The Role of Fe2O3 Species in Depressing the Formation of N2O in the Selective Reduction of NO by NH3 over V2O5/TiO2-Based Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Fe2O3-Promoted V2O5-WO3/TiO2 Catalysts
2.2. Textural Features of Fe2O3-Promoted V2O5-WO3/TiO2 Catalysts
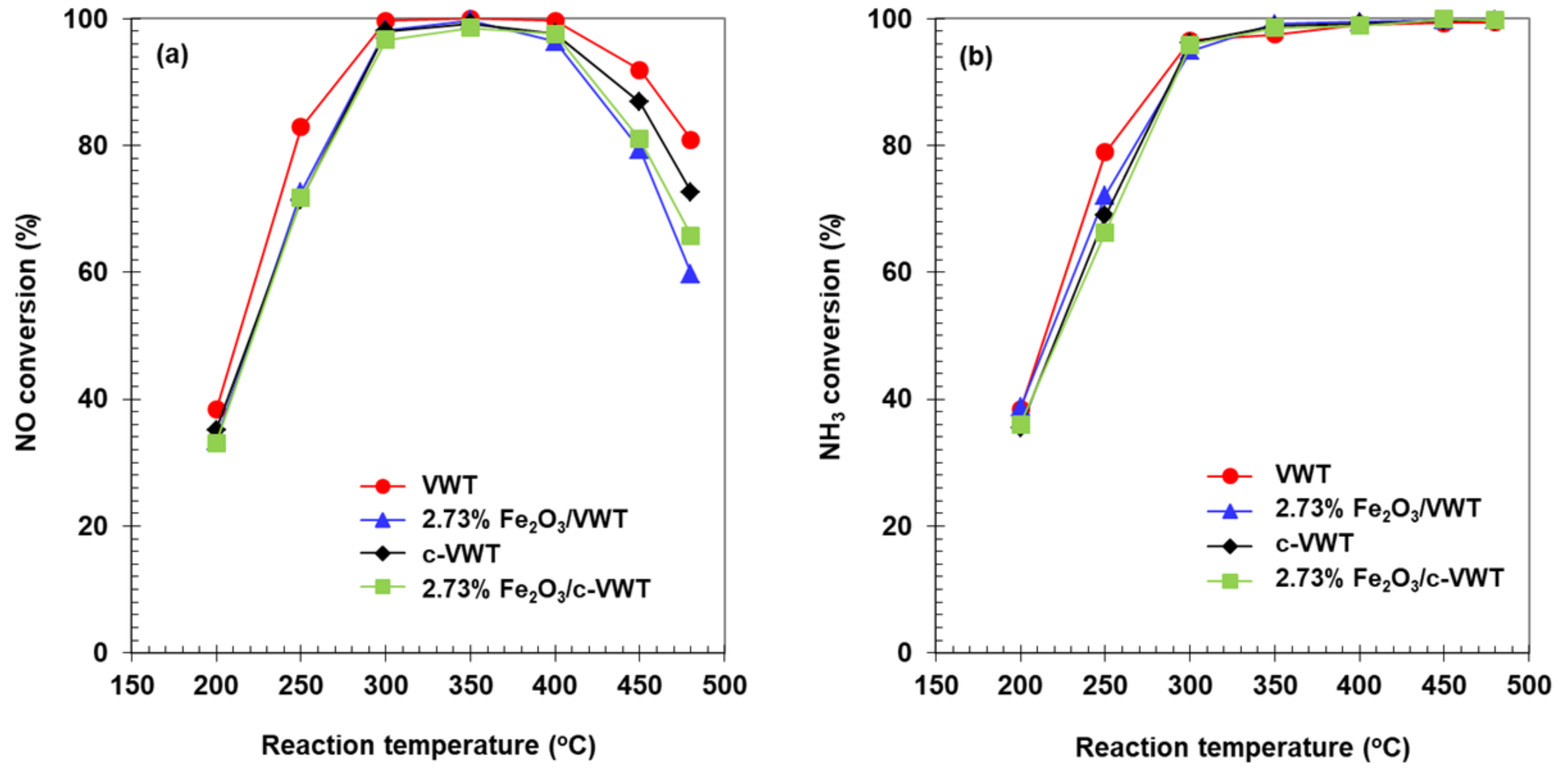
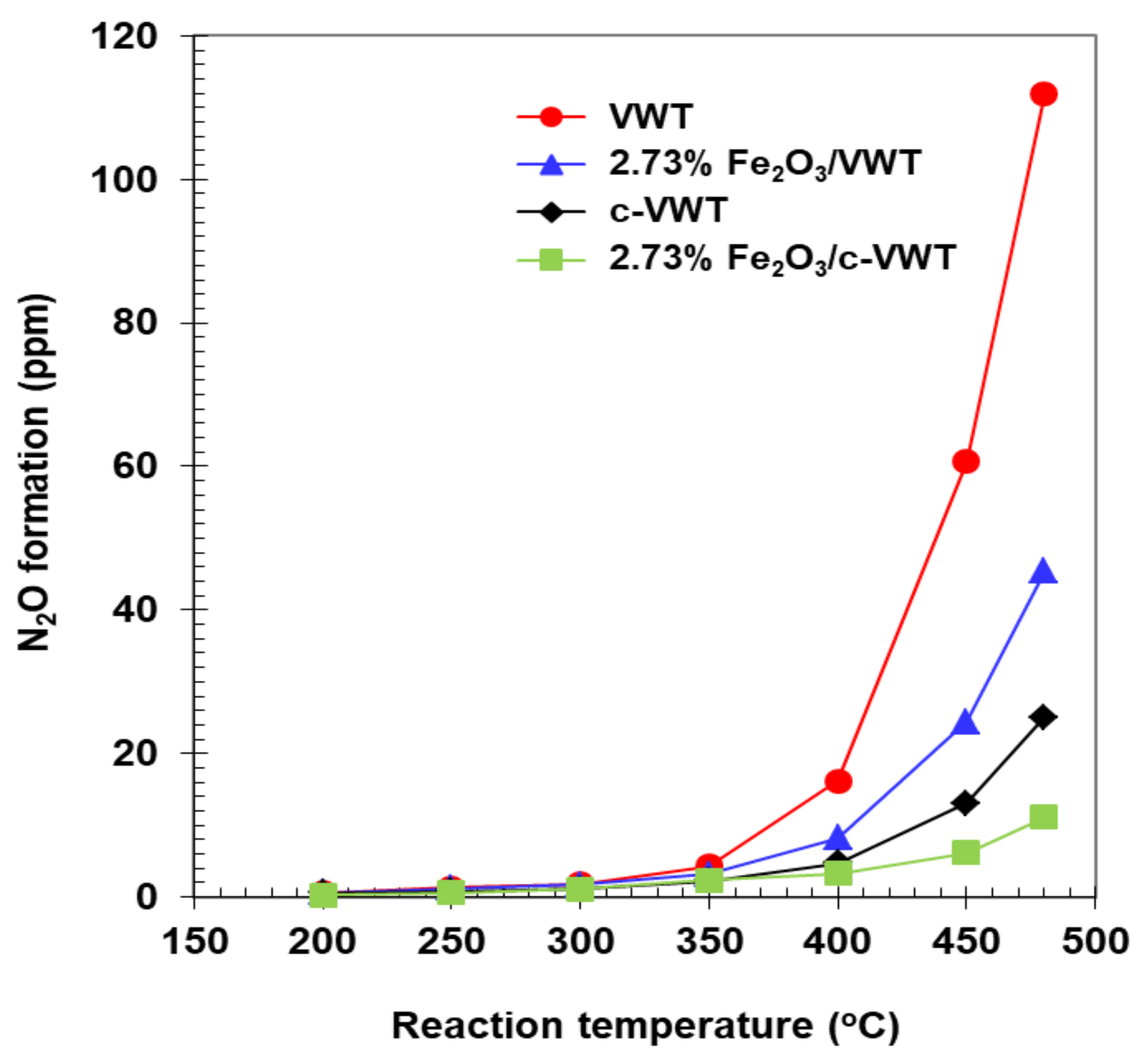
2.3. Effect of Fe2O3 Species on NH3-SCR Reaction and N2O Formation
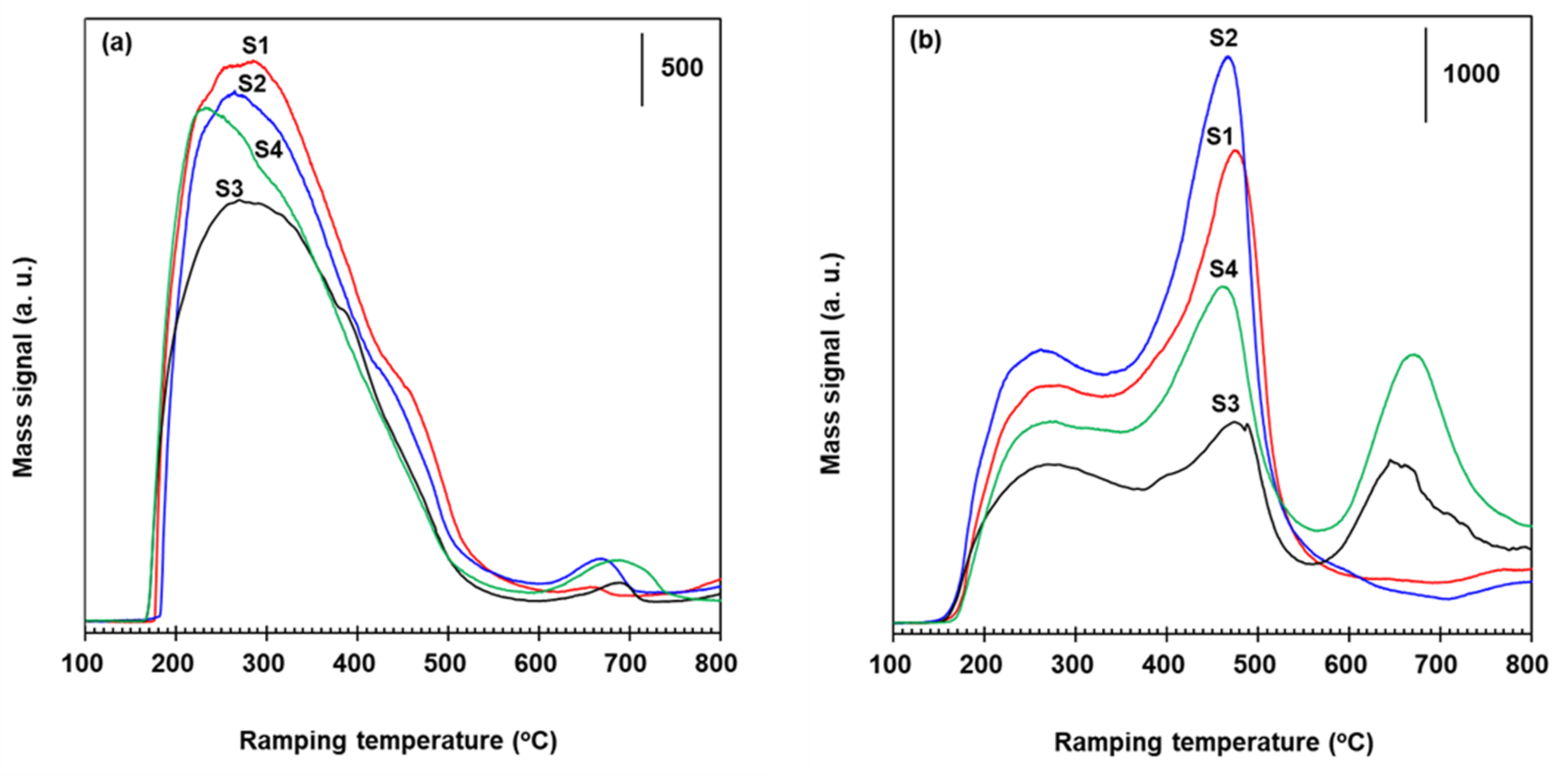
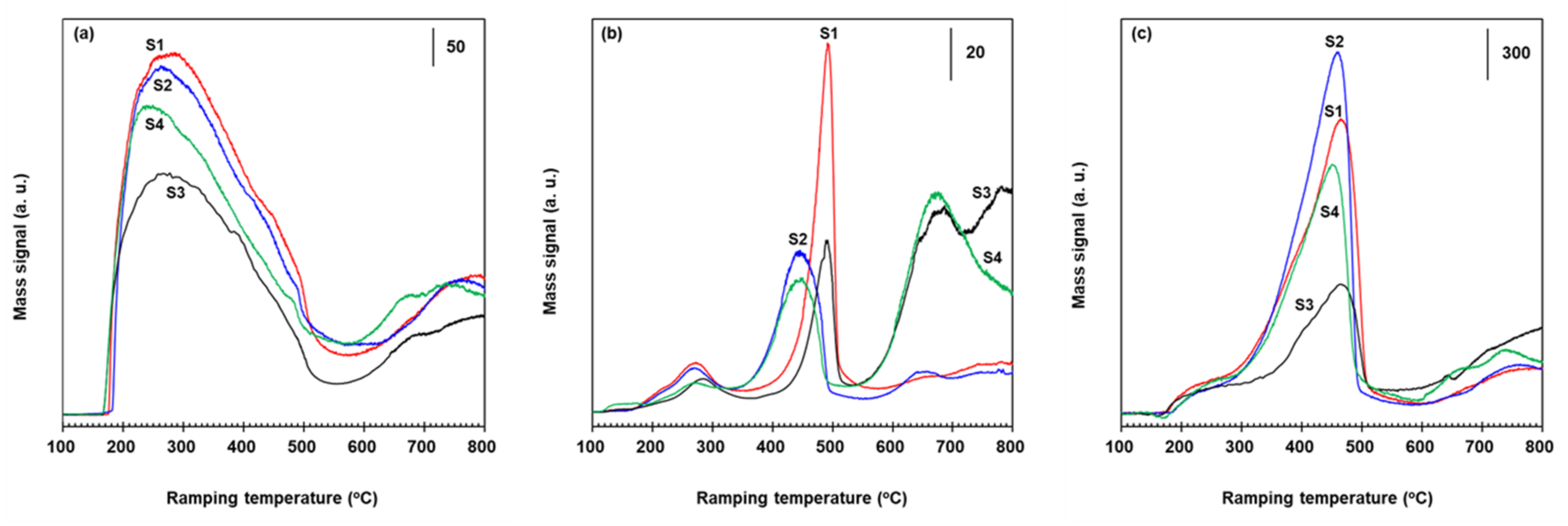
2.4. Role of Fe2O3 Species for the Suppression of N2O Formation
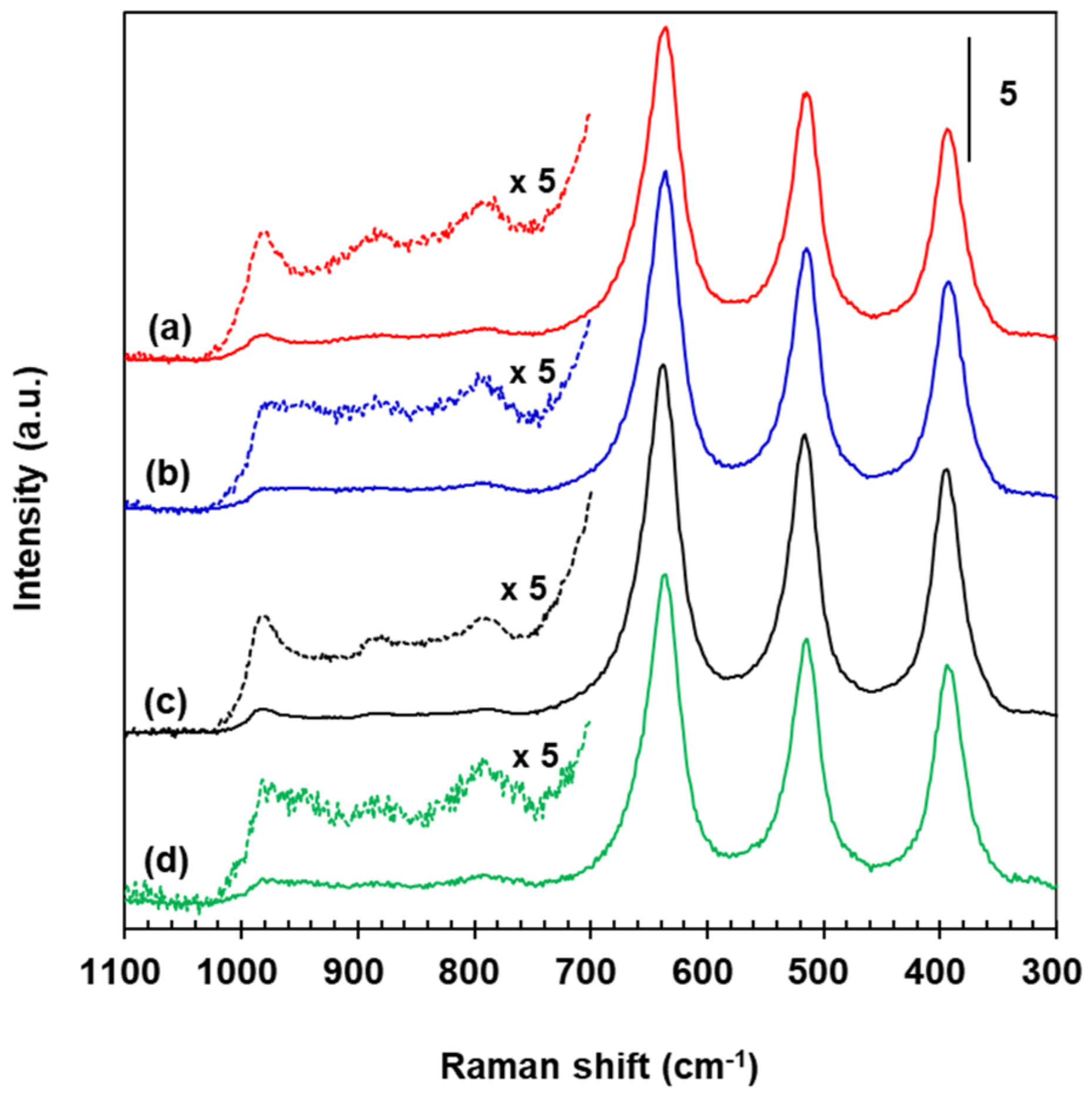
2.5. Surface Structure of Fe2O3, V2O5, and WO3 Species
3. Experimental
3.1. Preparation of Catalyst Samples
3.2. NH3-SCR deNOx Reaction and Determination of N2O Formation
3.3. Characterization of Catalyst Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marberger, A.; Elsener, M.; Ferri, D.; Krocher, O. VOx surface coverage optimization of V2O5/WO3-TiO2 SCR catalysts by variation of the V loading and by aging. Catalysts 2015, 5, 1704–1720. [Google Scholar] [CrossRef]
- He, Y.; Ford, M.E.; Zhu, M.; Liu, Q.; Tumuluri, U.; Wu, Z.; Wachs, I.E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3/TiO2 catalysts. Appl. Catal. B 2016, 193, 141–150. [Google Scholar] [CrossRef]
- Madia, G.; Elsener, M.; Koebel, M.; Raimondi, F.; Wokaun, A. Thermal stability of vanadia-tungsta-titania catalysts in the SCR process. Appl. Catal. B 2002, 39, 181–190. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Nova, I.; dall’Acqua, L.; Lietti, L.; Giamello, E.; Forzatti, P. Study of thermal deactivation of a de-NOx commercial catalyst. Appl. Catal. B 2001, 35, 31–42. [Google Scholar] [CrossRef]
- Kim, M.H.; Ham, S.W. Determination of N2O emissions levels in the selective reduction of NOx by NH3 over an on-site-used commercial V2O5-WO3/TiO2 catalyst using a modified gas cell. Top. Catal. 2010, 53, 597–607. [Google Scholar] [CrossRef]
- Kompio, P.G.W.A.; Bruckner, A.; Hipler, F.; Auer, G.; Loffler, E.; Grunert, W. A new view on the relations between tungsten and vanadium in V2O5-WO3/TiO2 catalysts for the selective reduction of NO with NH3. J. Catal. 2012, 286, 237–247. [Google Scholar] [CrossRef]
- Lietti, L.; Nova, I.; Ramis, G.; Dall’Acqua, L.; Busca, G.; Giamello, E.; Forzatti, P.; Bregani, F. Characterization and reactivity of V2O5–MoO3/TiO2 de-NOx SCR catalysts. J. Catal. 1999, 187, 419–435. [Google Scholar] [CrossRef]
- Nova, I.; Ciardelli, C.; Tronconi, E.; Chatterjee, D.; Weibel, M. Unifying redox kinetics for standard and fast NH3-SCR over a V2O5-WO3/TiO2 catalyst. AIChE J. 2009, 55, 1514–1529. [Google Scholar] [CrossRef]
- Nova, I.; Ciardelli, C.; Tronconi, E.; Chatterjee, D.; Bandl-Konrad, B. NH3-NO/NO2 chemistry over V-based catalysts and its role in the mechanism of the fast SCR reaction. Catal. Today 2006, 114, 3–12. [Google Scholar] [CrossRef]
- Xiong, S.; Xiao, X.; Liao, Y.; Dang, H.; Shan, W.; Yang, S. Global kinetic study of NO reduction by NH3 over V2O5–WO3/TiO2: Relationship between the SCR performance and the key factors. Ind. Eng. Chem. Res. 2015, 54, 11011–11023. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Madia, G.; Koebel, M.; Elsener, M.; Wokaun, A. Side reactions in the selective catalytic reduction of NOx with various NO2 fractions. Ind. Eng. Chem. Res. 2002, 41, 4008–4015. [Google Scholar] [CrossRef]
- Djerad, S.; Crocoll, M.; Kureti, S.; Tifouti, L.; Weisweiler, W. Effect of oxygen concentration on the NOx reduction with ammonia over V2O5–WO3/TiO2 catalyst. Catal. Today 2006, 113, 208–214. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.S. Effect of Fe-zeolite on formation of N2O in selective reduction of NO by NH3 over V2O5-WO3/TiO2 catalyst. Res. Chem. Intermed. 2016, 42, 171–184. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, S.W. Selective reduction of NO by NH3 over Fe-zeolite-promoted V2O5-WO3/TiO2-based catalysts: Great suppression of N2O formation and origin of NO removal activity loss. Catal. Commun. 2016, 86, 82–85. [Google Scholar] [CrossRef]
- Krocher, O.; Elsener, M. Combination of V2O5/WO3-TiO2, Fe-ZSM5, and Cu-ZSM5 catalysts for the selective catalytic reduction of nitric oxide with ammonia. Ind. Eng. Chem. Res. 2008, 47, 8588–8593. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Ma, L.; Peng, Y.; Qu, Z.; Yan, N.; Chen, J.; Chang, H.; Li, J. Substitution of WO3 in V2O5/WO3-TiO2 by Fe2O3 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2013, 3, 161–168. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, D.; Liu, X.; Shi, L.; Maitarad, P.; Li, H.; Zhang, J.; Cao, W. Enhanced catalytic performance of V2O5-WO3/Fe2O3/TiO2 microspheres for selective catalytic reduction of NO by NH3. Catal. Sci. Technol. 2013, 3, 191–199. [Google Scholar] [CrossRef]
- Devadas, M.; Krocher, O.; Elsener, M.; Wokaun, A.; Mitrikas, G.; Soger, N.; Pfeifer, M.; Demel, Y.; Mussmann, L. Characterization and catalytic investigation of Fe-ZSM5 for urea-SCR. Catal. Today 2007, 119, 137–144. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Ultra-active Fe/ZSM-5 catalyst for selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B 2005, 60, 13–22. [Google Scholar] [CrossRef]
- Rivallan, M.; Ricchiardi, G.; Bordiga, S.; Zecchina, A. Adsorption and reactivity of nitrogen oxides (NO2, NO, N2O) on Fe-zeolites. J. Catal. 2009, 264, 104–116. [Google Scholar] [CrossRef]
- Coq, B.; Mauvezin, M.; Delahay, G.; Butet, J.B.; Kieger, S. The simultaneous catalytic reduction of NO and N2O by NH3 using an Fe-zeolite-beta catalyst. Appl. Catal. B 2000, 27, 193–198. [Google Scholar] [CrossRef]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.S.; Shen, W. Rod-shaped Fe2O3 as an efficient catalyst for the selective reduction of nitrogen oxide by ammonia. Angew. Chem. Int. Ed. 2012, 51, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Miao, L.; Wang, H.; Fu, Q. Highly dispersed Fe2O3 on carbon nanotubes for low-temperature selective catalytic reduction of NO with NH3. Chem. Commun. 2015, 51, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Kim, M.H.; An, T.H. A commercial V2O5-WO3/TiO2 catalyst used at an NH3-SCR deNOx process in an oil-fired power plant: Cause of an increase in deNOxing and NH3 oxidation performances at low temperatures. Res. Chem. Intermed. 2011, 37, 1333–1344. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.; Zhang, Z.; Yang, X.; Hou, W.; Zhu, J.J. Polyaniline-intercalated layered vanadium oxide nanocomposites—One-pot hydrothermal synthesis and application in lithium battery. Nanoscale 2010, 2, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, H.; Fu, W.; Du, K.; Sui, Y.; Chen, J.; Zeng, Y.; Li, M.; Zou, G. Preparation and magnetic properties of magnetite nanoparticles by sol–gel method. J. Magn. Magn. Mater. 2007, 309, 307–311. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Silva, S.V.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Thommes, M.; Smarsly, B.; Groenewolt, M.; Ravikovitch, P.I.; Neimark, A.V. Adsorption hysteresis of nitrogen and argon in pore networks and characterization of novel micro- and mesoporous silicas. Langmuir 2006, 22, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Li, W.; Zhang, B.; Xie, Y. Low temperature vapor-phase preparation of TiO2 nanopowder. J. Mater. Sci. 1999, 34, 3505–3511. [Google Scholar] [CrossRef]
- Alemany, L.J.; Berti, F.; Busca, G.; Ramis, G.; Robba, D.; Toledo, G.P.; Trombetta, M. Characterization and composition of commercial V2O5-WO5-TiO2 SCR catalysts. Appl. Catal. B 1996, 10, 299–311. [Google Scholar] [CrossRef]
- Gutierrez, M.J.F.; Baxter, D.; Hunter, C.; Svoboda, K. Nitrous Oxide (N2O) emissions from waste and biomass to energy plants. Waste Manag. Res. 2005, 23, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Kompio, P.G.W.A.; Bruckner, A.; Hipler, F.; Manoylova, O.; Auer, G.; Mestl, G.; Grunert, W. V2O5-WO3/TiO2 catalysts under thermal stress: Responses of structure and catalytic behavior in the selective catalytic reduction of NO by NH3. Appl. Catal. B 2017, 217, 365–377. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Chang, H.; Peng, Y.; Li, J. Dispersion of tungsten oxide on SCR performance of V2O5-WO3/TiO2: Acidity, surface species and catalytic activity. Chem. Eng. J. 2013, 225, 520–527. [Google Scholar] [CrossRef]
- Lietti, L.; Nova, I.; Forzatti, P. Selective catalytic reduction (SCR) of NO by NH3 over TiO2-supported V2O5-WO3 and V2O5-MoO3 catalysts. Top. Catal. 2000, 11, 111–122. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Selective catalytic oxidation of ammonia to nitrogen over Fe2O3-TiO2 prepared with a sol–gel method. J. Catal. 2002, 207, 158–165. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Q.; He, C.; Ma, C.; Cheng, J.; Hao, Z. N2O catalytic reduction by NH3 over Fe-zeolites: Effective removal and active site. Catal. Commun. 2012, 18, 151–155. [Google Scholar] [CrossRef]
- Went, G.T.; Leu, L.J.; Rosin, R.R.; Bell, A.T. The effects of structure on the catalytic activity and selectivity of V2O5/TiO2 for the reduction of NO by NH3. J. Catal. 1992, 134, 492–505. [Google Scholar] [CrossRef]
- Usberti, N.; Jablonska, M.; Blasi, M.D.; Forzatti, P.; Lietti, L.; Beretta, A. Design of a “high-efficiency” NH3-SCR reactor for stationary applications. A kinetic study of NH3 oxidation and NH3-SCR over V-based catalysts. Appl. Catal. B 2015, 179, 185–195. [Google Scholar] [CrossRef]
- Giraud, F.; Geantet, C.; Guilhaume, N.; Loridant, S.; Gros, S.; Porcheron, L.; Kanniche, M.; Bianchi, D. Experimental microkinetic approach of de-NOx by NH3 on V2O5/WO3/TiO2 catalysts. 3. Impact of superficial WOz and VxOy/WOz groups on the heats of adsorption of adsorbed NH3 species. J. Phys. Chem. C 2015, 119, 15401–15413. [Google Scholar] [CrossRef]
- Kantcheva, M.M.; Hadjiivanov, K.I.; Klissurski, D.G. An IR spectroscopy study of the state and localization of vanadium-oxo species adsorbed on TiO2 (anatase). J. Catal. 1992, 134, 299–310. [Google Scholar] [CrossRef]
- Topsoe, N.Y. Characterization of the nature of surface sites on vanadia-titania catalysts by FTIR. J. Catal. 1991, 128, 499–511. [Google Scholar] [CrossRef]
- Lorenzelli, V.; Busca, G. Infrared studies of the surface of α-Fe2O3. Mater. Chem. Phys. 1985, 13, 261–281. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G.; Turco, M.; Kotur, E.; Willey, R.J. Adsorption, activation, and oxidation of ammonia over SCR catalysts. J. Catal. 1995, 157, 523–535. [Google Scholar] [CrossRef]
- Jung, S.M.; Grange, P. DRIFTS investigation of V=O behavior and its relations with the reactivity of ammonia oxidation and selective catalytic reduction of NO over V2O5 catalyst. Appl. Catal. B 2002, 36, 325–332. [Google Scholar] [CrossRef]
- Went, G.T.; Oyama, S.T.; Bell, A.T. Laser Raman spectroscopy of supported vanadium oxide catalysts. J. Phys. Chem. 1990, 94, 4240–4246. [Google Scholar] [CrossRef]
- Boulova, M.; Lucazeau, G. Crystallite nanosize effect on the structural transitions of WO3 studied by Raman spectroscopy. J. Solid State Chem. 2002, 167, 425–434. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kurti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Ostromecki, M.; Wachs, I.E. Surface structures of supported tungsten oxide catalysts under dehydrated conditions. J. Mol. Catal. A 1996, 106, 93–102. [Google Scholar] [CrossRef]
- Engweiler, J.; Harf, J.; Baiker, A. WOx/TiO2 catalysts prepared by grafting of tungsten alkoxides: Morphological properties and catalytic behavior in the selective reduction of NO by NH3. J. Catal. 1996, 159, 259–269. [Google Scholar] [CrossRef]
- Vuurman, M.A.; Wachs, I.E.; Hirt, A.M. Structural determination of supported V2O5-WO3/TiO2 catalysts by in situ Raman spectroscopy and X-ray photoelectron spectroscopy. J. Phys. Chem. 1991, 95, 9928–9937. [Google Scholar] [CrossRef]
- Reiche, M.A.; Burgi, T.; Baiker, A.; Scholz, A.; Schnyder, B.; Wokaun, A. Vanadia and tungsta grafted on TiO2: Influence of the grafting sequence on structural and chemical properties. Appl. Catal. A 2000, 198, 155–169. [Google Scholar] [CrossRef]
- Wachs, I.E.; Roberts, C.A. Monitoring surface metal oxide catalytic active sites with Raman spectroscopy. Chem. Soc. Rev. 2010, 39, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Amiridis, M.D.; Duevel, R.V.; Wachs, I.E. The effect of metal oxide additives on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of nitric oxide by ammonia. Appl. Catal. B 1999, 20, 111–122. [Google Scholar] [CrossRef]
- Colomban, P.; Cherifi, S.; Despert, G. Raman identification of corrosion products on automotive galvanized steel sheets. J. Raman Spectrosc. 2008, 39, 881–886. [Google Scholar] [CrossRef]
- Bourikas, K.; Fountzoula, C.; Kordulis, C. Monolayer transition metal supported on titania catalysts for the selective catalytic reduction of NO by NH3. App. Catal. B 2004, 52, 145–153. [Google Scholar] [CrossRef]
- Dunn, J.P.; Stenger, H.G., Jr.; Wachs, I.E. Oxidation of SO2 over supported metal oxide catalysts. J. Catal. 1999, 181, 233–243. [Google Scholar] [CrossRef]
- Amiridis, M.; Wachs, I.E.; Deo, G.; Jehng, J.M.; Kim, D.S. Reactivity of V2O5 catalysts for the selective catalytic reduction of NO by NH3: Influence of vanadia loading, H2O, and SO2. J. Catal. 1996, 161, 247–253. [Google Scholar] [CrossRef]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- Bond, G.C.; Bruckman, K. Selective oxidation of o-xylene by monolayer V2O5-TiO2 catalysts. Faraday Discuss. Chem. Soc. 1981, 72, 235–246. [Google Scholar] [CrossRef]
- Vermaire, D.C.; van Berge, P.C. The preparation of WO3/TiO2 and WO3/A12O3 and characterization by temperature-programmed reduction. J. Catal. 1989, 116, 309–317. [Google Scholar] [CrossRef]
- Yu, X.F.; Wu, N.Z.; Huang, H.Z.; Xie, Y.C.; Tang, Y.Q. A study on the monolayer dispersion of tungsten oxide on anatase. J. Mater. Chem. 2001, 11, 3337–3342. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Lian, Z.; Shan, W.; Xie, L.; Asakura, K.; Yang, W.; Deng, H. Highly dispersed iron vanadate catalyst supported on TiO2 for the selective catalytic reduction of NOx with NH3. J. Catal. 2013, 307, 340–351. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, M.H.; Ham, S.W. An on-line infrared spectroscopic system with a modified multipath White cell for direct measurements of N2O from NH3-SCR reaction. Korean J. Chem. Eng. 2010, 27, 1730–1737. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, I.H.; Park, J.H.; Choi, S.O.; Lee, I.S. Adsorption of CO2 and CO on H-zeolites with different framework topologies and chemical compositions and a correlation to probing protonic sites using NH3 adsorption. J. Porous Mater. 2016, 23, 291–299. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, I.H.; Choi, S.O.; Lee, I.S. Surface energetic heterogeneity of nanoporous solids for CO2 and CO adsorption: The key to an adsorption capacity and selectivity at low pressures. J. Nanosci. Nanotechnol. 2016, 16, 4474–4479. [Google Scholar] [CrossRef] [PubMed]



| Catalyst | Amount (%) | SBET (m2/g) | dm (Å) a | Vt (cm3/g) b | |
|---|---|---|---|---|---|
| V2O5 | WO3 | ||||
| WT | - | 10 | 91 | 127 | 0.30 |
| VWT | 1.6 | 10 | 67 | 142 | 0.26 |
| 2.73% Fe2O3/VWT | 1.6 | 10 | 60 | 139 | 0.23 |
| c-VWT | 1.44 | 9.42 | 70 | 144 | 0.27 |
| 2.73% Fe2O3/c-VWT | 1.44 | 9.42 | 89 | 126 | 0.31 |
| Catalyst | Surface Density (μmol/m2) | Surface Coverage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VOx | WOx | FeOx | MeOx a | VOx b | WOx c | FeOx d | MeOx e | ||
| WT | - | 4.74 | - | 4.74 | - | 0.86 ± 0.17 | - | 0.86 ± 0.17 | |
| VWT | 2.63 | 6.44 | - | 9.07 | 0.22 ± 0.02 | 1.16 ± 0.24 | - | 1.38 ± 0.26 | |
| 2.73% Fe2O3/VWT | 2.93 | 7.19 | 5.69 | 15.81 | 0.24 ± 0.03 | 1.30 ± 0.26 | 0.87 | 1.54 ± 0.29 | |
| c-VWT | 2.26 | 5.80 | - | 8.06 | 0.19 ± 0.02 | 1.05 ± 0.21 | - | 1.24 ± 0.23 | |
| 2.73% Fe2O3/c-VWT | 1.78 | 4.57 | 3.84 | 10.19 | 0.15 ± 0.01 | 0.82 ± 0.17 | 0.59 | 0.97 ± 0.18 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Yang, K.H. The Role of Fe2O3 Species in Depressing the Formation of N2O in the Selective Reduction of NO by NH3 over V2O5/TiO2-Based Catalysts. Catalysts 2018, 8, 134. https://doi.org/10.3390/catal8040134
Kim MH, Yang KH. The Role of Fe2O3 Species in Depressing the Formation of N2O in the Selective Reduction of NO by NH3 over V2O5/TiO2-Based Catalysts. Catalysts. 2018; 8(4):134. https://doi.org/10.3390/catal8040134
Chicago/Turabian StyleKim, Moon Hyeon, and Ki Hyuck Yang. 2018. "The Role of Fe2O3 Species in Depressing the Formation of N2O in the Selective Reduction of NO by NH3 over V2O5/TiO2-Based Catalysts" Catalysts 8, no. 4: 134. https://doi.org/10.3390/catal8040134
APA StyleKim, M. H., & Yang, K. H. (2018). The Role of Fe2O3 Species in Depressing the Formation of N2O in the Selective Reduction of NO by NH3 over V2O5/TiO2-Based Catalysts. Catalysts, 8(4), 134. https://doi.org/10.3390/catal8040134




