A New Approach to Deep Desulfurization of Light Cycle Oil over Ni2P Catalysts: Combined Selective Oxidation and Hydrotreating
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Properties of Catalysts
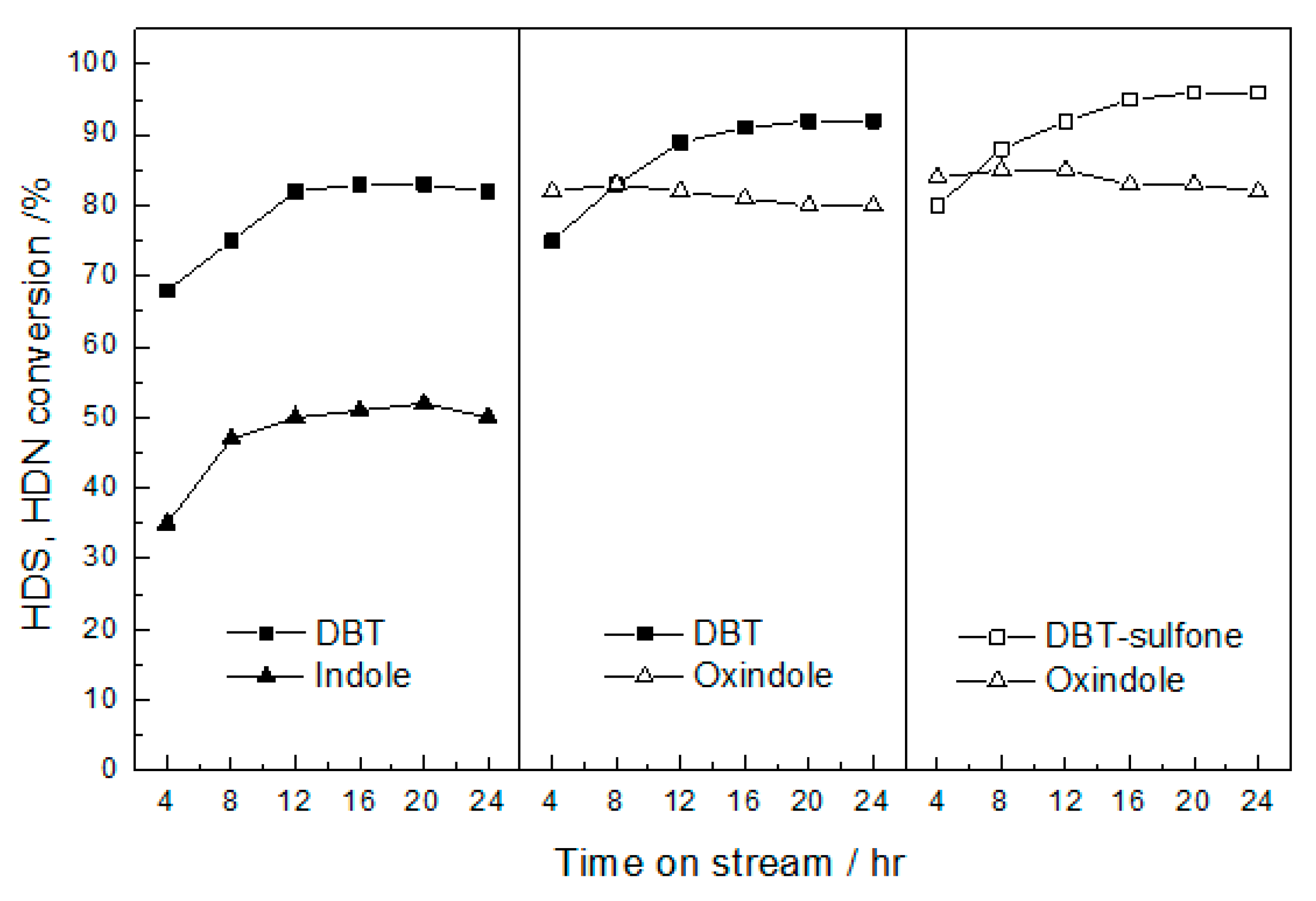
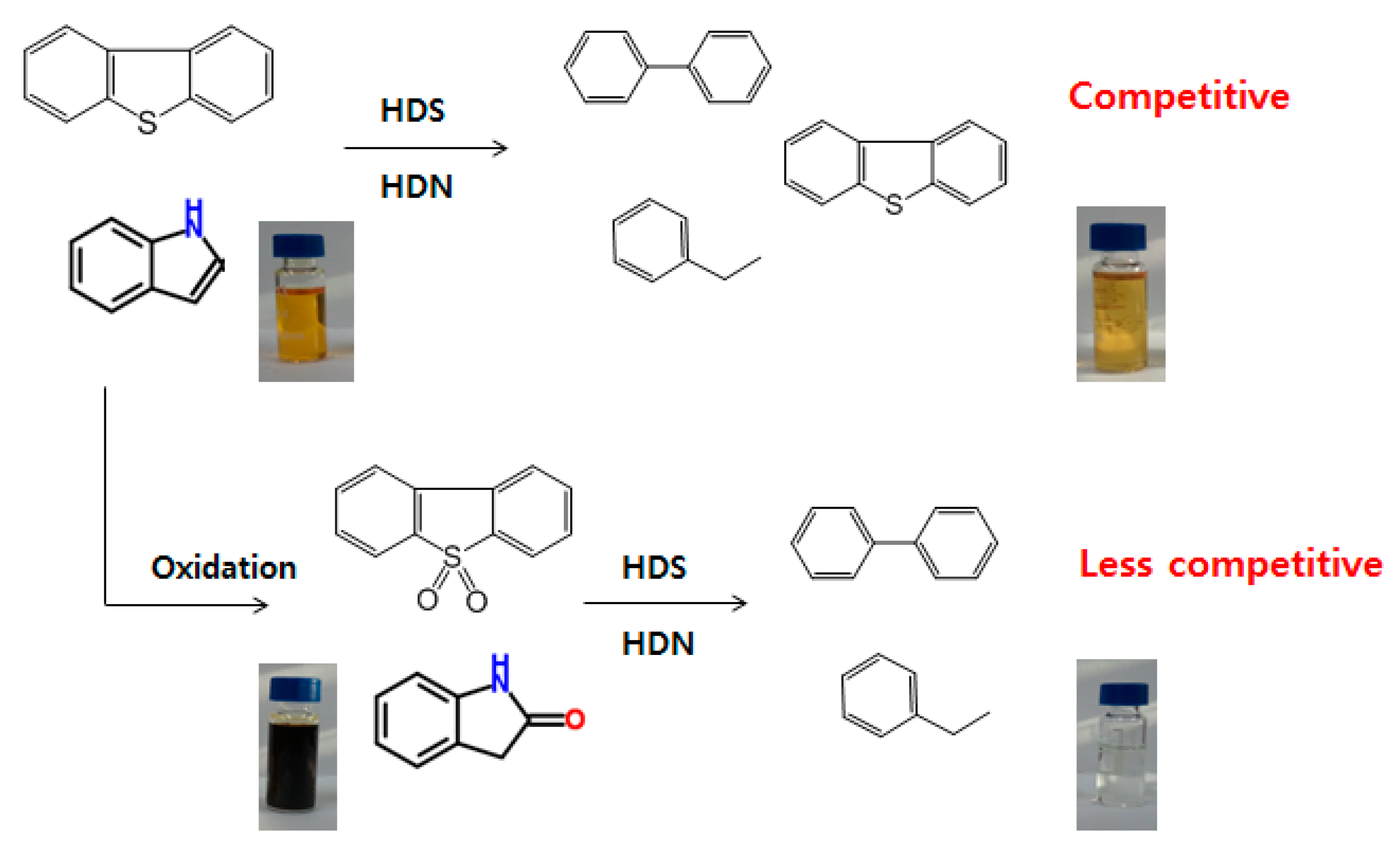
2.2. Activity Test of Catalysts
3. Experimental
3.1. Preparation of the Amphiphilic Catalyst
3.2. Synthesis of Ni2P and Ni-Mo-S Catalysts
3.3. Characterization of Catalyst Samples
3.4. Activity Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yun, G.N.; Lee, Y.K. Dispersion effects of Ni2P catalysts on hydrotreating of light cycle oil. Appl. Catal. B Environ. 2014, 150, 647–655. [Google Scholar] [CrossRef]
- Cho, K.S.; Lee, Y.K. Effects of nitrogen compounds, aromatics, and aprotic solvents on the oxidative desulfurization (ODS) of light cycle oil over Ti-SBA-15 catalyst. Appl. Catal. B Environ. 2014, 147, 35–42. [Google Scholar] [CrossRef]
- Breysse, M.; Djega-Mariadassou, G.; Pessayre, S.; Geantet, C.; Vrinat, M.; Pérot, G.; Lemaire, M. Deep desulfurization: Reactions, catalysts and technological challenges. Catal. Today 2003, 84, 129–138. [Google Scholar] [CrossRef]
- Song, C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal. Today 2003, 86, 211–263. [Google Scholar] [CrossRef]
- Ma, X.; Sakanishi, K.; Mochida, I. Hydrodesulfurization reactivities of various sulfur compounds in diesel fuel. Ind. Eng. Chem. Res. 1994, 33, 218–222. [Google Scholar] [CrossRef]
- Babich, I.; Moulijn, J. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Schulz, H.; Böhringer, W.; Waller, P.; Ousmanov, F. Gas oil deep hydrodesulfurization: Refractory compounds and retarded kinetics. Catal. Today 1999, 49, 87–97. [Google Scholar] [CrossRef]
- Manoli, J.-M.; Da Costa, P.; Brun, M.; Vrinat, M.; Maugé, F.; Potvin, C. Hydrodesulfurization of 4, 6-dimethyldibenzothiophene over promoted (Ni, P) alumina-supported molybdenum carbide catalysts: Activity and characterization of active sites. J. Catal. 2004, 221, 365–377. [Google Scholar] [CrossRef]
- Gomez, E.; Santos, V.E.; Alcon, A.; Martin, A.B.; Garcia-Ochoa, F. Oxygen-uptake and mass-transfer rates on the growth of pseudomonas p utida cect5279: Influence on biodesulfurization (BDS) capability. Energy Fuels 2006, 20, 1565–1571. [Google Scholar] [CrossRef]
- Zhou, A.; Ma, X.; Song, C. Effects of oxidative modification of carbon surface on the adsorption of sulfur compounds in diesel fuel. Appl. Catal. B Environ. 2009, 87, 190–199. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Li, X.; Ma, X. Oxidative desulfurization of dibenzothiophene and diesel over [bmim] 3 pmo 12 O 40. J. Catal. 2011, 279, 269–275. [Google Scholar] [CrossRef]
- Zhu, W.; Li, H.; Jiang, X.; Yan, Y.; Lu, J.; Xia, J. Oxidative desulfurization of fuels catalyzed by peroxotungsten and peroxomolybdenum complexes in ionic liquids. Energy Fuels 2007, 21, 2514–2516. [Google Scholar] [CrossRef]
- Huang, C.; Chen, B.; Zhang, J.; Liu, Z.; Li, Y. Desulfurization of gasoline by extraction with new ionic liquids. Energy Fuels 2004, 18, 1862–1864. [Google Scholar] [CrossRef]
- Yan, X.-M.; Su, G.-S.; Xiong, L. Oxidative desulfurization of diesel oil over ag-modified mesoporous HPW/SiO2 catalyst. J. Fuel Chem. Tech. 2009, 37, 318–323. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, G.; Zeng, D.; Tang, Y.; Wang, M.; Li, Y. Oxidative desulfurization of diesel fuel using amphiphilic quaternary ammonium phosphomolybdate catalysts. Fuel Process. Technol. 2009, 90, 1538–1542. [Google Scholar] [CrossRef]
- Toteva, V.; Georgiev, A.; Topalova, L. Investigation of the oxidative desulfurization of lco model mixture by GC-MS and FTIR spectroscopy. Fuel Process. Technol. 2012, 101, 101–105. [Google Scholar] [CrossRef]
- Haw, K.-G.; Bakar, W.A.W.A.; Ali, R.; Chong, J.-F.; Kadir, A.A.A. Catalytic oxidative desulfurization of diesel utilizing hydrogen peroxide and functionalized-activated carbon in a biphasic diesel–acetonitrile system. Fuel Process. Technol. 2010, 91, 1105–1112. [Google Scholar] [CrossRef]
- Bakar, W.A.W.A.; Ali, R.; Kadir, A.A.A.; Mokhtar, W.N.A.W. Effect of transition metal oxides catalysts on oxidative desulfurization of model diesel. Fuel Process. Technol. 2012, 101, 78–84. [Google Scholar] [CrossRef]
- Yun, G.N.; Lee, Y.K. Beneficial effects of polycyclic aromatics on oxidative desulfurization of light cycle oil over phosphotungstic acid (PTA) catalyst. Fuel Process. Technol. 2013, 114, 1–5. [Google Scholar] [CrossRef]
- Oyama, S.T.; Wang, X.; Lee, Y.-K.; Bando, K.; Requejo, F. Effect of phosphorus content in nickel phosphide catalysts studied by xafs and other techniques. J. Catal. 2002, 210, 207–217. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Oyama, S.T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating: EXAFS and FTIR studies. J. Catal. 2006, 239, 376–389. [Google Scholar] [CrossRef]
- Infantes-Molina, A.; Cecilia, J.; Pawelec, B.; Fierro, J.; Rodríguez-Castellón, E.; Jiménez-López, A. Ni2P and CoP catalysts prepared from phosphite-type precursors for hds–hdn competitive reactions. Appl. Catal. A Gen. 2010, 390, 253–263. [Google Scholar] [CrossRef]
- Ishihara, A.; Wang, D.H.; Dumeignil, F.; Amano, H.; Qian, E.W.H.; Kabe, T. Oxidative desulfurization and denitrogenation of a light gas oil using an oxidation/adsorption continuous flow process. Appl. Catal. A Gen. 2005, 279, 279–287. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Bérubé, F.; Kaliaguine, S. Calcination and thermal degradation mechanisms of triblock copolymer template in sba-15 materials. Microporous Mesoporous Mater. 2008, 115, 469–479. [Google Scholar] [CrossRef]
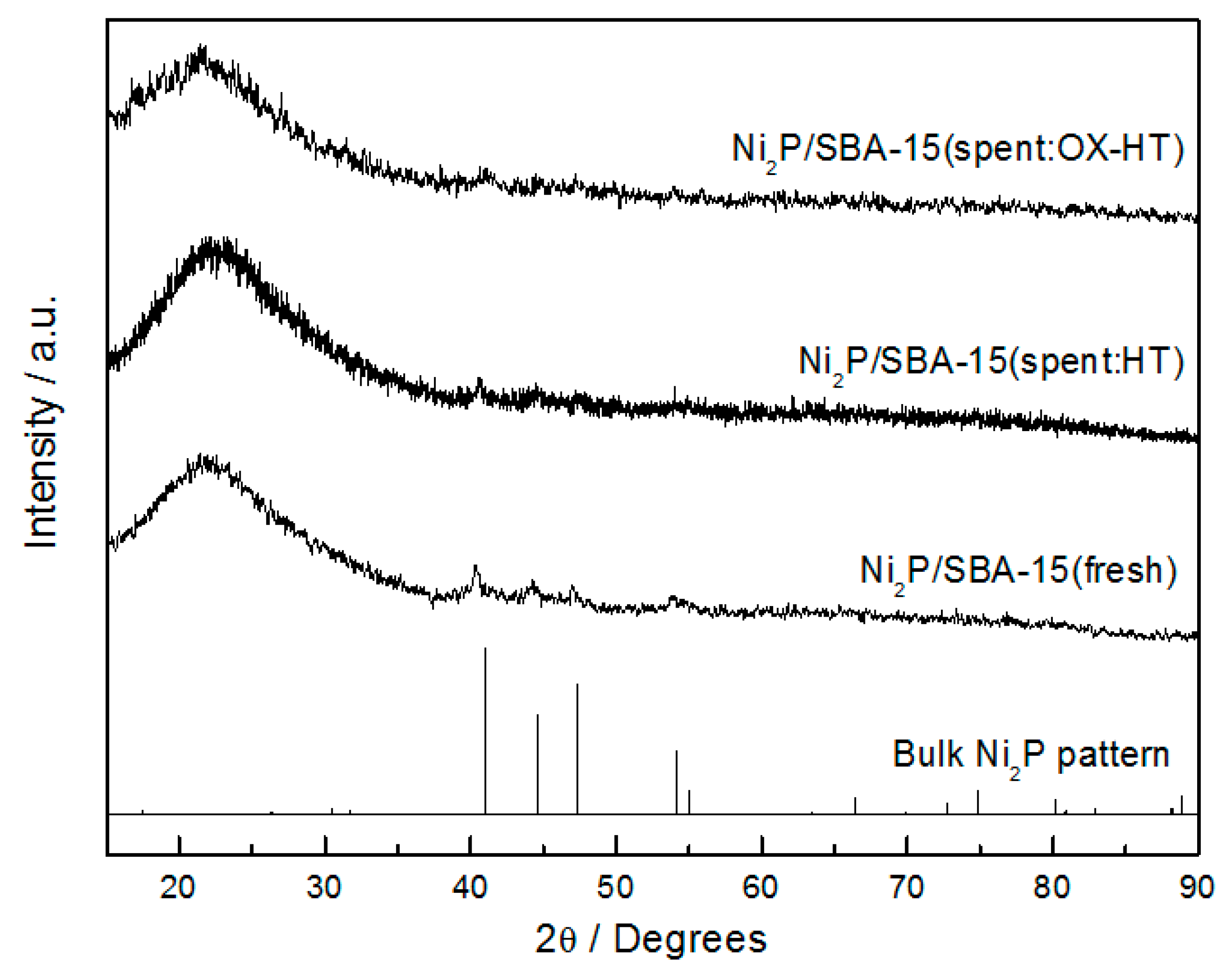
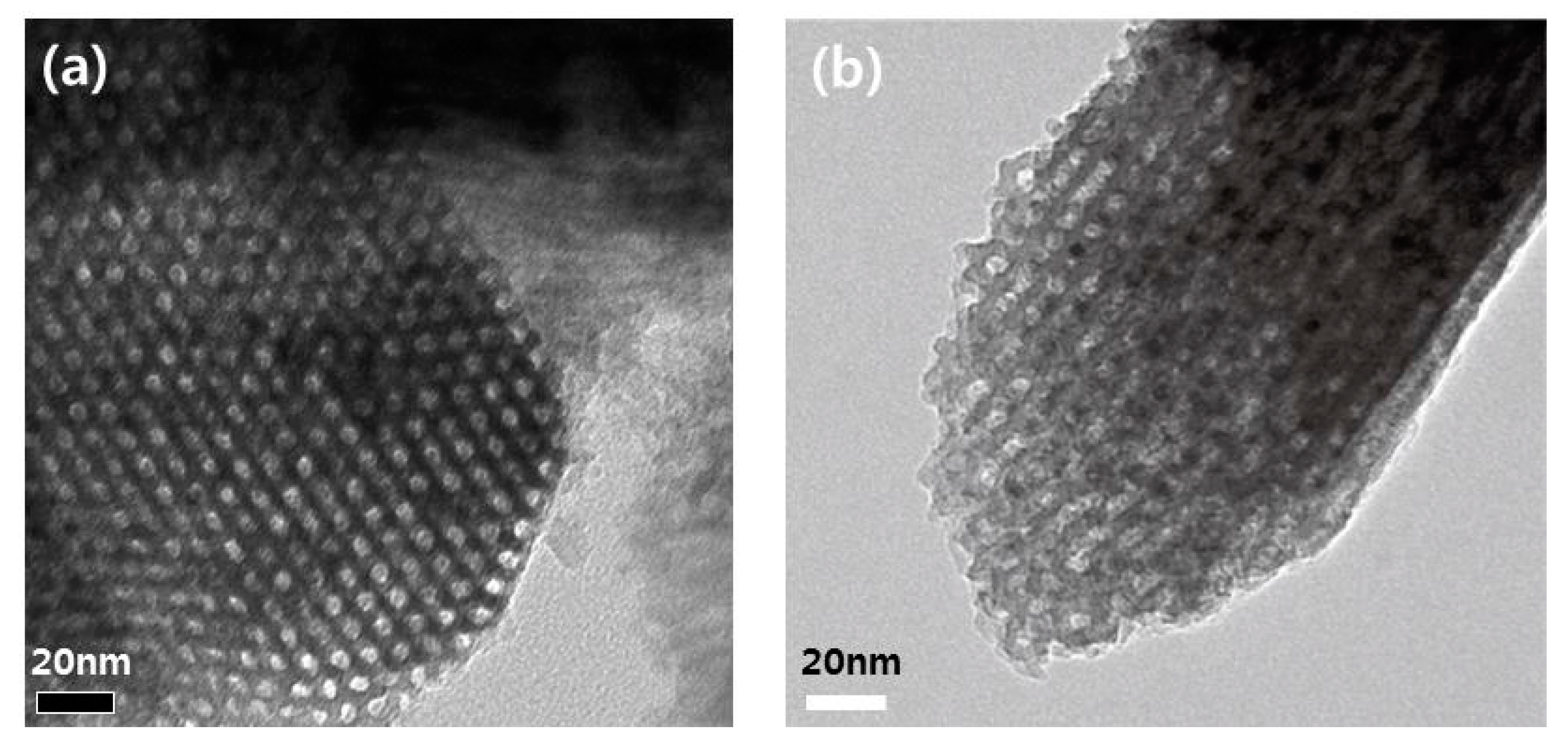

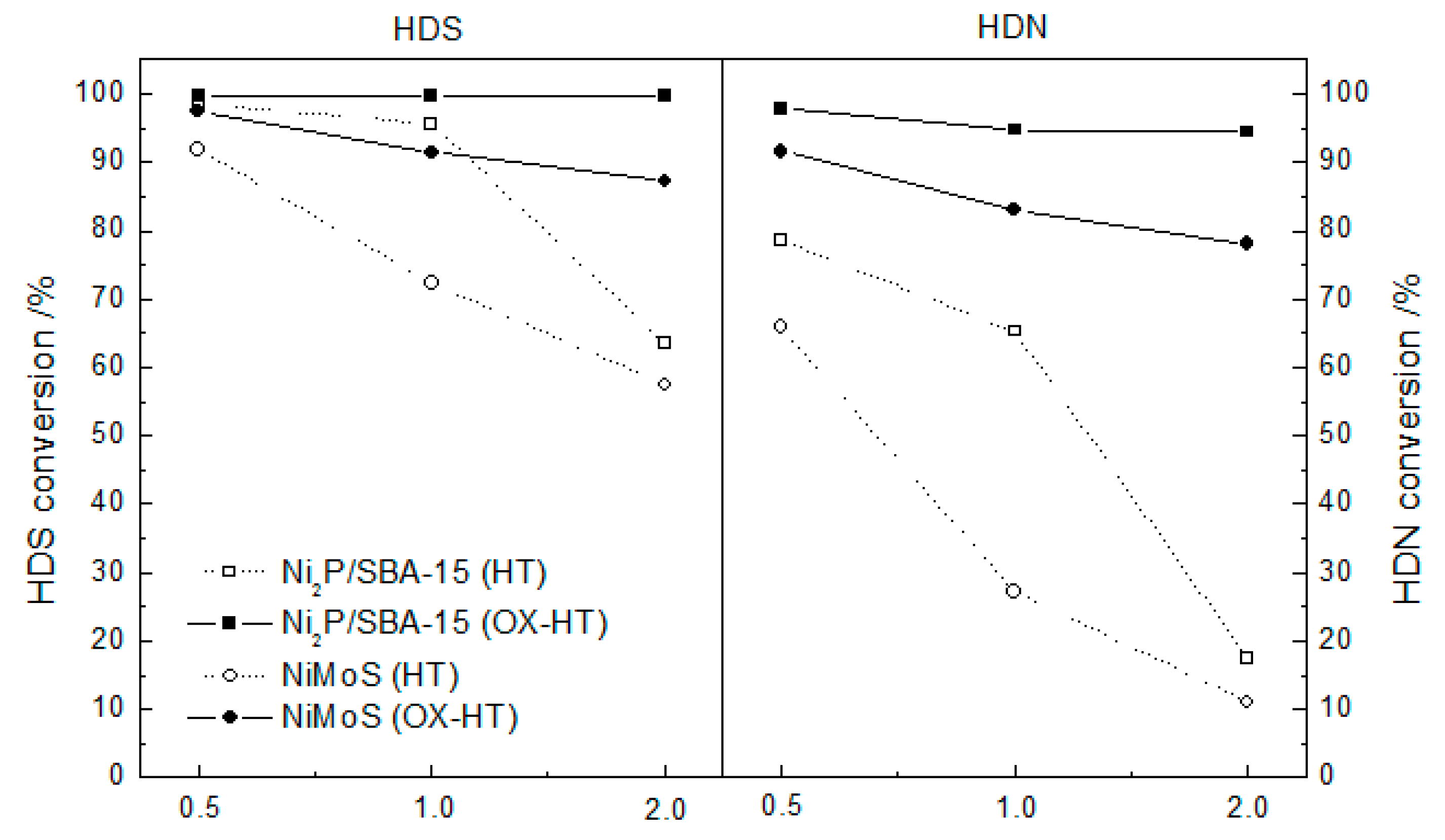
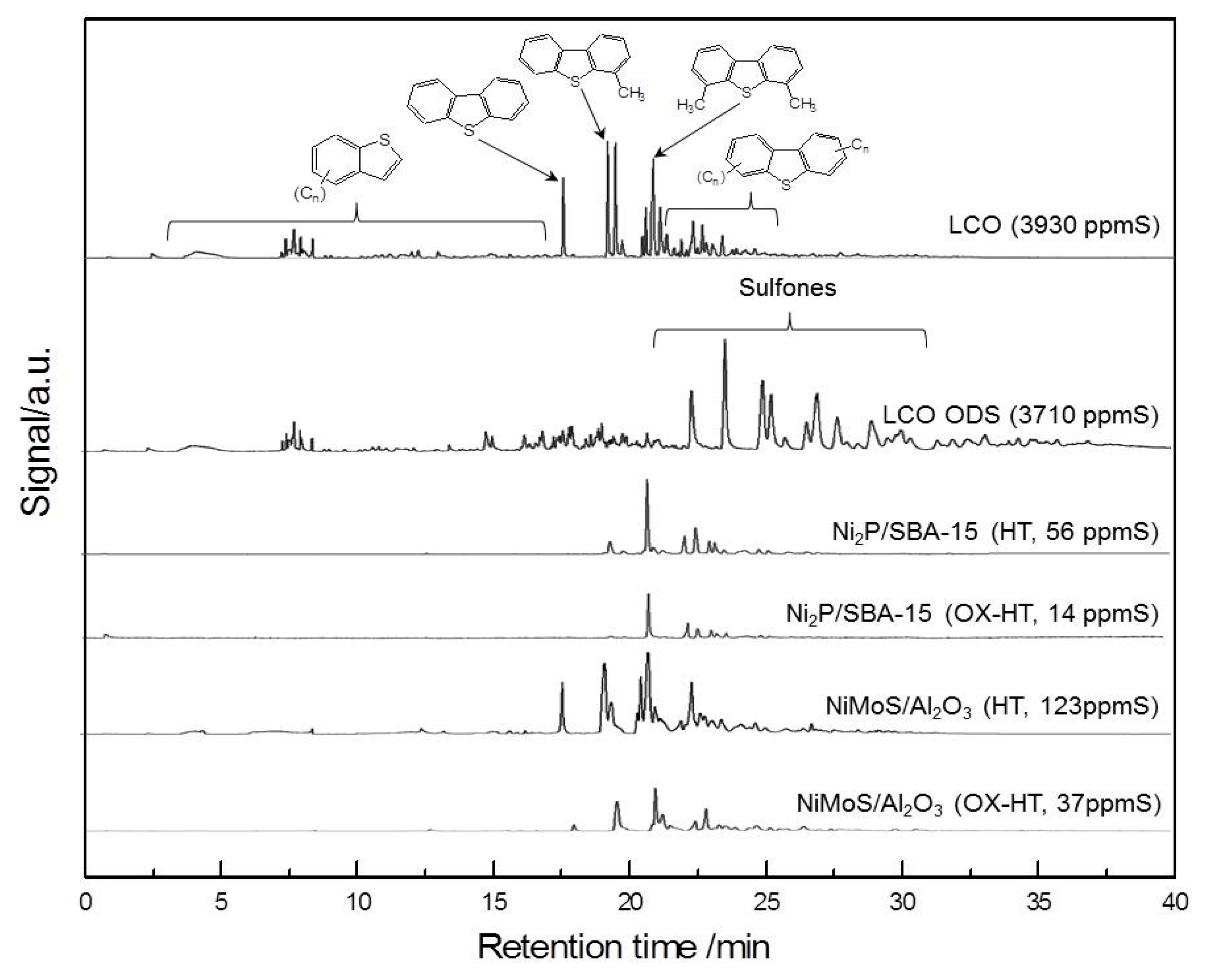
| Samples | Conditions | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | CO Uptake (μmol g−1) | Molar Ratios P/Ni Ni/Mo |
|---|---|---|---|---|---|
| SBA-15 support | calcined | 689.1 | 0.92 | - | - |
| Ni2P/SBA-15 | fresh | 374.3 | 0.53 | 149 | 1.11 |
| Ni2P/SBA-15 | spent (HDS) | 285.6 | 0.39 | 117 | 0.83 |
| Ni2P/SBA-15 | spent (ODS-HDS) | 293.5 | 0.42 | 122 | 0.90 |
| Al2O3 support | as received | 101.3 | 0.42 | - | - |
| NiMo/Al2O3 | fresh | 68.1 | 0.28 | 65 | 0.49 |
| NiMo/Al2O3 | spent (HDS) | 59.5 | 0.24 | 30 | 0.49 |
| Physical Properties | LCO | |
|---|---|---|
| S/ppm | 3930 | |
| N/ppm | 550 | |
| Color (ASTM) | L2.5 | |
| Aromatics/wt. % | Total | 74.3 |
| Mono | 14.3 | |
| Di | 40.6 | |
| Tri+ | 19.4 | |
| Cetane Index | 24.9 | |
| Distillation/K | IBP/5/10 | 498/529/535 |
| 30/40/50 | 557/565/581 | |
| 60/90/95 | 598/671/- | |
| EP | - | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, G.-N.; Cho, K.-S.; Kim, Y.-S.; Lee, Y.-K. A New Approach to Deep Desulfurization of Light Cycle Oil over Ni2P Catalysts: Combined Selective Oxidation and Hydrotreating. Catalysts 2018, 8, 102. https://doi.org/10.3390/catal8030102
Yun G-N, Cho K-S, Kim Y-S, Lee Y-K. A New Approach to Deep Desulfurization of Light Cycle Oil over Ni2P Catalysts: Combined Selective Oxidation and Hydrotreating. Catalysts. 2018; 8(3):102. https://doi.org/10.3390/catal8030102
Chicago/Turabian StyleYun, Gwang-Nam, Kye-Syng Cho, Yong-Su Kim, and Yong-Kul Lee. 2018. "A New Approach to Deep Desulfurization of Light Cycle Oil over Ni2P Catalysts: Combined Selective Oxidation and Hydrotreating" Catalysts 8, no. 3: 102. https://doi.org/10.3390/catal8030102
APA StyleYun, G.-N., Cho, K.-S., Kim, Y.-S., & Lee, Y.-K. (2018). A New Approach to Deep Desulfurization of Light Cycle Oil over Ni2P Catalysts: Combined Selective Oxidation and Hydrotreating. Catalysts, 8(3), 102. https://doi.org/10.3390/catal8030102







