Increased Aromatics Formation by the Use of High-Density Polyethylene on the Catalytic Pyrolysis of Mandarin Peel over HY and HZSM-5
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermogravimetric (TG) Analysis
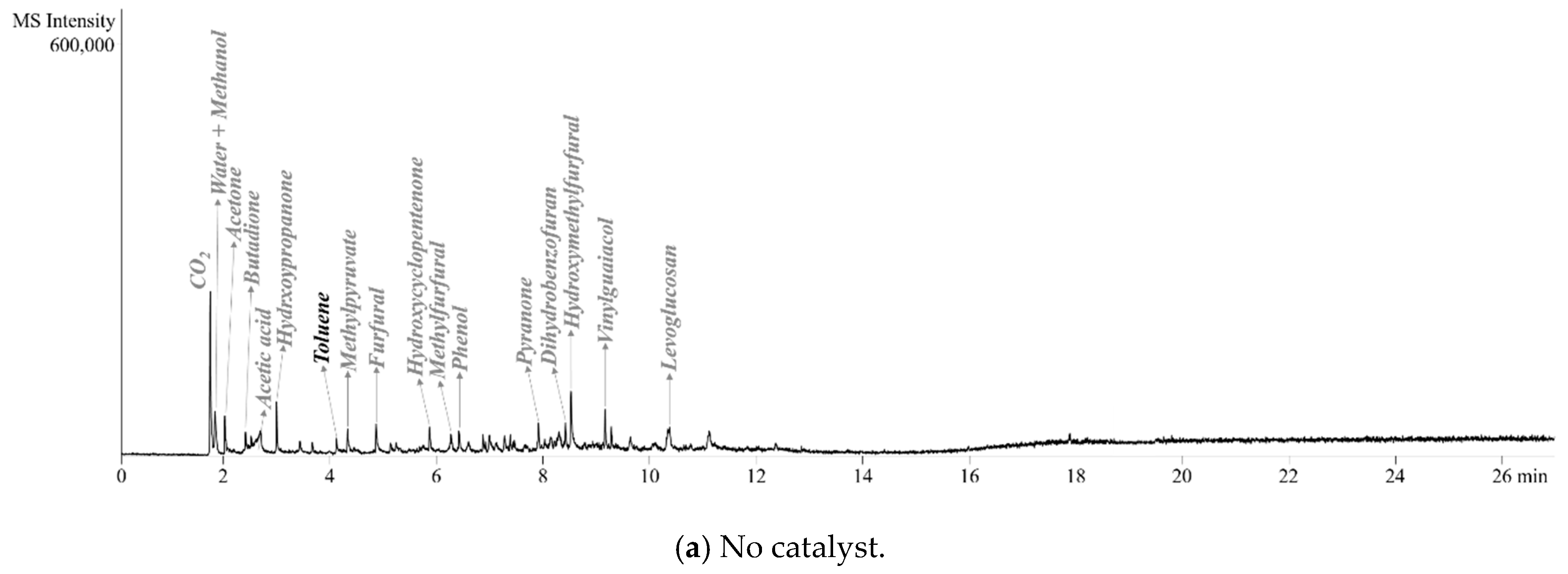
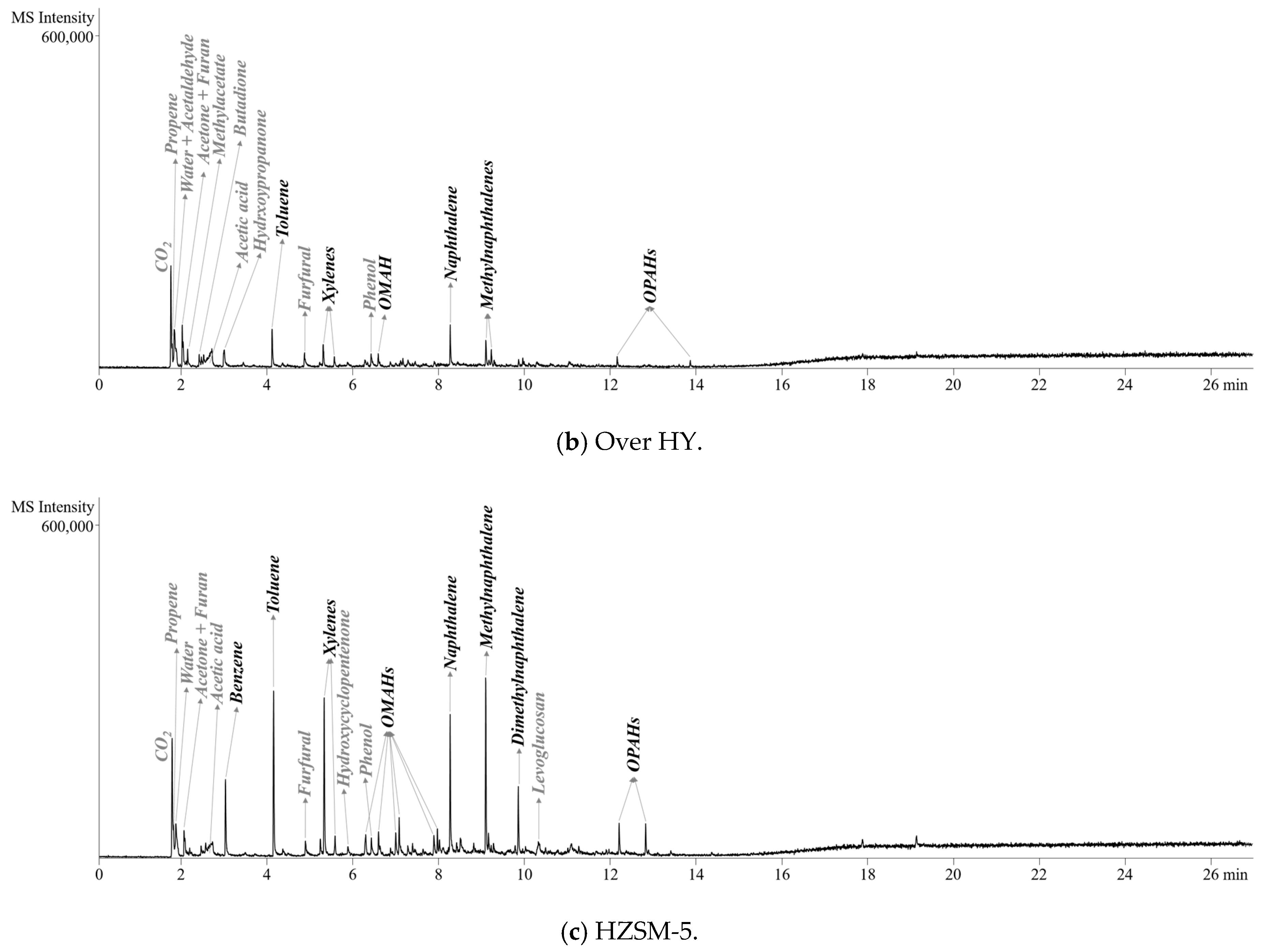
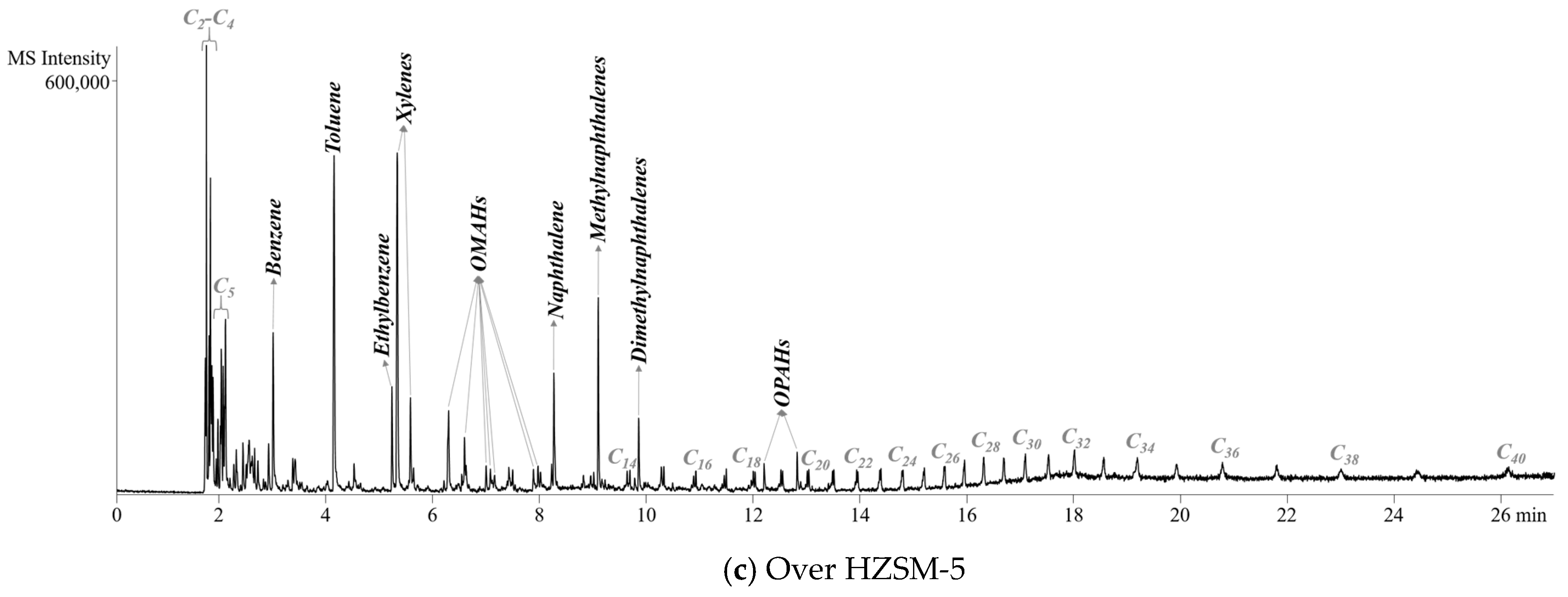
2.2. Pyrolyzer-Gas Chromatography/Mass Spectrometry (Py-GC/MS) Analysis
3. Materials and Methods
3.1. MP and HDPE
3.2. Catalyst
3.3. TG Analysis
3.4. Py-GC/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.M.; Park, S.; Kang, B.S.; Jae, J.; Rhee, G.H.; Jung, S.C.; Park, Y.K. Suppressed char agglomeration by rotary kiln reactor with alumina ball during the pyrolysis of kraft lignin. J. Ind. Eng. Chem. 2018, 66, 72–77. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.M.; Kim, S.; Ryu, C.; Park, S.H.; Park, Y.K. Review of the use of activated biochar for energy and environmental applications. Carbon Lett. 2018, 26, 1–10. [Google Scholar]
- Lee, H.; Kim, Y.M.; Lee, I.G.; Jeon, J.K.; Jung, S.C.; Chung, J.D.; Choi, W.G.; Park, Y.K. Recent advances in the catalytic hydrodeoxygenation of bio-oil. Korean J. Chem. Eng. 2016, 33, 3299–3315. [Google Scholar] [CrossRef]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2018, 98, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Lee, Y.G.; Khanal, S.K.; Park, B.J.; Bae, H.J. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl. Energy 2015, 140, 65–74. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.H.; Jung, J.; Jeon, J.K.; Ko, C.H.; Jeong, K.E.; Park, Y.K. Catalytic pyrolysis of mandarin residue from the mandarin juice processing industry. Bioresour. Technol. 2013, 136, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Lee, H.W.; Lee, S.H.; Kim, S.S.; Park, S.H.; Jeon, J.K.; Kim, S.; Park, Y.K. Pyrolysis properties and kinetics of mandarin peel. Korean J. Chem. Eng. 2011, 28, 2012–2016. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, H.; Cha, J.S.; Jeon, J.K.; Park, S.H.; Jung, S.C.; Kim, J.M.; Kim, S.C.; Park, Y.K. Catalytic pyrolysis of Citrus unshiu peel over mesoporous catalysts. Sci. Adv. Mater. 2017, 9, 1015–1019. [Google Scholar] [CrossRef]
- Miranda, R.; Bustos-Martinez, D.; Blanco, C.S.; Villarreal, M.H.G.; Cantu, M.E.R. Pyrolysis of sweet orange (Citrus sinensis) dry peel. J. Anal. Appl. Pyrolysis 2009, 86, 245–251. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.W.; Kim, S.; Watanabe, C.; Park, Y.K. Non-isothermal pyrolysis of Citrus unshiu peel. Bioenerg. Res. 2015, 8, 431–439. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil-A review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, Y.M.; Jae, J.; Watanabe, C.; Kim, S.; Jung, S.C.; Kim, S.C.; Park, Y.K. Pyrolysis and catalytic upgrading of Citrus unshiu peel. Bioresour. Technol. 2015, 194, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, H.; Chen, S.; Wu, J. Catalytic co-pyrolysis of lignocellulosic biomass with polymer: A critical review. Green Chem. 2016, 18, 4145–4169. [Google Scholar] [CrossRef]
- Xue, Y.; Kelkar, A.; Bai, X. Catalytic co-pyrolysis of biomass and polyethylene in a tandem micropyrolyzer. Fuel 2016, 166, 227–236. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Oh, D.; Hong, Y.; Kim, Y.M.; Jae, J.; Jung, S.C.; Jeon, J.K.; Park, Y.K. In-situ catalytic co-pyrolysis of yellow poplar and high-density polyethylene over mesoporous catalysts. Energy Convers. Manag. 2017, 151, 116–122. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.W.; Jae, J.; Jung, K.B.; Jung, S.C.; Watanabe, A.; Park, Y.K. Catalytic co-pyrolysis of biomass carbohydrates with LLDPE over Al-SBA-15 and mesoporous ZSM-5. Catal. Today 2017, 298, 46–52. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, Y.M.; Lee, H.W.; Jae, J.; Kim, D.H.; Jung, S.C.; Watanabe, C.; Park, Y.K. Catalytic copyrolysis of cellulose and thermoplastics over HZSM-5 and HY. ACS Sustain. Chem. Eng. 2016, 4, 1354–1363. [Google Scholar]
- Kim, Y.M.; Jae, J.; Lee, H.W.; Han, T.U.; Lee, H.; Park, S.H.; Kim, S.; Watanabe, C.; Park, Y.K. Ex-situ catalytic pyrolysis of citrus fruit peels over mesoporous MFI and Al-MCM-41. Energy Convers. Manag. 2016, 125, 277–289. [Google Scholar] [CrossRef]
- Aburto, J.; Moran, M.; Galano, A.; Torres-Garcia, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Alvarez, J.; Hooshdaran, B.; Cortazar, M.; Amutio, M.; Lopez, G.; Freire, F.B.; Haghshenasfard, M.; Hosseini, S.H.; Olazar, M. Valorization of citrus wastes by fast pyrolysis in a conical spouted bed reactor. Fuel 2018, 224, 111–120. [Google Scholar] [CrossRef]
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef] [PubMed]
- To, A.T.; Resasco, D.E. Role of a phenolic pool in the conversion of m-cresol to aromatics over HY and HZSM-5 zeolites. Appl. Catal. A-Gen. 2014, 487, 62–71. [Google Scholar] [CrossRef]
- Jae, J.; Tompsett, G.A.; Foster, A.J.; Hammond, K.D.; Auerbach, S.M.; Lobo, R.F.; Huber, G.W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal. 2011, 279, 257–268. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.M.; Jae, J.; Sung, B.H.; Jung, S.C.; Kim, S.C.; Jeon, J.K.; Park, Y.K. Catalytic pyrolysis of lignin using a two-stage fixed bed reactor comprised of in-situ natural zeolite and ex-situ HZSM-5. J. Anal. Appl. Pyrolysis 2016, 122, 282–288. [Google Scholar] [CrossRef]
- Park, Y.K.; Lee, B.; Watanabe, A.; Lee, H.W.; Lee, J.Y.; Kim, S.; Han, T.U.; Kim, Y.M. Catalytic copyrolysis of cork oak and waste plastic film over HBeta. Catalysts 2018, 8, 318. [Google Scholar] [CrossRef]
- Yan, G.; Jing, X.; Wen, H.; Xiang, S. Thermal cracking of virgin and waste plastics of PP and LDPE in a semibatch reactor under atmospheric pressure. Energy Fuels 2015, 29, 2289–2298. [Google Scholar] [CrossRef]
- Aho, A.; Kumar, N.; Eränen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure. Fuel 2008, 87, 2493–2501. [Google Scholar] [CrossRef]
- Carlson, T.R.; Tompsett, G.A.; Conner, W.C.; Huber, G.W. Aromatic production from catalytic fast pyrolysis of biomass-derived feedstocks. Top. Catal. 2009, 52, 241–252. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.M.; Lee, B.; Kim, S.; Jae, J.; Jung, S.C.; Kim, T.W.; Park, Y.K. Catalytic copyrolysis of torrefied cork oak and high density polyethylene over a mesoporous HY catalyst. Catal. Today 2018, 307, 301–307. [Google Scholar] [CrossRef]
- Zhang, H.; Carlson, T.R.; Xiao, R.; Huber, G.W. Catalytic fast pyrolysis of wood and alcohol mixture in a fluidized bed reactor. Green Chem. 2012, 14, 98–110. [Google Scholar] [CrossRef]
- Atutxa, A.; Aguado, R.; Gayubo, A.G.; Olazar, M.; Bilbao, J. Kinetic description of the catalytic pyrolysis of biomass in a conical spouted bed reactor. Energy Fuels 2005, 19, 765–774. [Google Scholar] [CrossRef]
- Asadieraghi, M.; Daud, W.M.A.W. In-situ catalytic upgrading of biomass pyrolysis vapor: Co-feeding with methanol in a multi-zone fixed bed reactor. Energy Convers. Manag. 2015, 92, 448–458. [Google Scholar] [CrossRef]
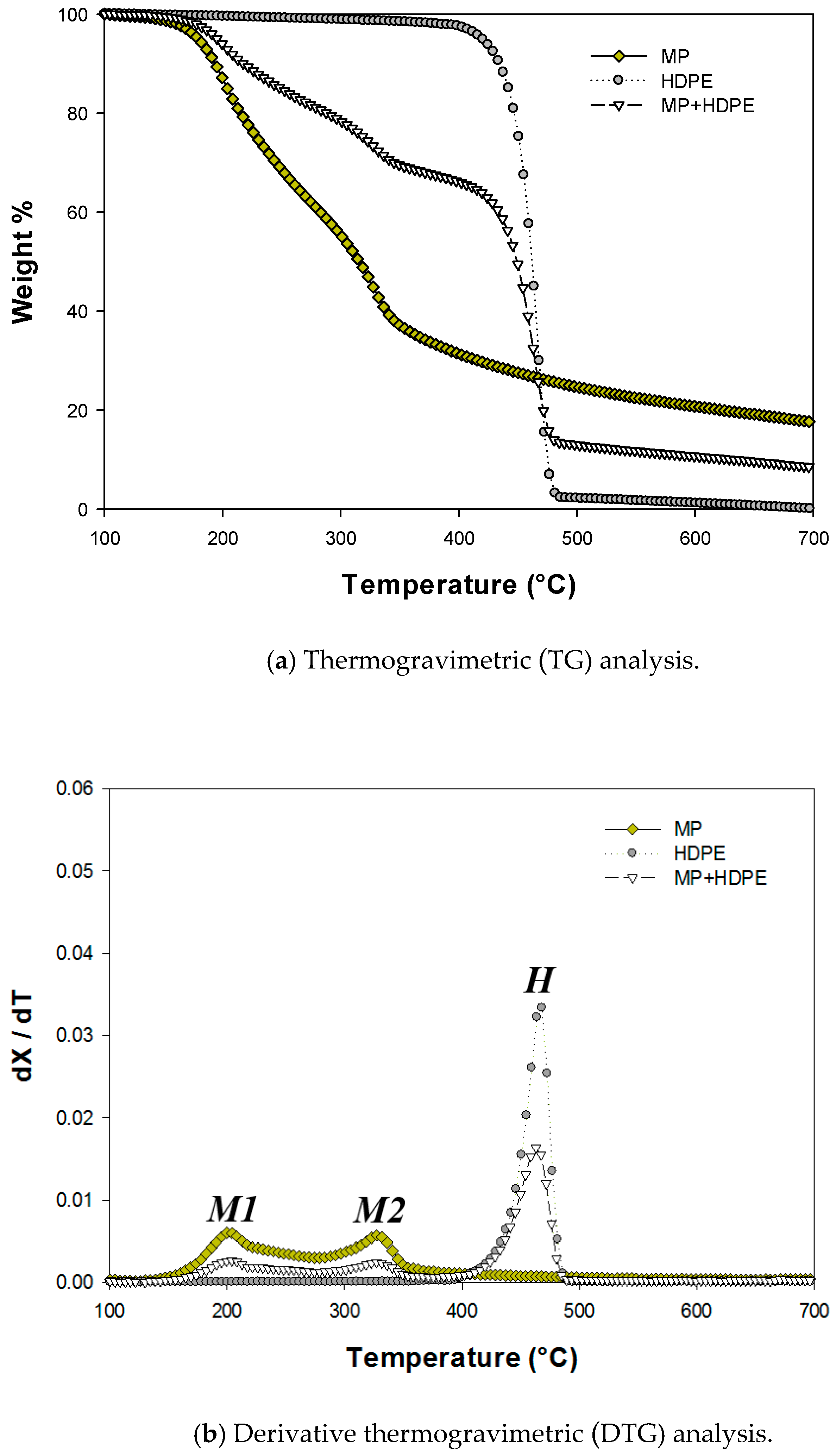
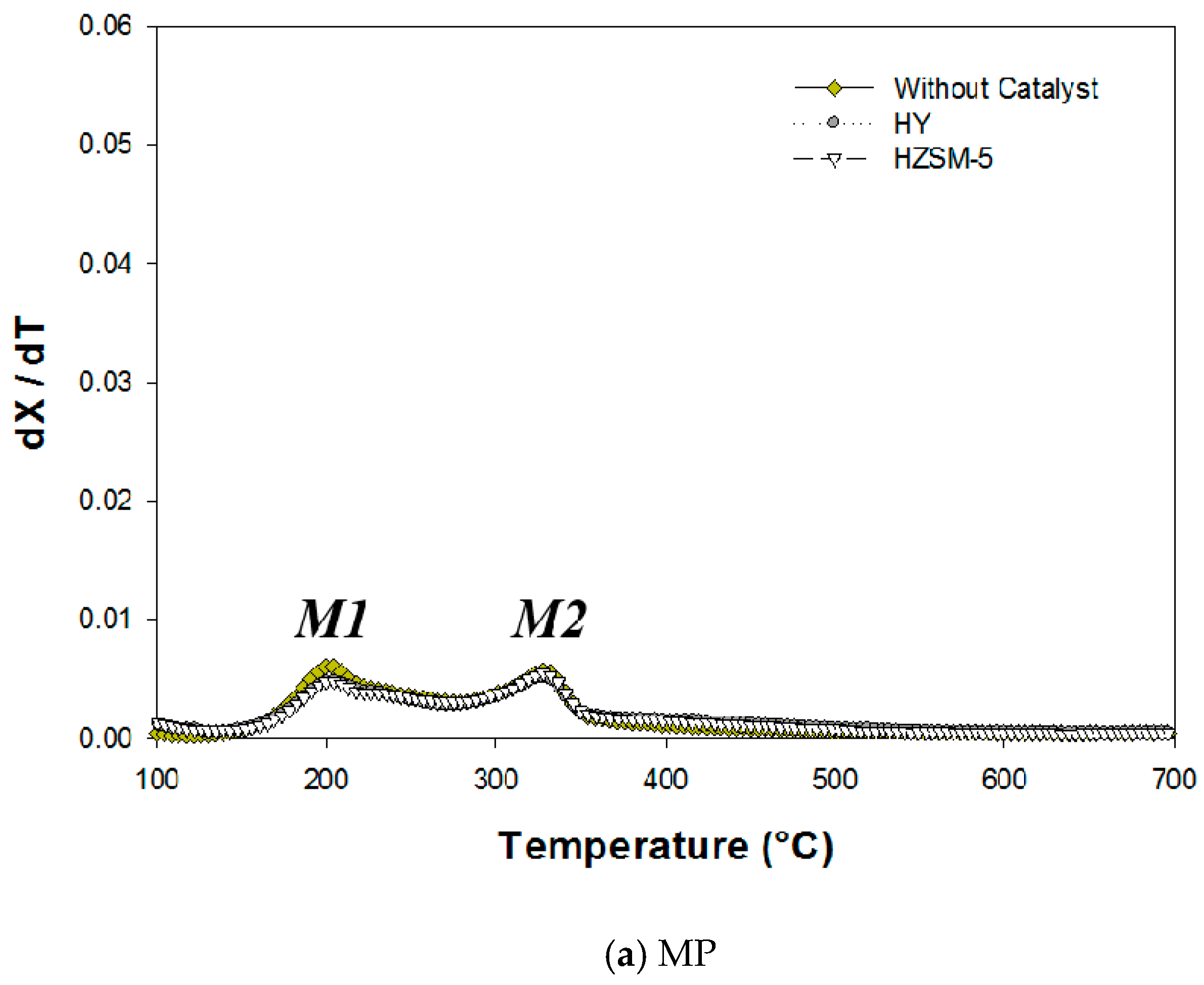
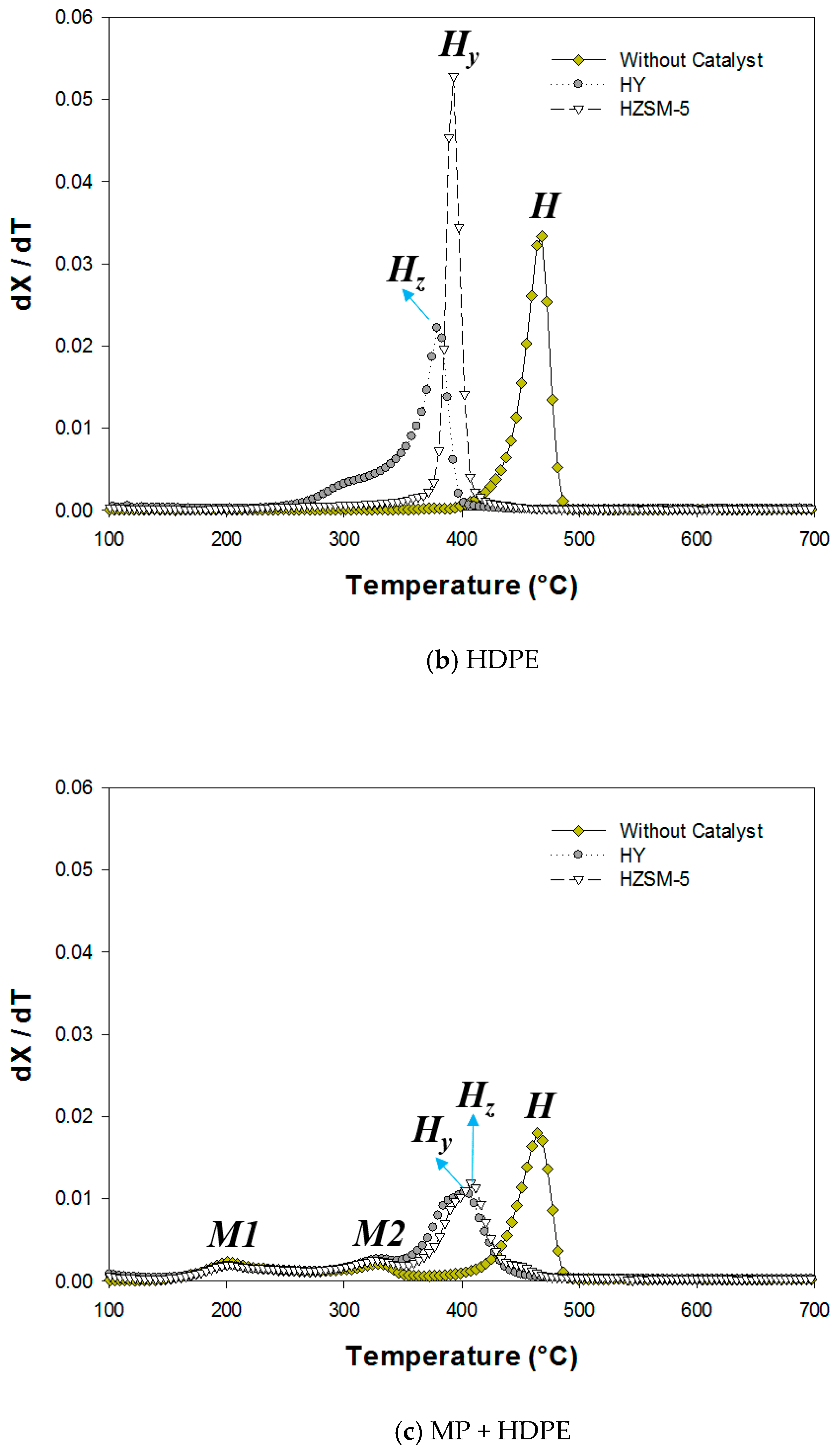




| M1 | M2 | H | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Catalyst | Tonset | Tmax | Tmax | Toffset | Tonset | Tmax | Toffset |
| MP | No catalyst | 160 | 204 | 328 | 354 | - | - | - |
| HDPE | No catalyst | - | - | - | - | 397 | 465 | 495 |
| MP + HDPE | No catalyst | 161 | 205 | 329 | 355 | 395 | 464 | 495 |
| MP | HY | 163 | 205 | 329 | 355 | - | - | - |
| MP | HZSM-5 | 162 | 204 | 328 | 354 | - | - | - |
| HDPE | HY | - | - | - | - | 327 | 379 | 406 |
| HDPE | HZSM-5 | - | - | - | - | 363 | 393 | 416 |
| MP + HDPE | HY | 160 | 204 | 328 | 345 | 350 | 402 | 459 |
| MP + HDPE | HZSM-5 | 161 | 205 | 329 | 346 | 351 | 408 | 460 |
| Catalyst | SiO2/Al2O3 | SBET (m2/g) | Pore Size (nm) |
|---|---|---|---|
| HY | 5.1 | 730 | 0.74 × 0.74 |
| HZSM-5 | 23 | 382 | 0.51 × 0.55 (100) 0.53 × 0.56 (010) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-K.; Siddiqui, M.Z.; Kang, Y.; Watanabe, A.; Lee, H.W.; Jeong, S.J.; Kim, S.; Kim, Y.-M. Increased Aromatics Formation by the Use of High-Density Polyethylene on the Catalytic Pyrolysis of Mandarin Peel over HY and HZSM-5. Catalysts 2018, 8, 656. https://doi.org/10.3390/catal8120656
Park Y-K, Siddiqui MZ, Kang Y, Watanabe A, Lee HW, Jeong SJ, Kim S, Kim Y-M. Increased Aromatics Formation by the Use of High-Density Polyethylene on the Catalytic Pyrolysis of Mandarin Peel over HY and HZSM-5. Catalysts. 2018; 8(12):656. https://doi.org/10.3390/catal8120656
Chicago/Turabian StylePark, Young-Kwon, Muhammad Zain Siddiqui, Yejin Kang, Atsushi Watanabe, Hyung Won Lee, Sang Jae Jeong, Seungdo Kim, and Young-Min Kim. 2018. "Increased Aromatics Formation by the Use of High-Density Polyethylene on the Catalytic Pyrolysis of Mandarin Peel over HY and HZSM-5" Catalysts 8, no. 12: 656. https://doi.org/10.3390/catal8120656
APA StylePark, Y.-K., Siddiqui, M. Z., Kang, Y., Watanabe, A., Lee, H. W., Jeong, S. J., Kim, S., & Kim, Y.-M. (2018). Increased Aromatics Formation by the Use of High-Density Polyethylene on the Catalytic Pyrolysis of Mandarin Peel over HY and HZSM-5. Catalysts, 8(12), 656. https://doi.org/10.3390/catal8120656









