | Peak Position (eV) | Peak Area | Mn3+/Mn4+ |
|---|---|---|---|---|---|
| MnO2-150-0.5 | 2P3/2 | Mn3+ | 642.35 | 51,009.41 | 2.16 |
| Mn4+ | 643.70 | 25,816.35 | |||
| 2P1/2 | Mn3+ | 653.95 | 30,811.95 | ||
| Mn4+ | 655.30 | 12,019.14 | |||
| MnO2-120-12 | 2P3/2 | Mn3+ | 642.35 | 41,672.21 | 1.96 |
| Mn4+ | 643.70 | 23,236.07 | |||
| 2P1/2 | Mn3+ | 653.95 | 25,282.41 | ||
| Mn4+ | 655.30 | 10,952.38 |
| Samples | Rs (Ω) | Rf (Ω) | Rct (Ω) | C (F) | Wo-R | Wo-T | Wo-P |
|---|---|---|---|---|---|---|---|
| MnO2-150-0.5/N-KB | 49.47 | 5.3 | 68.0 | 0.00108 | 10.94 | 0.01586 | 0.40993 |
| MnO2-120-12/N-KB | 48.32 | 7.2 | 72.2 | 0.00067 | 33.00 | 0.01125 | 0.32217 |
| Samples | Rs (Ω) | Rf (Ω) | Rct (Ω) | C (F) | CPE-T | CPE-P |
|---|---|---|---|---|---|---|
| MnO2-150-0.5/N-KB | 59.13 | 5.12 | 227.7 | 0.05920 | 0.002868 | 0.712 |
| MnO2-120-12/N-KB | 61.39 | 9.21 | 310.2 | 0.05640 | 0.001926 | 0.889 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment. Catalysts 2018, 8, 138. https://doi.org/10.3390/catal8040138
Wang M, Chen K, Liu J, He Q, Li G, Li F. Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment. Catalysts. 2018; 8(4):138. https://doi.org/10.3390/catal8040138
Chicago/Turabian StyleWang, Mei, Kui Chen, Jun Liu, Quanguo He, Guangli Li, and Fuzhi Li. 2018. "Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment" Catalysts 8, no. 4: 138. https://doi.org/10.3390/catal8040138
APA StyleWang, M., Chen, K., Liu, J., He, Q., Li, G., & Li, F. (2018). Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment. Catalysts, 8(4), 138. https://doi.org/10.3390/catal8040138






