Oriented Decoration in Metal-Functionalized Ordered Mesoporous Silicas and Their Catalytic Applications in the Oxidation of Aromatic Compounds
Abstract
:1. Introduction
2. The Categories of Metal Active Sites
2.1. Acidic–Basic Metal Active Sites
2.1.1. Acidic Active Sites
2.1.2. Basic Active Sites
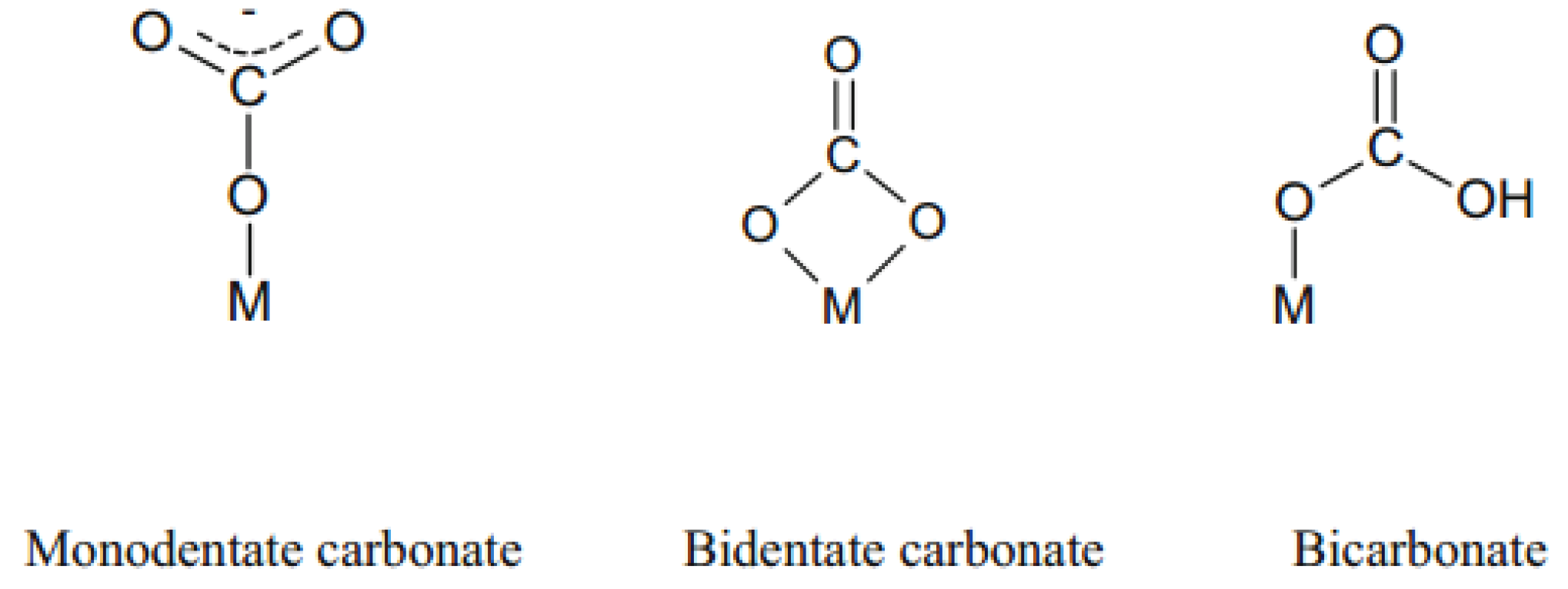
2.2. Redox Metal Active Sites
3. The Introduced Locations of Metal Active Sites in the OMSs
3.1. In the Mesoporous Channels of OMSs
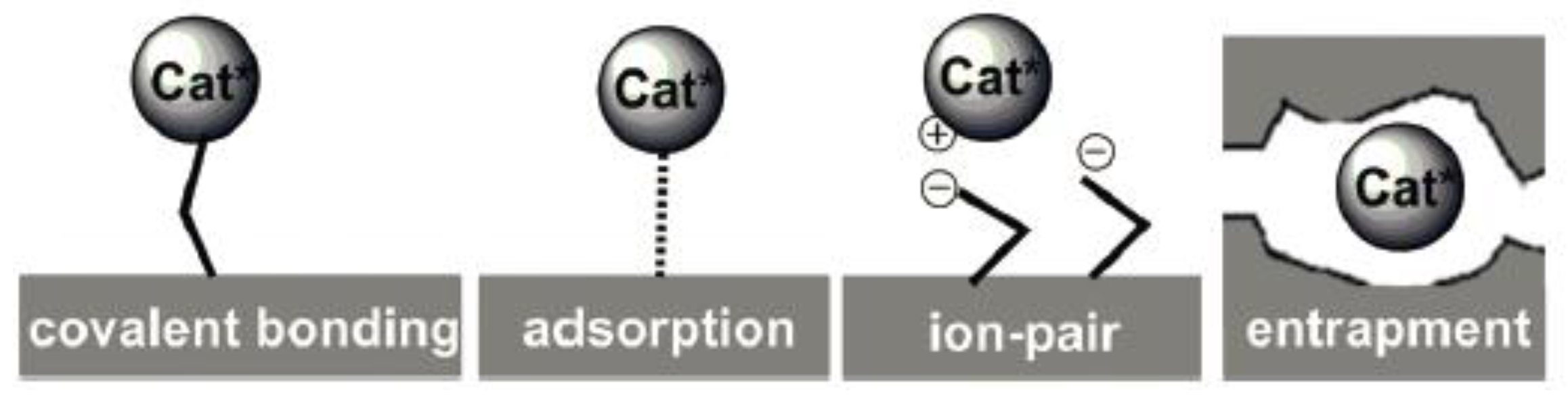
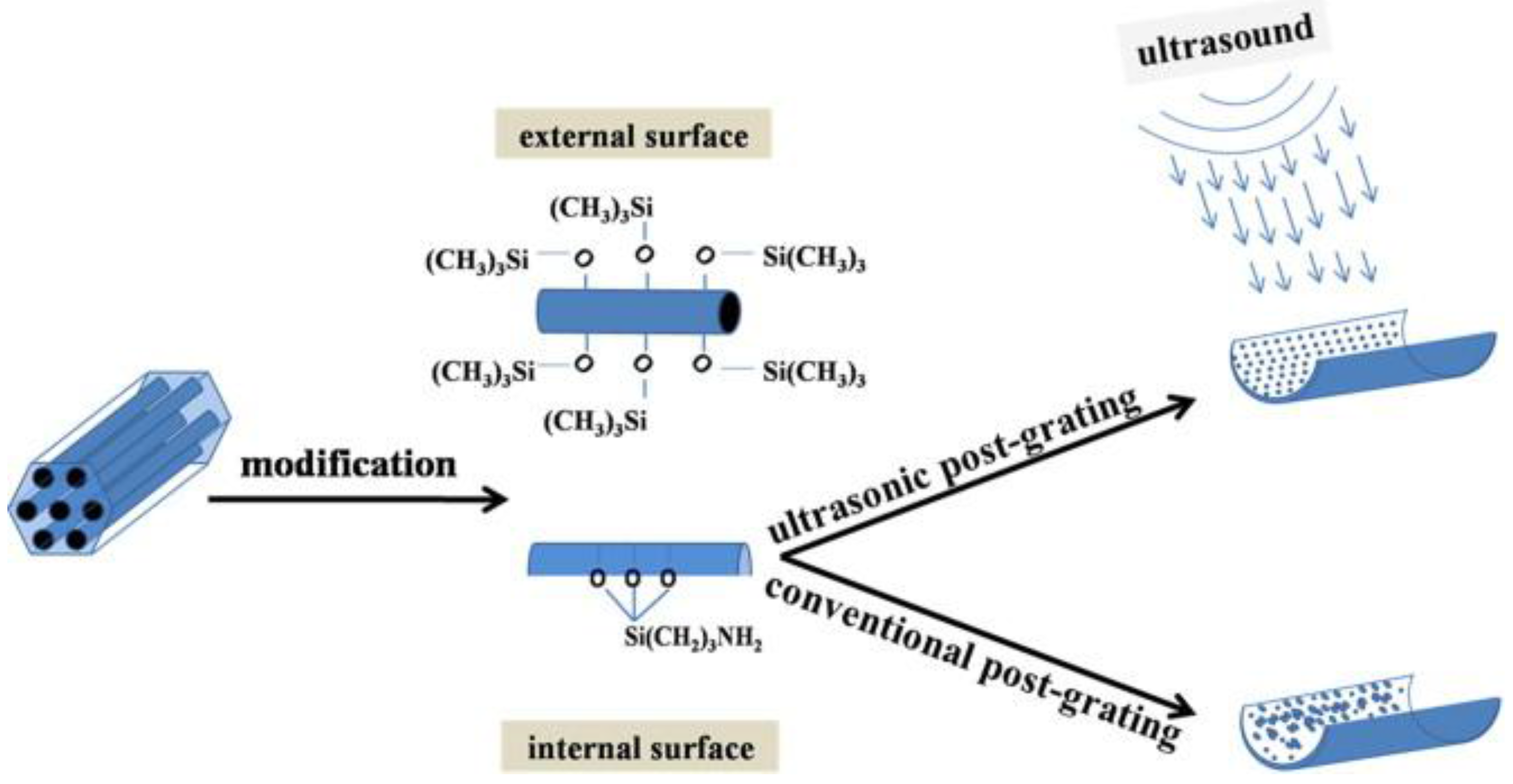
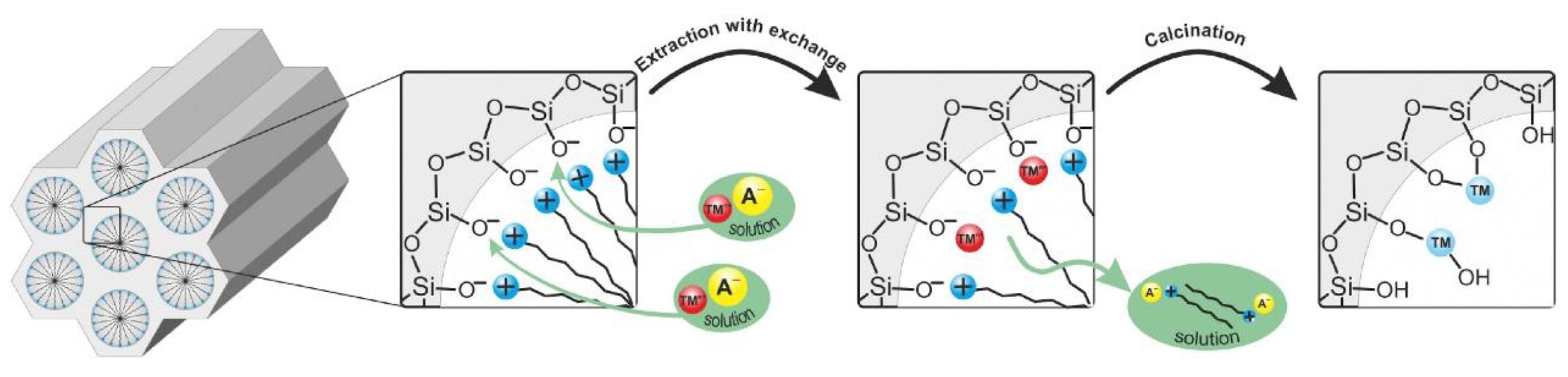
3.1.1. Post-Synthesis Method
3.1.2. Direct-Synthesis Method
Cationic Surfactant-Templated Route (S+X−M+I−)
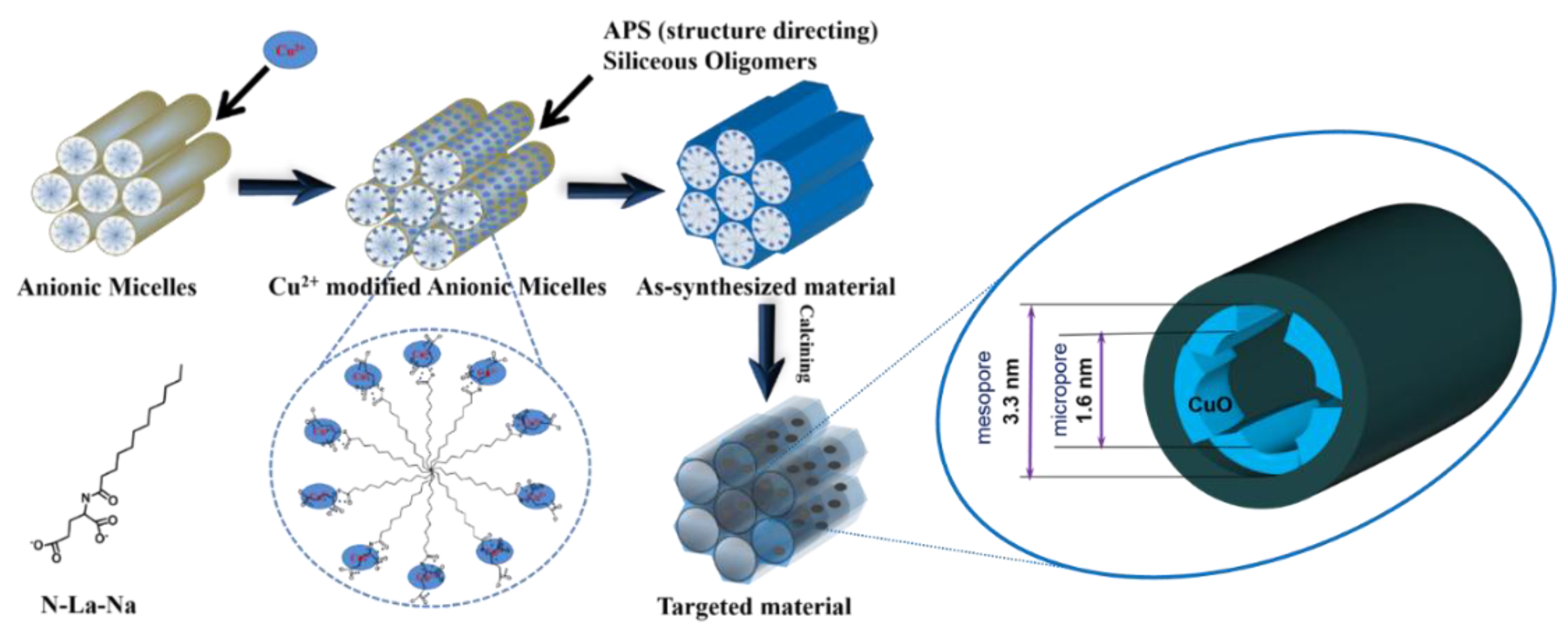
Anionic Surfactant-Templated Route (M+S−X+I− or S−[MN]+I−)
Neutral Surfactant-Templated Route (S0M+I−)
3.2. In the Framework of OMSs
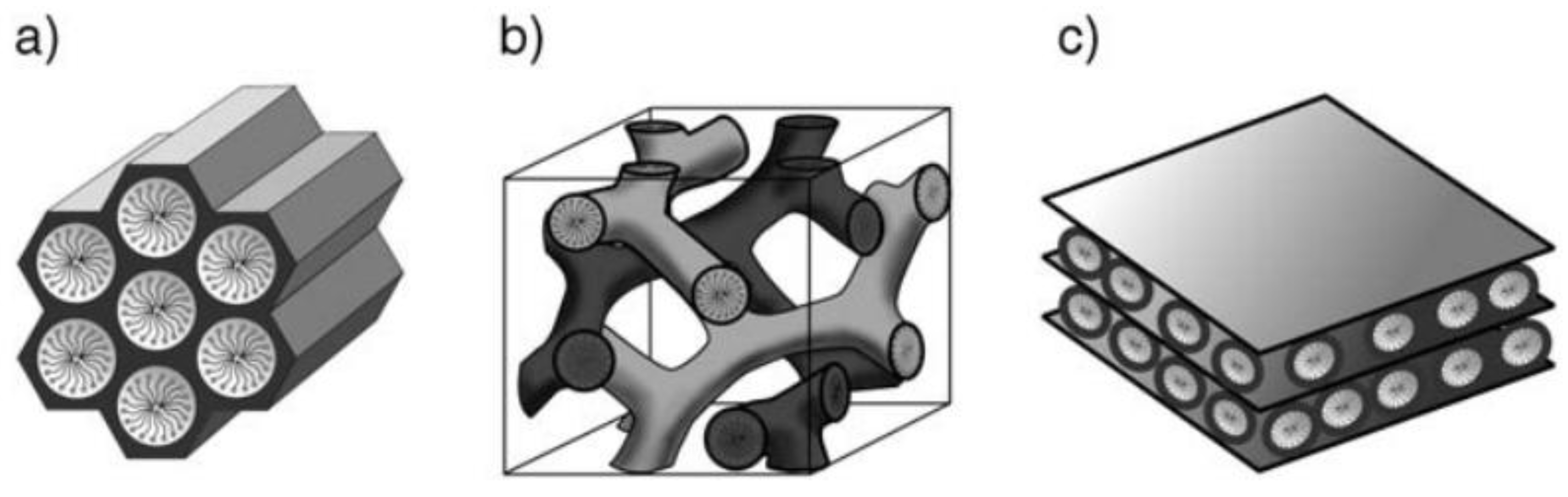
4. Morphology and Channel Structure in OMSs
4.1. Morphology
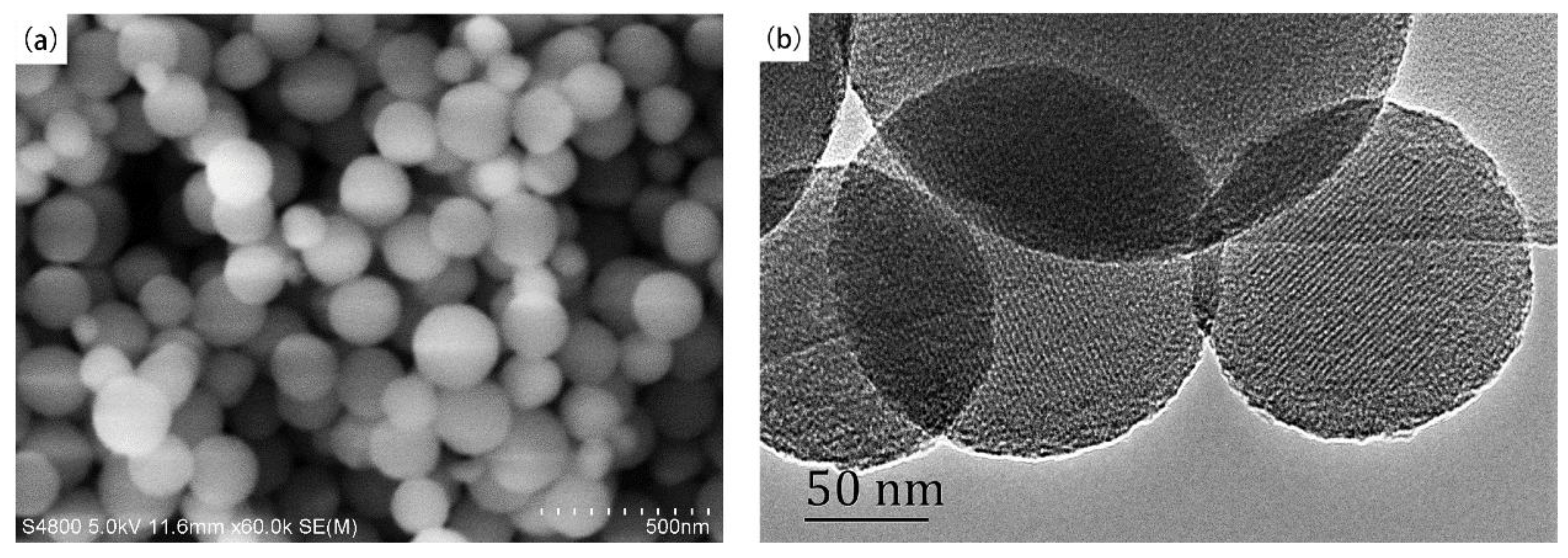
4.1.1. Nanosphere
4.1.2. Nanowire, Nanotube, Nanorod
4.2. Structure
5. OMSs in the Oxidation of Aromatic Compounds
5.1. Benzene Oxidation
5.2. Styrene Oxidation
5.3. Ethylbenzene Oxidation
5.4. Other Oxidation of Aromatic Derivatives
6. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sanz-Pérez, E.S.; Arencibia, A.; Calleja, G.; Sanz, R. Tuning the textural properties of HMS mesoporous silica. Functionalization towards CO2 adsorption. Microporous Mesoporous Mater. 2018, 260, 235–244. [Google Scholar] [CrossRef]
- Dündar-Tekkaya, E.; Yürüm, Y. Mesoporous MCM-41 material for hydrogen storage: A short review. Int. J. Hydrogen Energy 2016, 41, 9789–9795. [Google Scholar] [CrossRef]
- Chen, Z.; Luck, R.L. Oxidation of olefins using atmospheric oxygen atoms initiated by tert-butylhydroperoxide or hydrogen peroxide with silver nanoparticles deposited on MCM-41 as catalysts. Green Chem. 2016, 18, 3354–3359. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Zhang, J.; Linden, M.; Sahlgren, C. Mesoporous silica nanoparticles in tissue engineering—A perspective. Nanomedicine 2016, 11, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Tomer, V.K.; Duhan, S.; Adhyapak, P.V.; Mulla, I.S. Mn-loaded mesoporous silica nanocomposite: A highly efficient humidity sensor. J. Am. Ceram. Soc. 2015, 98, 741–747. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Xie, S.-M.; Zhang, M.; Zi, M.; He, P.-G.; Yuan, L.-M. Novel inorganic mesoporous material with chiral nematic structure derived from nanocrystalline cellulose for high-resolution gas chromatographic separations. Anal. Chem. 2014, 86, 9595–9602. [Google Scholar] [CrossRef] [PubMed]
- Sarvi, M.N.; Budianto Bee, T.; Gooi, C.K.; Woonton, B.W.; Gee, M.L.; O’Connor, A.J. Development of functionalized mesoporous silica for adsorption and separation of dairy proteins. Chem. Eng. J. 2014, 235, 244–251. [Google Scholar] [CrossRef]
- Newton, M.A.; Belver-Coldeira, C.; Martínez-Arias, A.; Fernández-García, M. Dynamic in situ observation of rapid size and shape change of supported Pd nanoparticles during Co/No cycling. Nat. Mater. 2007, 6, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.L.; Mundy, J.A.; Liu, Z.; Cabezas, R.; Hovden, R.; Kourkoutis, L.F.; Zhang, J.; Subramanian, N.P.; Makharia, R.; Wagner, F.T.; et al. Atomic-resolution spectroscopic imaging of ensembles of nanocatalyst particles across the life of a fuel cell. Nano Lett. 2012, 12, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Wynblatt, P.; Gjostein, N.A. Supported metal crystallites. Prog. Solid State Chem. 1975, 9, 21–58. [Google Scholar] [CrossRef]
- Campbell, C.T.; Parker, S.C.; Starr, D.E. The effect of size-dependent nanoparticle energetics on catalyst sintering. Science 2002, 298, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Veser, G. Exceptional high-temperature stability through distillation-like self-stabilization in bimetallic nanoparticles. Nat. Mater. 2009, 9, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.A.; Campbell, C.T. Ceria maintains smaller metal catalyst particles by strong metal-support bonding. Science 2010, 329, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhao, Q.; Zhang, D.; Liu, G. Transition-metal-functionalized ordered mesoporous silicas: An overview of sustainable chiral catalysts for enantioselective transformations. Green Chem. 2015, 17, 2100–2122. [Google Scholar] [CrossRef]
- Heitbaum, M.; Glorius, F.; Escher, I. Asymmetric heterogeneous catalysis. Angew. Chem. Int. Ed. 2006, 45, 4732–4762. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Vinu, A.; Hossain, K.Z.; Ariga, K. Recent advances in functionalization of mesoporous silica. J. Nanosci. Nanotechnol. 2005, 5, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Shah, S.; Santra, C.; Sen, D.; Sharma, S.; Pandey, J.K.; Mazumder, S.; Chowdhury, B. Controllable synthesis of niobium doped mesoporous silica materials with various morphologies and its activity for oxidative catalysis. Microporous Mesoporous Mater. 2016, 226, 169–178. [Google Scholar] [CrossRef]
- Xin, H.; Zhao, J.; Li, X.; Tang, J.; Yang, Q. An acid-free route for the facile synthesis of iron-functionalized mesoporous silicas: Transformation between hollow nanospheres and cage-like mesostructures. Microporous Mesoporous Mater. 2014, 190, 54–62. [Google Scholar] [CrossRef]
- Wan, Y.; Shi, Y.; Zhao, D. Designed synthesis of mesoporous solids via nonionic-surfactant-templating approach. Chem. Commun. 2007, 897–926. [Google Scholar] [CrossRef] [PubMed]
- Sayari, A.; Hamoudi, S. Periodic mesoporous silica-based organic−inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3151–3168. [Google Scholar] [CrossRef]
- Vu, X.; Armbruster, U.; Martin, A. Micro/mesoporous zeolitic composites: Recent developments in synthesis and catalytic applications. Catalysts 2016, 6, 183. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Wang, Y.M.; Wu, P. Pt nanoparticles supported on highly dispersed alumina coated inside SBA-15 for enantioselective hydrogenation. ChemCatChem 2010, 2, 1303–1311. [Google Scholar] [CrossRef]
- Jiménez-Morales, I.; Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst. Appl. Catal. B Environ. 2015, 164, 70–76. [Google Scholar] [CrossRef]
- Zepeda, T.A.; Infantes-Molina, A.; de Leon, J.N.D.; Obeso-Estrella, R.; Fuentes, S.; Alonso-Nuñez, G.; Pawelec, B. Synthesis and characterization of Ga-modified Ti-HMS oxide materials with varying Ga content. J. Mol. Catal. A Chem. 2015, 397, 26–35. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Mochizuki, T.; Abe, Y.; Toba, M.; Yoshimura, Y. Ti-incorporated SBA-15 mesoporous silica as an efficient and robust lewis solid acid catalyst for the production of high-quality biodiesel fuels. Appl. Catal. B Environ. 2014, 148–149, 344–356. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Puad, K.; Triwahyono, S.; Jalil, A.A.; Khayoon, M.S.; Atabani, A.E.; Ramli, Z.; Majid, Z.A.; Prasetyoko, D.; Hartanto, D. Transesterification of croton megalocarpus oil to biodiesel over WO3 supported on silica mesoporous-macroparticles catalyst. Chem. Eng. J. 2017, 316, 882–892. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Kleitz, F.; Fontaine, F.-G. Lewis acidity quantification and catalytic activity of Ti, Zr and Al-supported mesoporous silica. Dalton Trans. 2017, 46, 3864–3876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Song, X.; Liu, F. Highly active and recyclable mesoporous molecular sieves CaO(SrO,BaO)/SBA-15 with base sites as heterogeneous catalysts for methanolysis of polycarbonate. Catal. Lett. 2017, 147, 2940–2949. [Google Scholar] [CrossRef]
- Jiang, L.; Diao, Y.; Han, J.; Yan, R.; Zhang, X.; Zhang, S. MgO-SBA-15 supported Pd-Pb catalysts for oxidative esterification of methacrolein with methanol to methyl methacrylate. Chin. J. Chem. Eng. 2014, 22, 1098–1104. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Shen, K.; Chu, W.; Qian, W. Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen. Int. J. Hydrogen Energy 2013, 38, 9718–9731. [Google Scholar] [CrossRef]
- Tantirungrotechai, J.; Thananupappaisal, P.; Yoosuk, B.; Viriya-Empikul, N.; Faungnawakij, K. One-pot synthesis of calcium-incorporated MCM-41 as a solid base catalyst for transesterification of palm olein. Catal. Commun. 2011, 16, 25–29. [Google Scholar] [CrossRef]
- Sun, H.; Han, J.; Ding, Y.; Li, W.; Duan, J.; Chen, P.; Lou, H.; Zheng, X. One-pot synthesized mesoporous Ca/SBA-15 solid base for transesterification of sunflower oil with methanol. Appl. Catal. A Gen. 2010, 390, 26–34. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Jiang, Q.; Wang, Y.M.; Wang, H.J.; Sun, L.B.; Shi, L.Y.; Xu, J.H.; Wang, Y.; Chun, Y.; Zhu, J.H. Generating superbasic sites on mesoporous silica SBA-15. Chem. Mater. 2006, 18, 4600–4608. [Google Scholar] [CrossRef]
- Michalska, A.; Daturi, M.; Saussey, J.; Nowak, I.; Ziolek, M. The role of MCM-41 composition in the creation of basicity by alkali metal impregnation. Microporous Mesoporous Mater. 2006, 90, 362–369. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Maneerung, T.; Kawi, S. Highly active and durable Ca-doped Ce-SBA-15 catalyst for biodiesel production. Energy 2015, 89, 946–956. [Google Scholar] [CrossRef]
- Cano, L.A.; Cagnoli, M.V.; Bengoa, J.F.; Garcia-Fierro, J.L.; Marchetti, S.G. Synthesis and characterization of SBA-15 modified with alkali metals. J. Porous Mater. 2017, 24, 631–638. [Google Scholar] [CrossRef]
- Lee, G.V.d.; Ponec, V. On some problems of selectivity in syngas reactions on the group VIII metals. Catal. Rev. 1987, 29, 183–218. [Google Scholar] [CrossRef]
- Ngantsoue-Hoc, W.; Zhang, Y.; O’Brien, R.J.; Luo, M.; Davis, B.H. Fischer−tropsch synthesis: Activity and selectivity for group I alkali promoted iron-based catalysts. Appl. Catal. A Gen. 2002, 236, 77–89. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Xiao, P.; Liu, D.; Zou, C.J. Structure and catalytic performance of Mg-SBA-15-supported nickel catalysts for CO2 reforming of methane to syngas. Chem. Eng. Technol. 2013, 36, 1701–1707. [Google Scholar]
- Novak Tušar, N.; Laha, S.C.; Cecowski, S.; Arčon, I.; Kaučič, V.; Gläser, R. Mn-containing porous silicates as catalysts for the solvent-free selective oxidation of alkyl aromatics to aromatic ketones with molecular oxygen. Microporous Mesoporous Mater. 2011, 146, 166–171. [Google Scholar] [CrossRef]
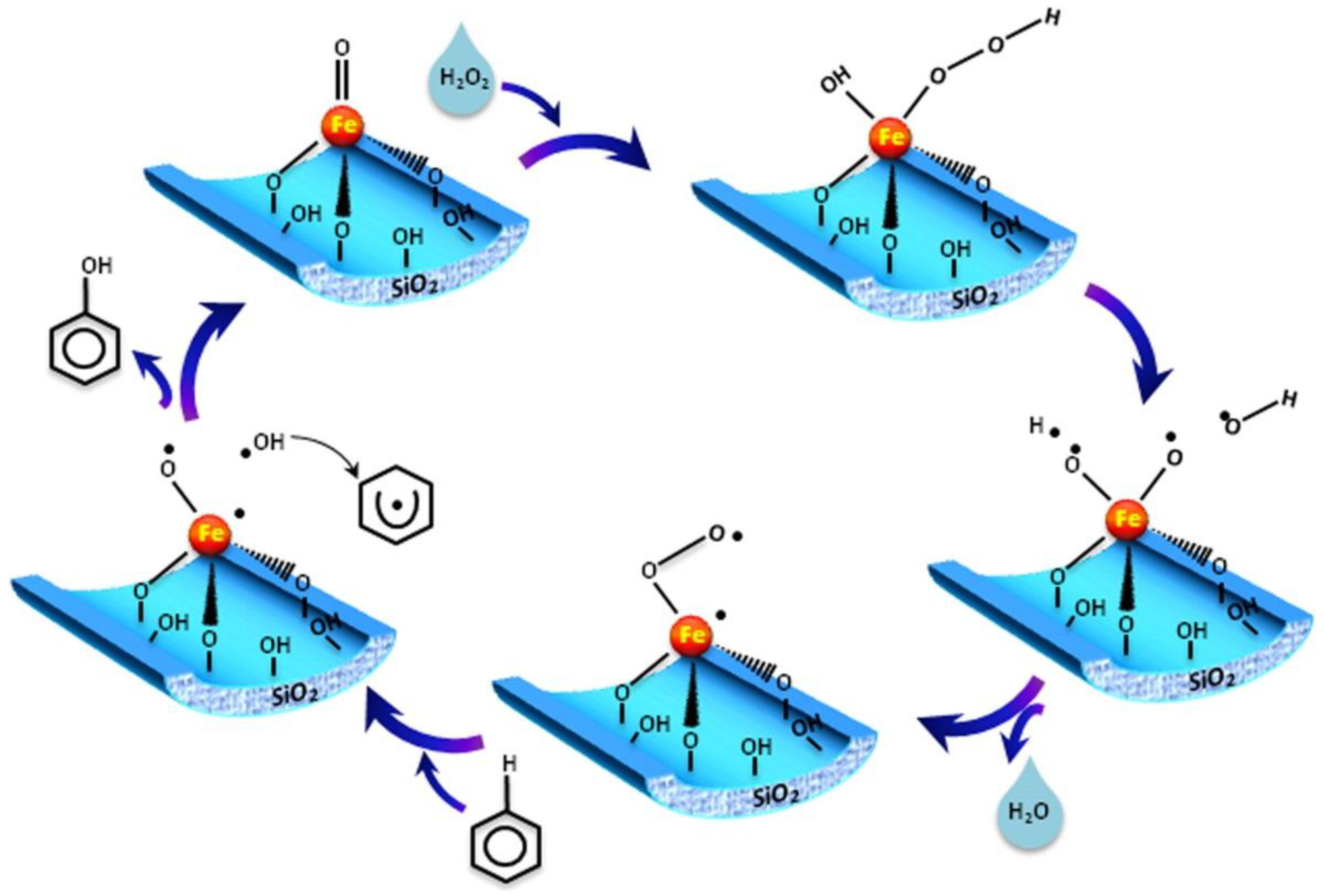
- Liang, X.; Yang, R.; Li, G.; Hu, C. Phenol hydroxylation over Fe-incorporated mesoporous materials prepared by coprecipitation. Microporous Mesoporous Mater. 2013, 182, 62–72. [Google Scholar] [CrossRef]
- Dong, Y.; Zhan, X.; Niu, X.; Li, J.; Yuan, F.; Zhu, Y.; Fu, H. Facile synthesis of Co-SBA-16 mesoporous molecular sieves with EISA method and their applications for hydroxylation of benzene. Microporous Mesoporous Mater. 2014, 185, 97–106. [Google Scholar] [CrossRef]
- Hu, L.; Yue, B.; Chen, X.; He, H. Direct hydroxylation of benzene to phenol on Cu–V bimetal modified HMS catalysts. Catal. Commun. 2014, 43, 179–183. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Koodali, R.T.; Hu, Y.; Xiao, Q.; Zhou, J.; Wang, X.; Guo, L. Activation of MCM-41 mesoporous silica by transition-metal incorporation for photocatalytic hydrogen production. Appl. Catal. B Environ. 2014, 150–151, 138–146. [Google Scholar] [CrossRef]
- Li, X.; Kong, Y.; Zhou, S.; Wang, B. In situ incorporation of well-dispersed Cu–Fe oxides in the mesochannels of AMS and their utilization as catalysts towards the fenton-like degradation of methylene blue. J. Mater. Sci. 2017, 52, 1432–1445. [Google Scholar] [CrossRef]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wang, H.; Chen, J.; Kong, Y. Spherical V-Fe-MCM-48: The synthesis, characterization and hydrothermal stability. Materials 2015, 8, 1752–1765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Scott, M.J.; Hu, Z.; Peng, G.; Munck, E.; Holm, R.H. Synthesis and comparative reactivity and electronic structural features of [MFe3S4]z+ cubane-type clusters (M = iron, cobalt, nickel). J. Am. Chem. Soc. 1992, 114, 10843–10854. [Google Scholar] [CrossRef]
- Niu, K.; Liang, L.; Geng, H.; Hou, W.; Tian, H.; Liu, S. Chelating template-assisted fabrication of cobalt oxide/mesoporous silica composites with diverse mesophases. Mater. Lett. 2013, 107, 325–328. [Google Scholar] [CrossRef]
- Niu, K.; Liang, L.; Gu, Y.; Ke, L.; Duan, F.; Chen, M. Fabrication and photoluminescent properties of ZnO/mesoporous silica composites templated by a chelating surfactant. Langmuir 2011, 27, 13820–13827. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Shi, D.; Dong, W.; Chen, M.; Ni, Z. Chelating template-induced encapsulation of NiO cluster in mesoporous silica via anionic surfactant-templated route. J. Colloid Interface Sci. 2011, 362, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Li, J.; Zhang, B.; Yuan, X.; Asakura, H.; Tanaka, T.; Teramura, K.; Xie, J.; Yan, N. The support effect on the size and catalytic activity of thiolated Au25 nanoclusters as precatalysts. Nanoscale 2015, 7, 6325–6333. [Google Scholar] [CrossRef] [PubMed]
- Da'na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Lim, W.Q.; Phua, S.Z.F.; Xu, H.V.; Sreejith, S.; Zhao, Y. Recent advances in multifunctional silica-based hybrid nanocarriers for bioimaging and cancer therapy. Nanoscale 2016, 8, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Albela, B.; Bonneviot, L. Surface molecular engineering in the confined space of templated porous silica. New J. Chem. 2016, 40, 4115–4131. [Google Scholar] [CrossRef]
- Iliade, P.; Miletto, I.; Coluccia, S.; Berlier, G. Functionalization of mesoporous MCM-41 with aminopropyl groups by co-condensation and grafting: A physico-chemical characterization. Res. Chem. Intermed. 2012, 38, 785–794. [Google Scholar] [CrossRef]
- Ursachi, I.; Stancu, A.; Vasile, A. Magnetic α-Fe2O3/MCM-41 nanocomposites: Preparation, characterization, and catalytic activity for methylene blue degradation. J. Colloid Interface Sci. 2012, 377, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, N.; Wang, G.; Dong, W.; Yang, M.; Luan, Y.; Shi, Z. Synthesis of highly loaded and well dispersed CuO/SBA-15 via an ultrasonic post-grafting method and its application as a catalyst for the direct hydroxylation of benzene to phenol. Microporous Mesoporous Mater. 2013, 177, 47–53. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Borcuch, A.; Michalik, M.; Rutkowska, M.; Gil, B.; Sojka, Z.; Indyka, P.; Chmielarz, L. MCM-41 modified with transition metals by template ion-exchange method as catalysts for selective catalytic oxidation of ammonia to dinitrogen. Microporous Mesoporous Mater. 2017, 240, 9–21. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Luz, I.; Soukri, M.; Van Goethem, C.; Vankelecom, I.F.J.; Lail, M.; De Vos, D.E. Boosting the catalytic performance of metal–organic frameworks for steroid transformations by confinement within a mesoporous scaffold. Angew. Chem. Int. Ed. 2017, 56, 13302–13306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fröba, M.; Wang, J.; Tanev, P.T.; Wong, J.; Pinnavaia, T.J. Mesoporous titanosilicate molecular sieves prepared at ambient temperature by electrostatic (S+I−, S+X−I+) and neutral (S0I0) assembly pathways: A comparison of physical properties and catalytic activity for peroxide oxidations. J. Am. Chem. Soc. 1996, 118, 9164–9171. [Google Scholar] [CrossRef]
- Che, S.; Garcia-Bennett, A.E.; Yokoi, T.; Sakamoto, K.; Kunieda, H.; Terasaki, O.; Tatsumi, T. A novel anionic surfactant templating route for synthesizing mesoporous silica with unique structure. Nat. Mater. 2003, 2, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Burkett, S.L.; Li, H.-X.; Davis, M.E. Studies on mesoporous materials II. Synthesis mechanism of MCM-41. Microporous Mater. 1993, 2, 27–34. [Google Scholar] [CrossRef]
- Singha, S.; Parida, K.M.; Dash, A.C. Fe(III)-salim anchored MCM-41: Synthesis, characterization and catalytic activity towards liquid phase cyclohexane oxidation. J. Porous Mater. 2011, 18, 707–714. [Google Scholar] [CrossRef]
- Yang, F.; Gao, S.; Xiong, C.; Long, S.; Li, X.; Xi, T.; Kong, Y. Direct templating assembly route for the preparation of highly-dispersed vanadia species encapsulated in mesoporous MCM-41 channel. RSC Adv. 2015, 5, 72099–72106. [Google Scholar] [CrossRef]
- Han, L.; Che, S. Anionic surfactant templated mesoporous silicas (AMSs). Chem. Soc. Rev. 2013, 42, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Yoshitake, H.; Tatsumi, T. Synthesis of anionic-surfactant-templated mesoporous silica using organoalkoxysilane-containing amino groups. Chem. Mater. 2003, 15, 4536–4538. [Google Scholar] [CrossRef]
- Niu, K.; Dong, W.; Chen, M.; Ni, Z. Synthesis and characterization of NiO/mesoporous silica nanocomposite. Integr. Ferroelectr. 2011, 128, 135–141. [Google Scholar] [CrossRef]
- Long, S.; Zhou, S.; Yang, F.; Lu, K.; Xi, T.; Kong, Y. An iron-based micropore-enriched silica catalyst: In situ confining of Fe2O3 in the mesopores and its improved catalytic properties. RSC Adv. 2016, 6, 76064–76074. [Google Scholar] [CrossRef]
- Yang, F.; Wang, B.; Zhou, S.; Long, S.; Liu, X.; Kong, Y. Micropore-enriched CuO-based silica catalyst directly prepared by anionic template-induced method and its boosting catalytic activity in olefins epoxidation. Microporous Mesoporous Mater. 2017, 246, 215–224. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, S.; Yang, F.; Long, S.; Kong, Y. A facile method for the direct introduction of FeOx in mesoporous AMS through a templating route (S−[MN]+I−) and its catalytic application. ChemistrySelect 2016, 1, 1305–1313. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, S.; Wang, H.; Long, S.; Liu, X.; Kong, Y. A metal-assisted templating route (S0M+I-) for fabricating thin-layer CoO covered on the channel of nanospherical-HMS with improved catalytic properties. Dalton Trans. 2016, 45, 6371–6382. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, S.; Gao, S.; Liu, X.; Long, S.; Kong, Y. In situ embedding of ultra-fine nickel oxide nanoparticles in HMS with enhanced catalytic activities of styrene epoxidation. Microporous Mesoporous Mater. 2017, 238, 69–77. [Google Scholar] [CrossRef]
- Yang, F.; Wang, B.; Zhou, S.; Yang, X.; Kong, Y. Template-induced in situ dispersion of enhanced basic-sites on sponge-like mesoporous silica and its improved catalytic property. RSC Adv. 2016, 6, 91968–91980. [Google Scholar] [CrossRef]
- Yang, F.; Ding, Y.; Tang, J.; Zhou, S.; Wang, B.; Kong, Y. Oriented surface decoration of (Co-Mn) bimetal oxides on nanospherical porous silica and synergetic effect in biomass-derived 5-hydroxymethylfurfural oxidation. Mol. Catal. 2017, 435, 144–155. [Google Scholar] [CrossRef]
- Betiha, M.A.; Hassan, H.M.A.; Al-Sabagh, A.M.; Khder, A.E.R.S.; Ahmed, E.A. Direct synthesis and the morphological control of highly ordered mesoporous AlSBA-15 using urea-tetrachloroaluminate as a novel aluminum source. J. Mater. Chem. 2012, 22, 17551–17559. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Wan, H.; Kong, Y.; Wu, X.; Dong, L.; Li, B.; Chen, Y. The states of vanadium species in V-SBA-15 synthesized under different pH values. Microporous Mesoporous Mater. 2008, 110, 508–516. [Google Scholar] [CrossRef]
- Gómez, S.; Garces, L.J.; Villegas, J.; Ghosh, R.; Giraldo, O.; Suib, S.L. Synthesis and characterization of TM-MCM-48 (TM=Mn, V, Cr) and their catalytic activity in the oxidation of styrene. J. Catal. 2005, 233, 60–67. [Google Scholar] [CrossRef]
- Piumetti, M.; Armandi, M.; Garrone, E.; Bonelli, B. An IR spectroscopy assessment of the surface acidity of mesoporous VOx–SiO2 catalysts. Microporous Mesoporous Mater. 2012, 164, 111–119. [Google Scholar] [CrossRef]
- Wang, H.; Qian, W.; Chen, J.; Wu, Y.; Xu, X.; Wang, J.; Kong, Y. Spherical V-MCM-48: The synthesis, characterization and catalytic performance in styrene oxidation. RSC Adv. 2014, 4, 50832–50839. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, F.; Wan, H.; Wu, C.; Kong, Y.; Wu, X.; Zhao, B.; Dong, L.; Chen, Y. Synthesis, characterization of bimetallic Ce-Fe-SBA-15 and its catalytic performance in the phenol hydroxylation. Microporous Mesoporous Mater. 2008, 113, 393–401. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Cheng, J.; Li, Z.; Wang, H.; Sun, Q.; Han, B.; Kong, Y. Synthesis, characterization and catalytic activity of binary metallic titanium and iron containing mesoporous silica. Microporous Mesoporous Mater. 2012, 162, 51–59. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, J.; Yang, J.; Kapoor, M.P.; Inagaki, S.; Li, C. Synthesis, characterization, and catalytic activity of sulfonic acid-functionalized periodic mesoporous organosilicas. J. Catal. 2004, 228, 265–272. [Google Scholar] [CrossRef]
- Polarz, S.; Kuschel, A. Preparation of a periodically ordered mesoporous organosilica material using chiral building blocks. Adv. Mater. 2006, 18, 1206–1209. [Google Scholar] [CrossRef]
- Alauzun, J.; Mehdi, A.; Reye, C.; Corriu, R.J.P. Direct synthesis of bifunctional mesoporous organosilicas containing chelating groups in the framework and reactive functional groups in the channel pores. J. Mater. Chem. 2007, 17, 349–356. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, F.; Huang, J.; Zhang, F.; Li, H. Phenyl@Rh(I)-bridged periodic mesoporous organometalsilica with high catalytic efficiency in water-medium organic reactions. Chem. Mater. 2009, 21, 4925–4933. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, F.; He, W.; Zhang, F.; Wang, W.; Li, H. Periodic mesoporous organometallic silicas with unary or binary organometals inside the channel walls as active and reusable catalysts in aqueous organic reactions. J. Am. Chem. Soc. 2010, 132, 1492–1493. [Google Scholar] [CrossRef] [PubMed]
- Corriu, R.J.P.; Mehdi, A.; Reye, C.; Thieuleux, C. Control of coordination chemistry in both the framework and the pore channels of mesoporous hybrid materials. New J. Chem. 2003, 27, 905–908. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, B.; Gao, S.; Ding, Y.; Kong, Y. The structure-property relationship of oxovanadium(IV) complexes in the wall framework of PMOs and their catalytic applications. Appl. Surf. Sci. 2017, 397, 183–191. [Google Scholar] [CrossRef]
- Zhang, G.; Qin, L.; Wu, Y.; Xu, Z.; Guo, X. Iron oxide nanoparticles immobilized to mesoporous NH2-SiO2 spheres by sulfonic acid functionalization as highly efficient catalysts. Nanoscale 2015, 7, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Quan, X.; Chen, S.; Wang, J.; Muhammad, D. Synthesis of manganese incorporated hierarchical mesoporous silica nanosphere with fibrous morphology by facile one-pot approach for efficient catalytic ozonation. J. Hazard. Mater. 2016, 318, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Sumida, Y.; Fujiwara, K.; Yamashita, H. Facile synthesis of yolk–shell nanostructured photocatalyst with improved adsorption properties and molecular-sieving properties. ChemCatChem 2016, 8, 2781–2788. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamanishi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. Activity, recyclability, and stability of lipases immobilized on oil-filled spherical silica nanoparticles with different silica shell structures. ChemCatChem 2013, 5, 2527–2536. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamanishi, T.; Kamegawa, T.; Mori, K.; Che, M.; Yamashita, H. Lipase-embedded silica nanoparticles with oil-filled core-shell structure: Stable and recyclable platforms for biocatalysts. Chem. Commun. 2012, 48, 2882–2884. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, H.T.; Ling, Y.; Han, B.; Xia, K.S.; Zhou, C.G. Synthesis of MnSiO3 decorated hollow mesoporous silica spheres and its promising application in environmental remediation. Microporous Mesoporous Mater. 2017, 241, 409–417. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Tong, L.; Li, J.; Luo, R.; Qi, J.; Li, Y.; Wang, L. Iron-copper bimetallic nanoparticles supported on hollow mesoporous silica spheres: An effective heterogeneous fenton catalyst for orange II degradation. RSC Adv. 2015, 5, 69593–69605. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, S.; He, F.; Ding, S.; Li, L.; Yang, P. In situ assembly of well-dispersed Ni nanoparticles on silica nanotubes and excellent catalytic activity in 4-nitrophenol reduction. Nanoscale 2014, 6, 11181–11188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Sun, Y.; Mu, J.; Zhang, M.; Zhang, P.; Guo, Z.; Liang, P.; Wang, C.; Liu, Y. Tubular nanocomposite catalysts based on size-controlled and highly dispersed silver nanoparticles assembled on electrospun silica nanotubes for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 1387–1395. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, M.; Shinkai, S. Fabrication of silica nanotubes by using self-assembled gels and their applications in environmental and biological fields. Chem. Soc. Rev. 2010, 39, 4286–4302. [Google Scholar] [CrossRef] [PubMed]
- Tuan, H.-Y.; Ghezelbash, A.; Korgel, B.A. Silicon nanowires and silica nanotubes seeded by copper nanoparticles in an organic solvent. Chem. Mater. 2008, 20, 2306–2313. [Google Scholar] [CrossRef]
- Bian, S.-W.; Ma, Z.; Zhang, L.-S.; Niu, F.; Song, W.-G. Silica nanotubes with mesoporous walls and various internal morphologies using hard/soft dual templates. Chem. Commun. 2009, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, W.; Gao, S.; Du, J.; Chen, J.; Kong, Y.; Wang, J. Controllable synthesis and catalytic performance of mesoporous silica nanotube micro-reactor. Appl. Catal. A Gen. 2015, 504, 228–237. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Xiong, C.; Gao, S.; Wang, J.; Kong, Y. One-pot synthesis of iron-containing nanoreactors with controllable catalytic activity based on multichannel mesoporous silica. ChemCatChem 2015, 7, 3855–3864. [Google Scholar] [CrossRef]
- Dou, J.; Zeng, H.C. Integrated networks of mesoporous silica nanowires and their bifunctional catalysis–sorption application for oxidative desulfurization. ACS Catal. 2014, 4, 566–576. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic–inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Deng, S.; Wang, J.; Sun, P.; Chen, T. Hierarchically mesoporous silica single-crystalline nanorods with three dimensional cubic Fm-3m mesostructure. J. Mater. Chem. A 2013, 1, 14555–14561. [Google Scholar] [CrossRef]
- Wang, L.-Z.; Jiang, L.; Xu, C.-C.; Zhang, J.-L. Influence of Cr-MCM-48 and Cr-KIT-6 matrixes synthesized in alkaline and acidic conditions to the visible-light driven photocatalytic performance of loaded TiO2. J. Phys. Chem. C 2012, 116, 16454–16460. [Google Scholar] [CrossRef]
- Kumar, A.; Srinivas, D. Aminolysis of epoxides catalyzed by three-dimensional, mesoporous titanosilicates, Ti-SBA-12 and Ti-SBA-16. J. Catal. 2012, 293, 126–140. [Google Scholar] [CrossRef]
- Kumar, A.; Srinivas, D.; Ratnasamy, P. Synthesis of framework Ti-substituted, 3-D hexagonal, mesoporous Ti-SBA-12 for selective catalytic oxidation. Chem. Commun. 2009, 6484–6486. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, W.; Liu, N.; Xu, X.; Wang, D.; Zhang, M.; Sun, P.; Chen, T. Low temperature oxidative desulfurization with hierarchically mesoporous titaniumsilicate Ti-SBA-2 single crystals. Chem. Commun. 2015, 51, 11500–11503. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Zhao, D.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Room temperature synthesis of Ti-MCM-48 and Ti-MCM-41 mesoporous materials and their performance on photocatalytic splitting of water. J. Phys. Chem. C 2012, 116, 1605–1613. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.-G.; Xu, J.-X.; Liu, J.-Y.; Zhou, H.-J.; Sun, P.-C.; Chen, T.-H. Synthesis of hydrothermally stable, hierarchically mesoporous aluminosilicate Al-SBA-1 and their catalytic properties. Nanoscale 2012, 4, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Grams, J.; Potrzebowska, N.; Goscianska, J.; Michalkiewicz, B.; Ruppert, A.M. Mesoporous silicas as supports for Ni catalyst used in cellulose conversion to hydrogen rich gas. Int. J. Hydrogen Energy 2016, 41, 8656–8667. [Google Scholar] [CrossRef]
- Jourshabani, M.; Badiei, A.; Shariatinia, Z.; Lashgari, N.; Mohammadi Ziarani, G. Fe-supported SBA-16 type cagelike mesoporous silica with enhanced catalytic activity for direct hydroxylation of benzene to phenol. Ind. Eng. Chem. Res. 2016, 55, 3900–3908. [Google Scholar] [CrossRef]
- Hu, L.; Yue, B.; Wang, C.; Chen, X.; He, H. Enhanced catalytic activity over vanadium-containing silylated SBA-15 catalysts for styrene epoxidation and benzene hydroxylation. Appl. Catal. A Gen. 2014, 477, 141–146. [Google Scholar] [CrossRef]
- Khatri, P.K.; Singh, B.; Jain, S.L.; Sain, B.; Sinha, A.K. Cyclotriphosphazene grafted silica: A novel support for immobilizing the oxo-vanadium Schiff base moieties for hydroxylation of benzene. Chem. Commun. 2011, 47, 1610–1612. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Xu, X.; Wu, Y.; Zhang, R.; Wang, J. Effect of promoters on the catalytic activity of MCM-41 with high copper content in benzene hydroxylation. Chin. J. Catal. 2008, 29, 385–390. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Y.; Shi, L.; Sun, Q. Hydrothermal glucose modified C/V-SiO2 as a reusable heterogeneous catalyst for benzene oxidation to phenol by O2. Catal. Lett. 2017, 147, 2799–2806. [Google Scholar] [CrossRef]
- Li, B.; Wu, N.; Wu, K.; Liu, J.; Han, C.; Li, X. Bimetallic V and Ti incorporated MCM-41 molecular sieves and their catalytic properties. RSC Adv. 2015, 5, 16598–16603. [Google Scholar] [CrossRef]
- Yamada, M.; Karlin, K.D.; Fukuzumi, S. One-step selective hydroxylation of benzene to phenol with hydrogen peroxide catalysed by copper complexes incorporated into mesoporous silica-alumina. Chem. Sci. 2016, 7, 2856–2863. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y.; Yamada, Y.; Fukuzumi, S. Selective hydroxylation of benzene derivatives and alkanes with hydrogen peroxide catalysed by a manganese complex incorporated into mesoporous silica-alumina. Chem. Commun. 2015, 51, 4662–4665. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, J.; Wang, B.; Wu, J.; Zhao, C.; Gao, G.; Wu, P. Influences of fluorine implantation on catalytic performance and porosity of MOR-type titanosilicate. J. Catal. 2014, 320, 160–169. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Kong, Y.; Wang, J. Improvement on the mesostructural ordering and catalytic activity of Co-MCM-41 with ascorbic acid as auxiliary. Mater. Lett. 2013, 100, 159–162. [Google Scholar] [CrossRef]
- Sanjini, N.S.; Velmathi, S. Cuo impregnated mesoporous silica KIT-6: A simple and efficient catalyst for benzene hydroxylation by C–H activation and styrene epoxidation reactions. J. Porous Mater. 2016, 23, 1527–1535. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, W.; Wang, F.; Xu, J. Preparation and characterization of vanadium(IV) oxide supported on SBA-15 and its catalytic performance in benzene hydroxylation to phenol using molecular oxygen. J. Nat. Gas Chem. 2012, 21, 481–487. [Google Scholar] [CrossRef]
- Samran, B.; Aungkutranont, S.; White, T.J.; Wongkasemjit, S. Room temperature synthesis of Ti-SBA-15 from silatrane and titanium-glycolate and its catalytic performance towards styrene epoxidation. J. Sol-Gel Sci. Technol. 2011, 57, 221–228. [Google Scholar] [CrossRef]
- Fadhli, M.; Khedher, I.; Fraile, J.M. Modified Ti/MCM-41 catalysts for enantioselective epoxidation of styrene. J. Mol. Catal. A Chem. 2016, 420, 282–289. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, Y.; Zhao, L.; Zhu, Y. Adsorption synthesized cobalt-containing mesoporous silica SBA-15 as highly active catalysts for epoxidation of styrene with molecular oxygen. Catal. Commun. 2011, 12, 417–420. [Google Scholar] [CrossRef]
- Yang, G.; Chen, X.; Wang, X.; Xing, W.; Xu, N. Nickel(II) complex anchored on MCM-41 for the epoxidation of styrene by oxygen. Chin. J. Catal. 2013, 34, 1326–1332. [Google Scholar] [CrossRef]
- Qi, B.; Lou, L.-L.; Wang, Y.; Yu, K.; Yang, Y.; Liu, S. Comparison of different prepared Mn-MCM-41 catalysts in the catalytic epoxidation of alkenes with 30% H2O2. Microporous Mesoporous Mater. 2014, 190, 275–283. [Google Scholar] [CrossRef]
- Rahman, S.; Farooqui, S.A.; Rai, A.; Kumar, R.; Santra, C.; Prabhakaran, V.C.; Bhadu, G.R.; Sen, D.; Mazumder, S.; Maity, S.; et al. Mesoporous TUD-1 supported indium oxide nanoparticles for epoxidation of styrene using molecular O2. RSC Adv. 2015, 5, 46850–46860. [Google Scholar] [CrossRef]
- Li, B.; Luo, X.; Zhu, Y.; Wang, X. Immobilization of Cu(II) in KIT-6 supported Co3O4 and catalytic performance for epoxidation of styrene. Appl. Surf. Sci. 2015, 359, 609–620. [Google Scholar] [CrossRef]
- Li, B.; Luo, X.; Huang, J.; Wang, X.; Liang, Z. One-pot synthesis of ordered mesoporous Cu-KIT-6 and its improved catalytic behavior for the epoxidation of styrene: Effects of the pH value of the initial gel. Chin. J. Catal. 2017, 38, 518–528. [Google Scholar] [CrossRef]
- Mandal, S.; Rahman, S.; Kumar, R.; Bando, K.K.; Chowdhury, B. XAFS, XPS characterization of cerium promoted Ti-TUD-1 catalyst and it's activity for styrene oxidation reaction. Catal. Commun. 2014, 46, 123–127. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Yang, J.; Chen, R.; Zhang, Y.; Yin, D.; Wang, J. Ti containing mesoporous silica submicrometer-sphere, with tunable particle size for styrene oxidation. Appl. Surf. Sci. 2013, 283, 794–801. [Google Scholar] [CrossRef]
- Li, B.; Zhu, Y.; Jin, X. Synthesis of cobalt-containing mesoporous catalysts using the ultrasonic-assisted “pH-adjusting” method: Importance of cobalt species in styrene oxidation. J. Solid State Chem. 2015, 221, 230–239. [Google Scholar] [CrossRef]
- Yang, C.; Fu, L.; Zhu, R.; Liu, Z. Influence of cobalt species on the catalytic performance of Co-N-C/SiO2 for ethylbenzene oxidation. Phys. Chem. Chem. Phys. 2016, 18, 4635–4642. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhao, S.; Chen, Y.; Liu, Z. One-pot synthesis of mesoporous silica hollow spheres with Mn-N-C integrated into the framework for ethylbenzene oxidation. Chem. Commun. 2016, 52, 5577–5580. [Google Scholar] [CrossRef] [PubMed]
- Bhoware, S.S.; Shylesh, S.; Kamble, K.R.; Singh, A.P. Cobalt-containing hexagonal mesoporous molecular sieves (Co-HMS): Synthesis, characterization and catalytic activity in the oxidation reaction of ethylbenzene. J. Mol. Catal. A Chem. 2006, 255, 123–130. [Google Scholar] [CrossRef]
- Jermy, B.R.; Kim, S.Y.; Kim, D.K.; Park, D.W. Optimization of key parameters for effective vanadium substitution into cubic SBA-16 in the presence of co-surfactant at low acidity: Application in the selective oxidation of ethylbenzene. J. Ind. Eng. Chem. 2011, 17, 130–137. [Google Scholar] [CrossRef]
- Li, S.; Zhai, S.-R.; An, Q.-D.; Li, M.-H.; Song, Y.; Song, X.-W. Designed synthesis of multifunctional Fe3O4@SiO2–NH2@Cs–Co(II) towards efficient oxidation of ethylbenzene. Mater. Res. Bull. 2014, 60, 665–673. [Google Scholar] [CrossRef]
- Imran, G.; Pachamuthu, M.P.; Maheswari, R.; Ramanathan, A.; Sardhar Basha, S.J. Catalytic activity of MnTUD-1 for liquid phase oxidation of ethylbenzene with tert-butyl hydroperoxide. J. Porous Mater. 2012, 19, 677–682. [Google Scholar] [CrossRef]
- Dapurkar, S.E.; Kawanami, H.; Yokoyama, T.; Ikushima, Y. Solvent-free selective oxidation of benzylic compounds over chromium containing mesoporous molecular sieve catalyst at 1atm O2. Catal. Commun. 2009, 10, 1025–1028. [Google Scholar] [CrossRef]
- Ghiaci, M.; Molaie, F.; Sedaghat, M.E.; Dorostkar, N. Metalloporphyrin covalently bound to silica. Preparation, characterization and catalytic activity in oxidation of ethyl benzene. Catal. Commun. 2010, 11, 694–699. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, Z.; Dai, J.; Zhong, W.; Xu, Q.; Mao, L.; Yin, D. Manganese-containing hollow TS-1: Description of the catalytic sites and surface properties for solvent-free oxidation of ethylbenzene. Chem. Eng. J. 2017, 313, 1382–1395. [Google Scholar] [CrossRef]
- Sujandi; Prasetyanto, E.A.; Han, D.-S.; Lee, S.-C.; Park, S.-E. Immobilization of Co(III) using tethered cyclam ligand on SBA-15 mesoporous silica for aerial oxidation of ethylbenzene. Catal. Today 2009, 141, 374–377. [Google Scholar] [CrossRef]
- Ma, H.; Xu, J.; Chen, C.; Zhang, Q.; Ning, J.; Miao, H.; Zhou, L.; Li, X. Catalytic aerobic oxidation of ethylbenzene over Co/SBA-15. Catal. Lett. 2007, 113, 104–108. [Google Scholar] [CrossRef]
- Parida, K.M.; Dash, S.S. Manganese containing MCM-41: Synthesis, characterization and catalytic activity in the oxidation of ethylbenzene. J. Mol. Catal. A Chem. 2009, 306, 54–61. [Google Scholar] [CrossRef]
- Vetrivel, S.; Pandurangan, A. Side-chain oxidation of ethylbenzene with tert-butylhydroperoxide over mesoporous Mn-MCM-41 molecular sieves. J. Mol. Catal. A Chem. 2004, 217, 165–174. [Google Scholar] [CrossRef]
- Visuvamithiran, P.; Shanthi, K.; Palanichamy, M.; Murugesan, V. Direct synthesis of Mn-Ti-SBA-15 catalyst for the oxidation of ethylbenzene. Catal. Sci. Technol. 2013, 3, 2340–2348. [Google Scholar] [CrossRef]
- Pachamuthu, M.P.; Rajalakshmi, R.; Maheswari, R.; Ramanathan, A. Direct glycol assisted synthesis of an amorphous mesoporous silicate with framework incorporated Co2+: Characterization and catalytic application in ethylbenzene oxidation. RSC Adv. 2014, 4, 29909–29916. [Google Scholar] [CrossRef]
- Gago, S.; Bruno, S.M.; Queirós, D.C.; Valente, A.A.; Gonçalves, I.S.; Pillinger, M. Oxidation of ethylbenzene in the presence of an MCM-41-supported or ionic liquid-standing bischlorocopper(II) complex. Catal. Lett. 2011, 141, 1009–1017. [Google Scholar] [CrossRef]
- Jiang, S.; Kong, Y.; Wang, J.; Ren, X.; Yan, Q. Synthesis, characterization of bimetallic Sn-Zn-MCM41 and its catalytic performance in the hydroxylation of phenol. J. Porous Mater. 2006, 13, 341–346. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H.; Fu, X.; Liu, P.; Lefebvre, F.; Basset, J.-M. Characterization and catalytic properties of tin-containing mesoporous silicas prepared by different methods. J. Mol. Catal. A Chem. 2005, 238, 185–191. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, C.; Lv, Y.; Sun, C.; Gao, F.; Dong, L.; Chen, Y. Synthesis, characterization, and catalytic performance of copper-containing SBA-15 in the phenol hydroxylation. J. Colloid Interface Sci. 2012, 380, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.M.; Rath, D. Surface characterization and catalytic evaluation of copper-promoted Al-MCM-41 toward hydroxylation of phenol. J. Colloid Interface Sci. 2009, 340, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chaliha, S.; Bhattacharyya, K.G. Using Mn(II)-MCM41 as an environment-friendly catalyst to oxidize phenol, 2-chlorophenol, and 2-nitrophenol in aqueous solution. Ind. Eng. Chem. Res. 2008, 47, 1370–1379. [Google Scholar] [CrossRef]
- Hong, L.; Guanzhong, L.; Yanglong, G.; Yun, G.; Junsong, W. Synthesis of framework-substituted Fe-HMS and its catalytic performance for phenol hydroxylation. Nanotechnology 2006, 17, 997. [Google Scholar]
- Choi, J.-S.; Yoon, S.-S.; Jang, S.-H.; Ahn, W.-S. Phenol hydroxylation using Fe-MCM-41 catalysts. Catal. Today 2006, 111, 280–287. [Google Scholar] [CrossRef]
- Andas, J.; Adam, F.; Rahman, I.A. Sol–gel derived mesoporous cobalt silica catalyst: Synthesis, characterization and its activity in the oxidation of phenol. Appl. Surf. Sci. 2014, 315, 154–162. [Google Scholar] [CrossRef]
- He, H.; Gao, F.; Gao, S.; Wang, H.; Liu, X.; Dong, L.; Kong, Y. The synergistic effect of bimetallic Zn-Ti in MCM-41 support for the improvement of catalytic activity. J. Nanosci. Nanotechnol. 2016, 16, 7742–7749. [Google Scholar] [CrossRef]
- Xia, M.; Long, M.; Yang, Y.; Chen, C.; Cai, W.; Zhou, B. A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for fenton oxidation of phenol solution. Appl. Catal. B Environ. 2011, 110, 118–125. [Google Scholar] [CrossRef]
- Zhong, W.; Kirk, S.R.; Yin, D.; Li, Y.; Zou, R.; Mao, L.; Zou, G. Solvent-free selective oxidation of toluene by oxygen over MnOx/SBA-15 catalysts: Relationship between catalytic behavior and surface structure. Chem. Eng. J. 2015, 280, 737–747. [Google Scholar] [CrossRef]
- Liu, C.-C.; Lin, T.-S.; Chan, S.I.; Mou, C.-Y. A room temperature catalyst for toluene aliphatic C–H bond oxidation: Tripodal tridentate copper complex immobilized in mesoporous silica. J. Catal. 2015, 322, 139–151. [Google Scholar] [CrossRef]
- Szegedi, Á.; Popova, M.; Minchev, C. Catalytic activity of Co/MCM-41 and Co/SBA-15 materials in toluene oxidation. J. Mater. Sci. 2009, 44, 6710. [Google Scholar] [CrossRef]
- Suh, M.-J.; Ihm, S.-K. Preparation of copper oxide with high surface area associated with mesoporous silica. Top. Catal. 2010, 53, 447–454. [Google Scholar] [CrossRef]
- Li, W.B.; Zhuang, M.; Xiao, T.C.; Green, M.L.H. MCM-41 supported Cu−Mn catalysts for catalytic oxidation of toluene at low temperatures. J. Phys. Chem. B 2006, 110, 21568–21571. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Bu, Y.; Qin, Y.; Wang, Y.; Fu, Q. The effects of alkali metal on structure of manganese oxide supported on SBA-15 for application in the toluene catalytic oxidation. Chem. Eng. J. 2012, 209, 163–169. [Google Scholar] [CrossRef]
- Popova, M.; Szegedi, Á.; Cherkezova-Zheleva, Z.; Mitov, I.; Kostova, N.; Tsoncheva, T. Toluene oxidation on titanium- and iron-modified MCM-41 materials. J. Hazard. Mater. 2009, 168, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Cánepa, A.L.; Elías, V.R.; Vaschetti, V.M.; Sabre, E.V.; Eimer, G.A.; Casuscelli, S.G. Selective oxidation of benzyl alcohol through eco-friendly processes using mesoporous V-MCM-41, Fe-MCM-41 and Co-MCM-41 materials. Appl. Catal. A Gen. 2017, 545, 72–78. [Google Scholar] [CrossRef]
- Cang, R.; Lu, B.; Li, X.; Niu, R.; Zhao, J.; Cai, Q. Iron-chloride ionic liquid immobilized on SBA-15 for solvent-free oxidation of benzyl alcohol to benzaldehyde with H2O2. Chem. Eng. Sci. 2015, 137, 268–275. [Google Scholar] [CrossRef]
- Rana, B.S.; Jain, S.L.; Singh, B.; Bhaumik, A.; Sain, B.; Sinha, A.K. Click on silica: Systematic immobilization of Co(II) Schiff bases to the mesoporous silica via click reaction and their catalytic activity for aerobic oxidation of alcohols. Dalton Trans. 2010, 39, 7760–7767. [Google Scholar] [CrossRef] [PubMed]
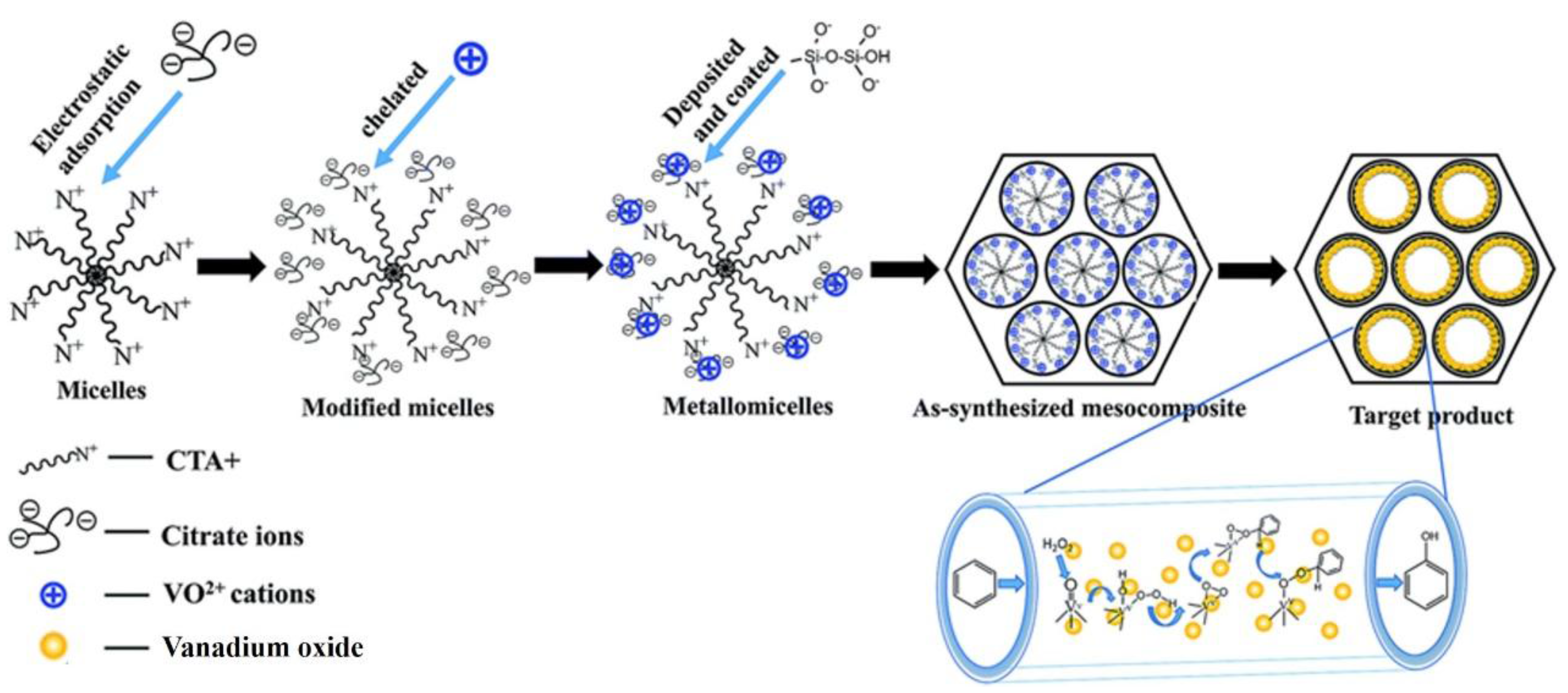
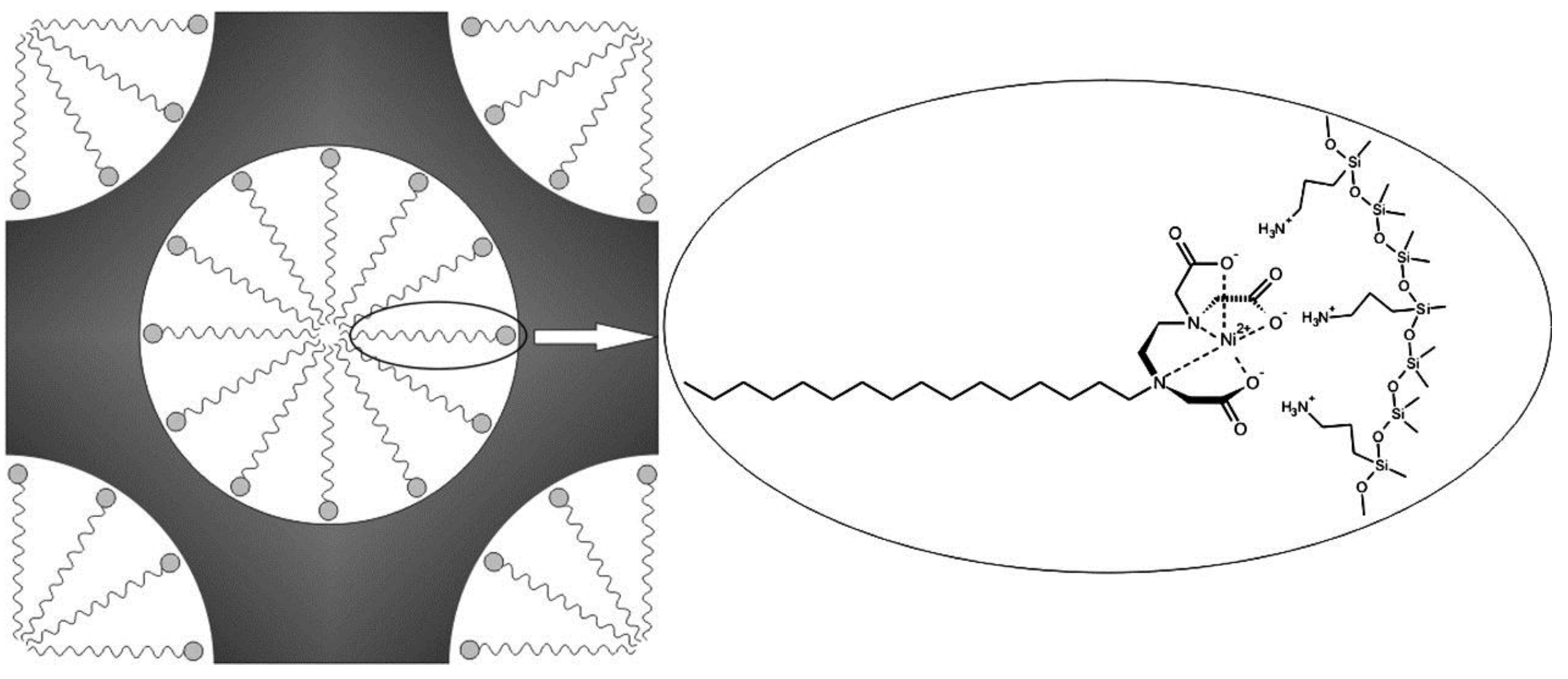

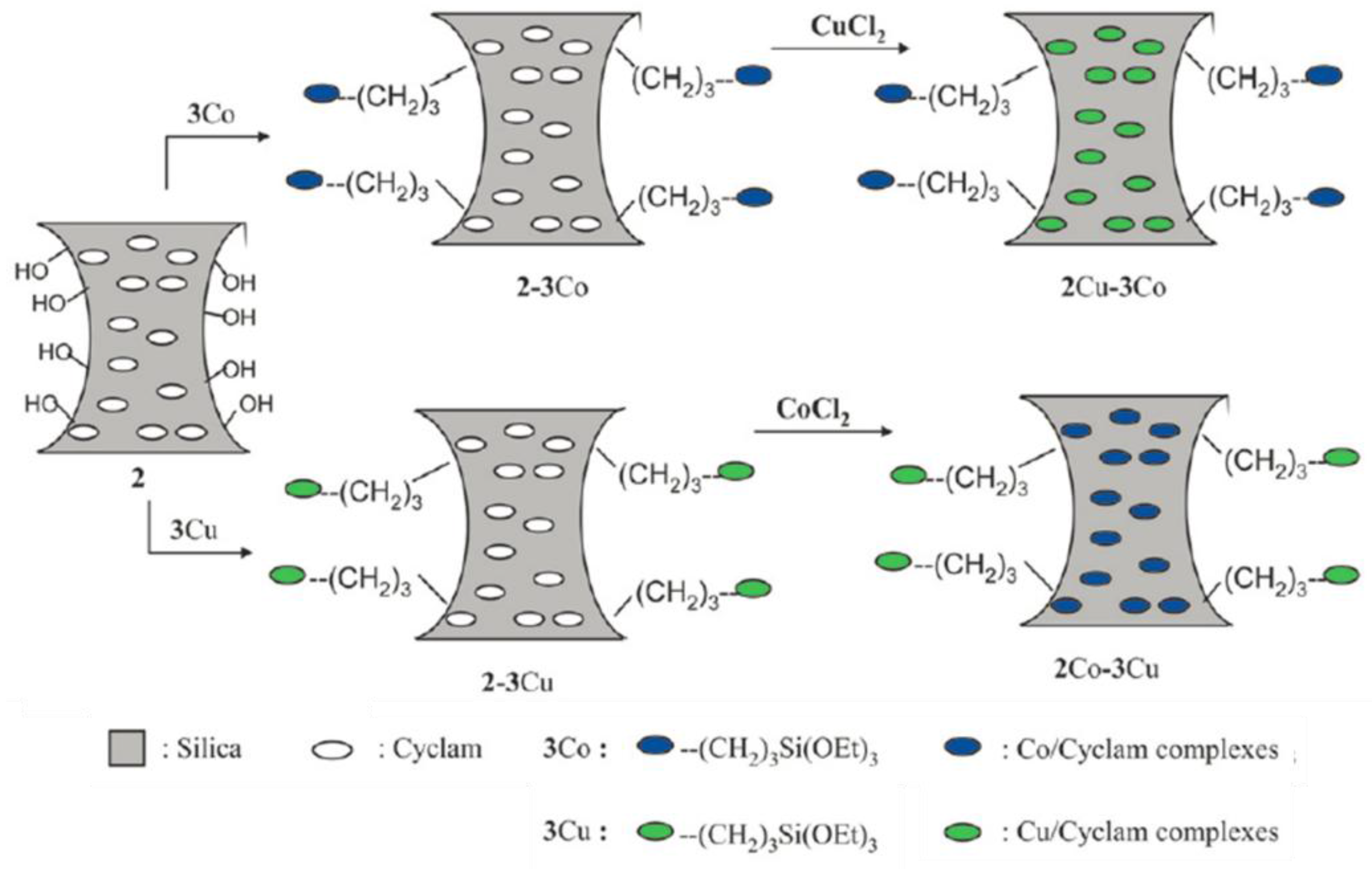
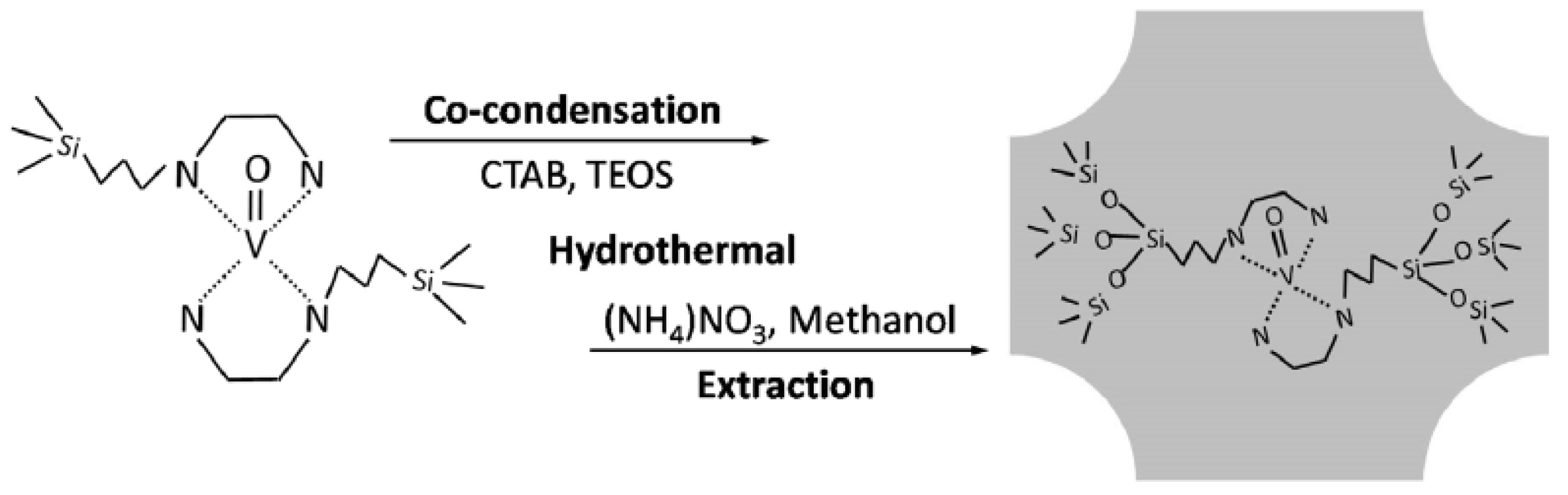

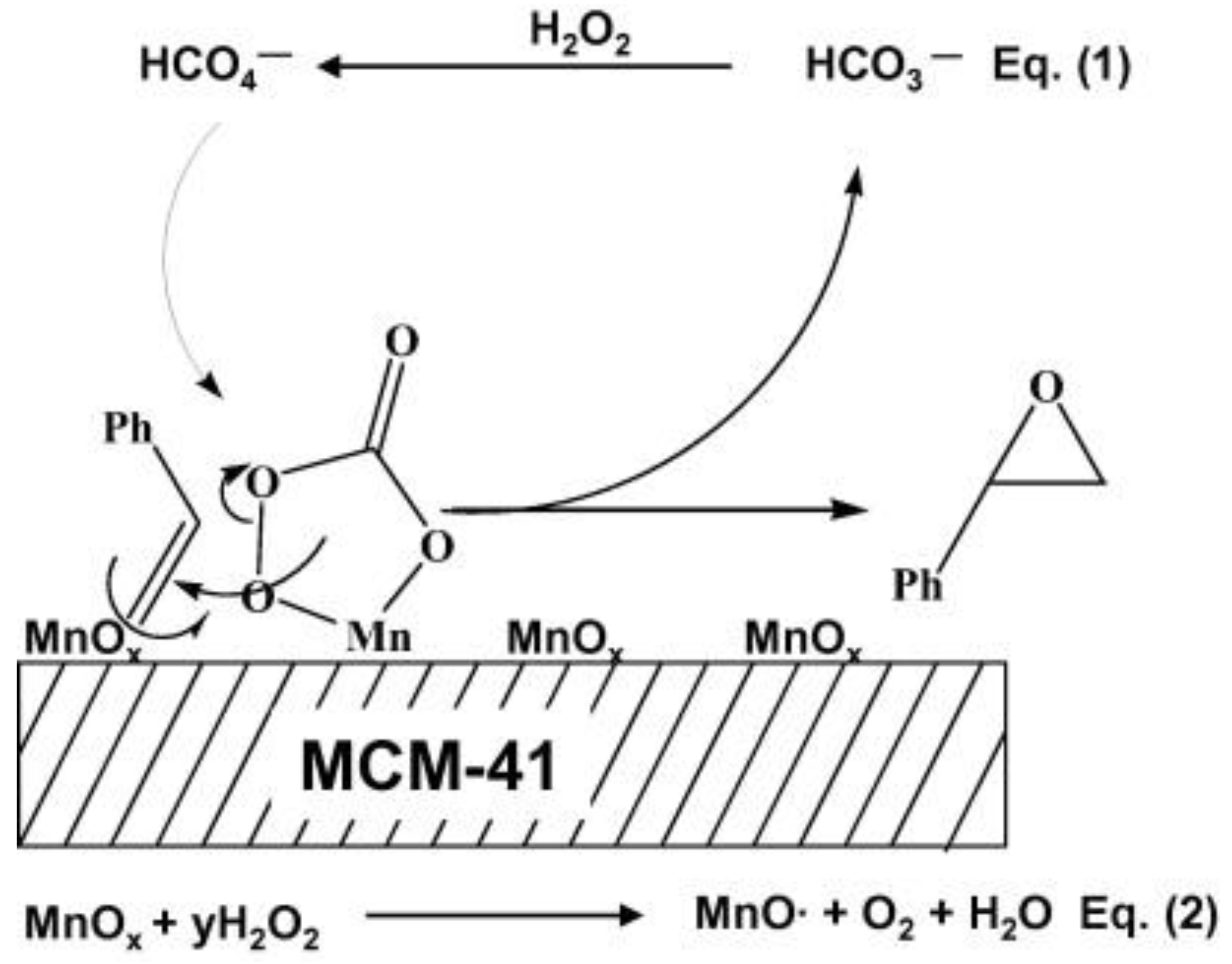

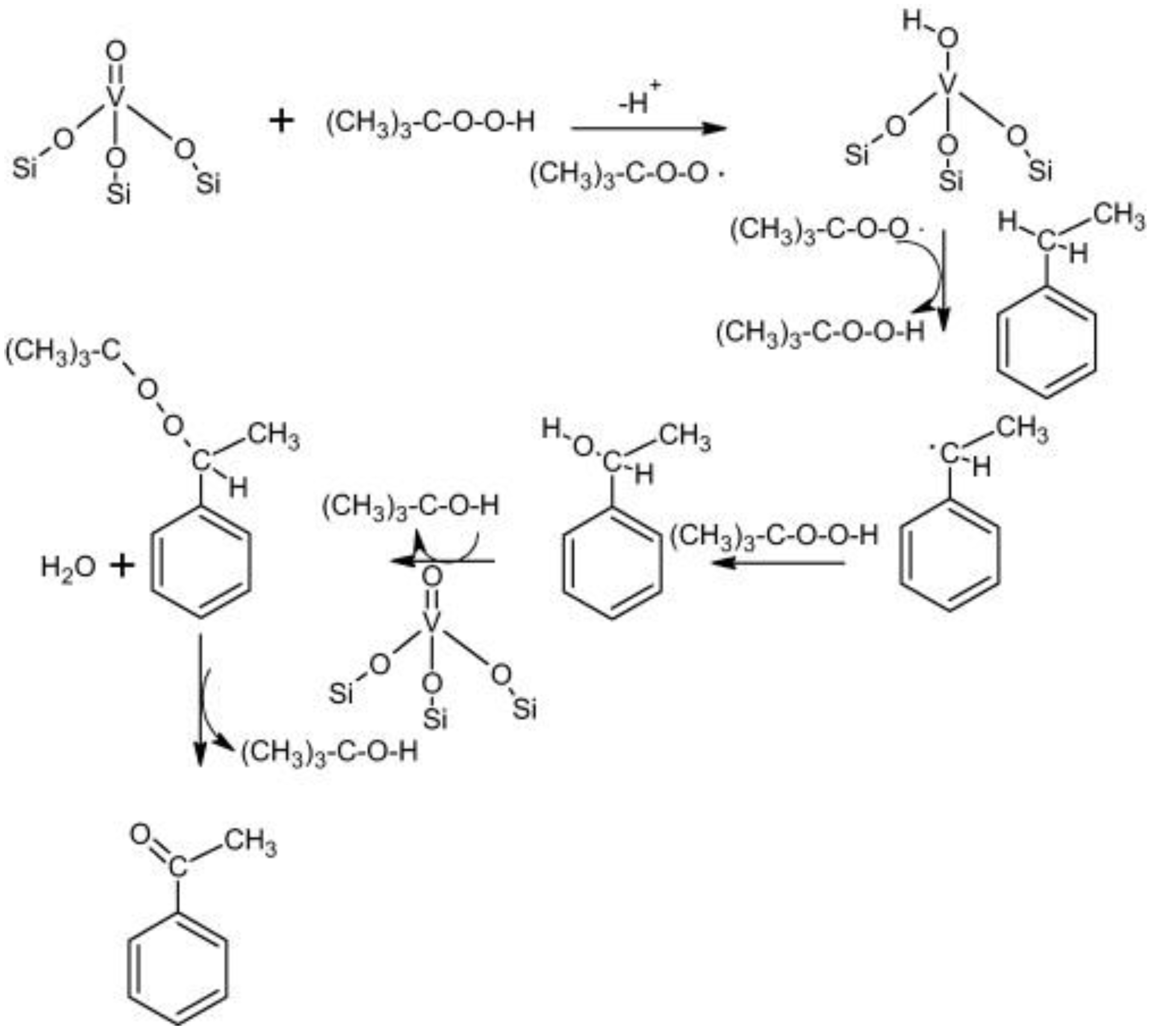
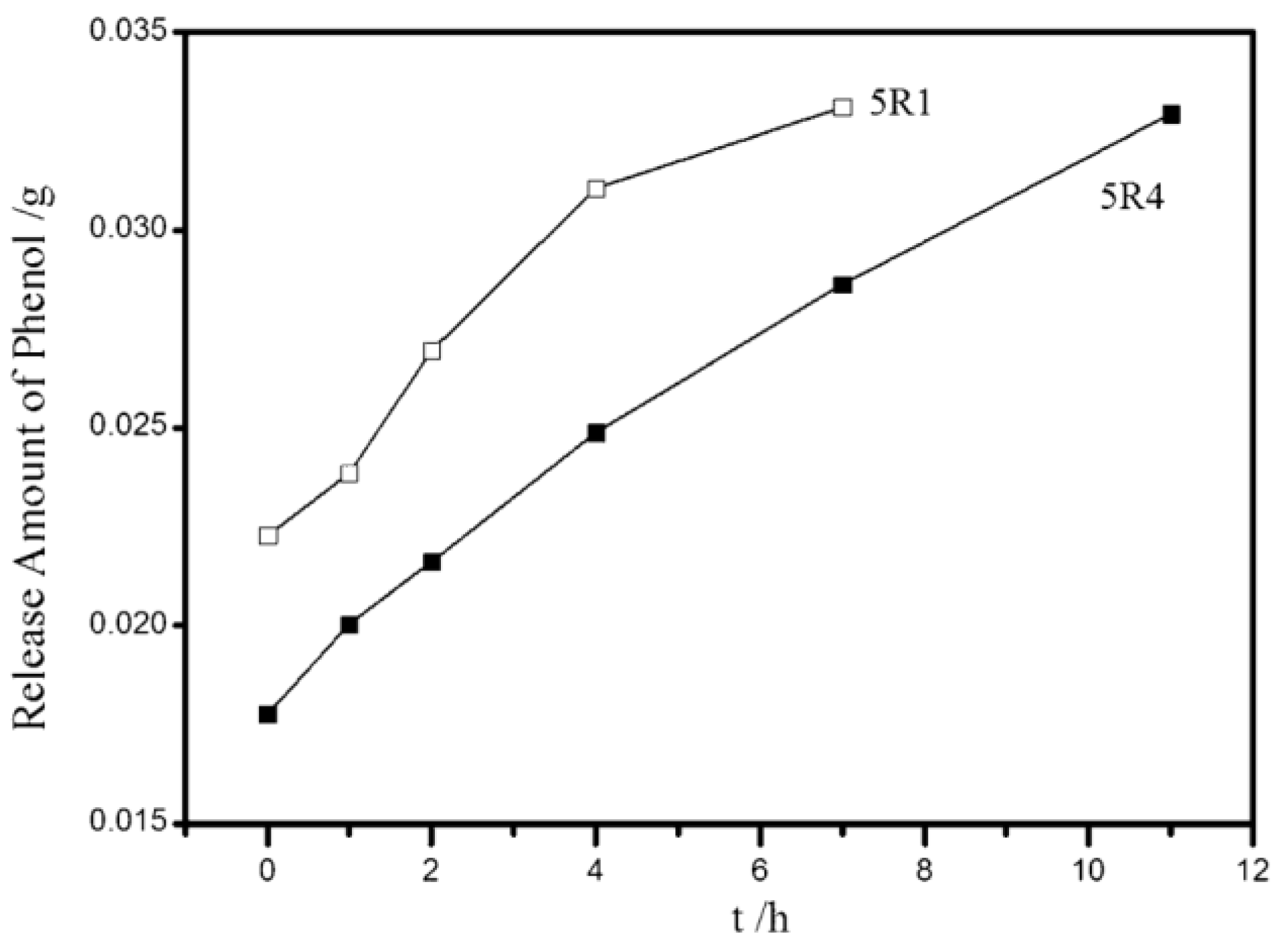
| Catalyst | Oxidant | Solvent | Temperature °C | Time h | Conv. % | Phenol Sel. % | Ref. |
|---|---|---|---|---|---|---|---|
| Fe/SBA-16 | H2O2 | CH3CN | 65 | 8 | 12.1 | 96.4 | [116] |
| V-Ti-MCM-41 | H2O2 | - | 30 | 5 | 22.3 | 94.7 | [121] |
| V/DMDS-x/SBA-15 | H2O2 | HAc | 70 | 3.5 | 34.1 | 93.2 | [117] |
| Cux-V-HMS | H2O2 | HAc | 70 | 3.5 | 31.5 | 29 | [44] |
| V-Schiff base SBA-15 | H2O2 | CH3CN | 50 | 8 | 37.35 | 60 | [118] |
| [CuII(tmpa)]2+@Al-MCM-41 | H2O2 | Acetone | 25 | 50 | 11.7 | >99 | [122] |
| [(tpa)MnII]2+@Al-MCM-41 | H2O2 | CH3CN | 25 | 25 | 11 | 100 | [123] |
| F-Ti-M-M | H2O2 | Butanone | 60 | 30 | 96.6 | >99 | [124] |
| Co-SBA-16 | H2O2 | CH3CN | 70 | 4 | 28.8 | 96.6 | [43] |
| Co-MCM-41 | H2O2 | HAc | 65 | 12 | 35.4 | 100 | [125] |
| CuO-KIT-6 | H2O2 | CH3CN | 65 | 4 | 51 | 100 | [126] |
| 20Cu-MCM-41 | H2O2 | CH3CN | 65 | 2.5 | 52.9 | 58.9 | [119] |
| 1Cr20Cu-MCM-41 | H2O2 | CH3CN | 65 | 2.5 | 61.0 | 51.7 | [119] |
| 1Al20Cu-MCM-41 | H2O2 | CH3CN | 65 | 2.5 | 33.0 | 100 | [119] |
| 60V/MCM | H2O2 | CH3CN | 30 | 10 | 29.1 | 90 | [67] |
| C/V-SiO2 | O2 | CH3CN | 90 | 10 | 12.0 | 100 | [120] |
| V-SBA-15 | O2 | HAc | 140 | 15 | 4.6 | 61 | [127] |
| Catalyst | Oxidant | Solvent | Temperature °C | Time h | Conv. % | SO Sel. % | Ref. |
|---|---|---|---|---|---|---|---|
| In2O3/TUD-1 | O2 | DMF | 130 | 8 | 25 | 60 | [133] |
| Co/SBA-15 | O2 | DMF | 100 | 6 | 93.9 | 65.5 | [130] |
| MCM-41-Ni | O2 | DMF | 100 | 6 | 95.2 | 66.7 | [131] |
| Cu(II)-Co3O4/KIT-6 | TBHP | DMF | 70 | 24 | 100 | 45.2 | [134] |
| Ti/MCM-41 | TBHP | DMF | 70 | 24 | 68 | 44 | [129] |
| Cu-KIT-6 | TBHP | CH3CN | 70 | 6 | 43.5 | 86.6 | [135] |
| CuO/AMS | TBHP | CH3CN | 70 | 6 | 94.5 | 91.7 | [72] |
| Ce-Ti-TUD-1 | H2O2 | DMF | 80 | 24 | 20.84 | 41.93 | [136] |
| Ti-MSSs | H2O2 | DMF | 60 | 6 | 44.7 | 17.7 | [137] |
| Co-SBA-15 | H2O2 | DMF | 70 | 10 | 34.7 | 88.2 | [138] |
| Co-MCM-41 | H2O2 | DMF | 70 | 10 | 27.0 | 84.0 | [138] |
| Ti-SBA-15 | H2O2 | DMF | 25 | 6 | 25.8 | 34.2 | [128] |
| Mn-MCM-41 | H2O2 | DMF | 25 | 1 | 99.5 | 90.2 | [132] |
| V/DMDS-x/SBA-15 | H2O2 | DMF | 70 | 5 | 67.1 | 88.5 | [117] |
| Catalyst | Oxidant | Solvent | Temperature °C | Time h | Conv. % | Sel. % | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| AP | PE | BP | |||||||
| MnOx/TS-1 | O2 | \ | 150 | 1 | 37.1 | 68.6 | 20.4 | 3.4 | [147] |
| Mn-N-C@SiO2 | O2 | \ | 120 | 5 | 15.7 | 73.6 | 19 | 7.4 | [140] |
| Co(III)(cyclam)py2-SBA-15 | O2 | \ | RT | 5 | 30 | 70 | - | - | [148] |
| Co-N-C/SiO2 | O2 | \ | 120 | 5 | 12.9 | 76.1 | 17.5 | 6.3 | [139] |
| Co/SBA-15 | O2 | \ | 120 | 9 | 70.1 | 83.7 | 4.1 | 0.1 | [149] |
| MnMCM-41 | O2 | \ | 110 | 6 | 34 | 88 | - | - | [41] |
| CrMCM-41 | O2 | \ | 95 | 24 | 66 | 90 | - | - | [145] |
| SF-ATPS-Mn(III)TMCPP | TBHP | - | 150 | 24 | 40.8 | 96.6 | 2.2 | 1.2 | [146] |
| MnTUD-1 | TBHP | CH3CN | 80 | 8 | 19.8 | 60.8 | 26.6 | 12.6 | [144] |
| Mn-MCM-41 | TBHP | CH3CN | 80 | 6 | 57.7 | 82.2 | - | 18 | [150] |
| Mn-MCM-41 | TBHP | - | 80 | 24 | 66.1 | 43.8 | 40.8 | 5.1 | [151] |
| Mn-Ti-MCM-41 | TBHP | CH3CN | 80 | 2 | 92 | 87 | 9 | 4 | [152] |
| Co-HMS | TBHP | - | 80 | 24 | 49 | 60 | 25 | 15 | [141] |
| CoTUD-1 | TBHP | CH3CN | 80 | 8 | 38 | 74 | 17 | 9 | [153] |
| V-SBA-16 | TBHP | CH3CN | 80 | 24 | 53.9 | 81.3 | 18.5 | - | [142] |
| MCM-41-L/CuCl2 | TBHP | CH3CN | 30 | 24 | 45 | 98 | - | - | [154] |
| Fe3O4@SiO2-NH2@CS-Co(II) | H2O2 | HOAc | 70 | 1 | 82.5 | 80.4 | - | - | [143] |
| Samples | Length (μm) | Iron Content a (wt. %) | X b (%) | S c (%) | Y d (%) | Cat./Hyd. e |
|---|---|---|---|---|---|---|
| 5 R1 | 0.15 | 0.68 | 35.5 | 61.8 | 21.9 | 1.55 |
| 5 R3 | 0.60 | 0.65 | 48.8 | 55.6 | 27.1 | 1.49 |
| 5 R4 | 0.80 | 0.65 | 50.1 | 50.5 | 25.3 | 1.48 |
| 5 R5 | 1.20 | 0.65 | 40.6 | 47.2 | 19.2 | 1.42 |
| Samples a | Pore Size b (nm) | Fe/Si c (wt. %) | Fe/Si d (wt. %) | X(Ph) e (%) | S(CAT) f (%) | S(HQ) g (%) |
|---|---|---|---|---|---|---|
| 0.05Fe/AMS | 1.72 | 1.11 | 0.92 | 29.8 | 44.3 | 28.4 |
| 0.1Fe/AMS | 1.72 | 2.23 | 1.62 | 34.8 | 49.4 | 29.7 |
| 0.15Fe/AMS | 1.72 | 3.50 | 2.53 | 44.3 | 52.2 | 30.4 |
| 0.20Fe/AMS | 1.67 | 4.67 | 2.89 | 43.6 | 50.1 | 30.3 |
| 0.15Fe/MCM-41(p) h | 2.40 | 3.50 | 2.52 | 38.6 | 32.8 | 18.6 |
| 0.15Fe/SBA-15(p) | 5.40 | 3.50 | 2.61 | 40.1 | 26.1 | 19.4 |
| 0.15Fe/AMS(p) | 2.60 | 3.50 | 2.50 | 36.7 | 31.4 | 19.6 |
| TS-1 | 0.54 | - | - | 29 | 50.2 | 49.8 |
| Fe-ZSM-5 | 0.50 | 2.00 | 1.54 | 32.9 | 60.5 | 39.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Yang, F.; Wang, B.; Su, H.; Lu, K.; Ding, Y.; Lei, K.; Xu, M.; Shao, B.; Wang, Y.; et al. Oriented Decoration in Metal-Functionalized Ordered Mesoporous Silicas and Their Catalytic Applications in the Oxidation of Aromatic Compounds. Catalysts 2018, 8, 80. https://doi.org/10.3390/catal8020080
Zhou S, Yang F, Wang B, Su H, Lu K, Ding Y, Lei K, Xu M, Shao B, Wang Y, et al. Oriented Decoration in Metal-Functionalized Ordered Mesoporous Silicas and Their Catalytic Applications in the Oxidation of Aromatic Compounds. Catalysts. 2018; 8(2):80. https://doi.org/10.3390/catal8020080
Chicago/Turabian StyleZhou, Shijian, Fu Yang, Bangbang Wang, Hang Su, Kangchao Lu, Yun Ding, Kai Lei, Man Xu, Bo Shao, Yun Wang, and et al. 2018. "Oriented Decoration in Metal-Functionalized Ordered Mesoporous Silicas and Their Catalytic Applications in the Oxidation of Aromatic Compounds" Catalysts 8, no. 2: 80. https://doi.org/10.3390/catal8020080
APA StyleZhou, S., Yang, F., Wang, B., Su, H., Lu, K., Ding, Y., Lei, K., Xu, M., Shao, B., Wang, Y., & Kong, Y. (2018). Oriented Decoration in Metal-Functionalized Ordered Mesoporous Silicas and Their Catalytic Applications in the Oxidation of Aromatic Compounds. Catalysts, 8(2), 80. https://doi.org/10.3390/catal8020080




