N-Heterocyclic Carbene Catalysis under Oxidizing Conditions
Abstract
1. Introduction
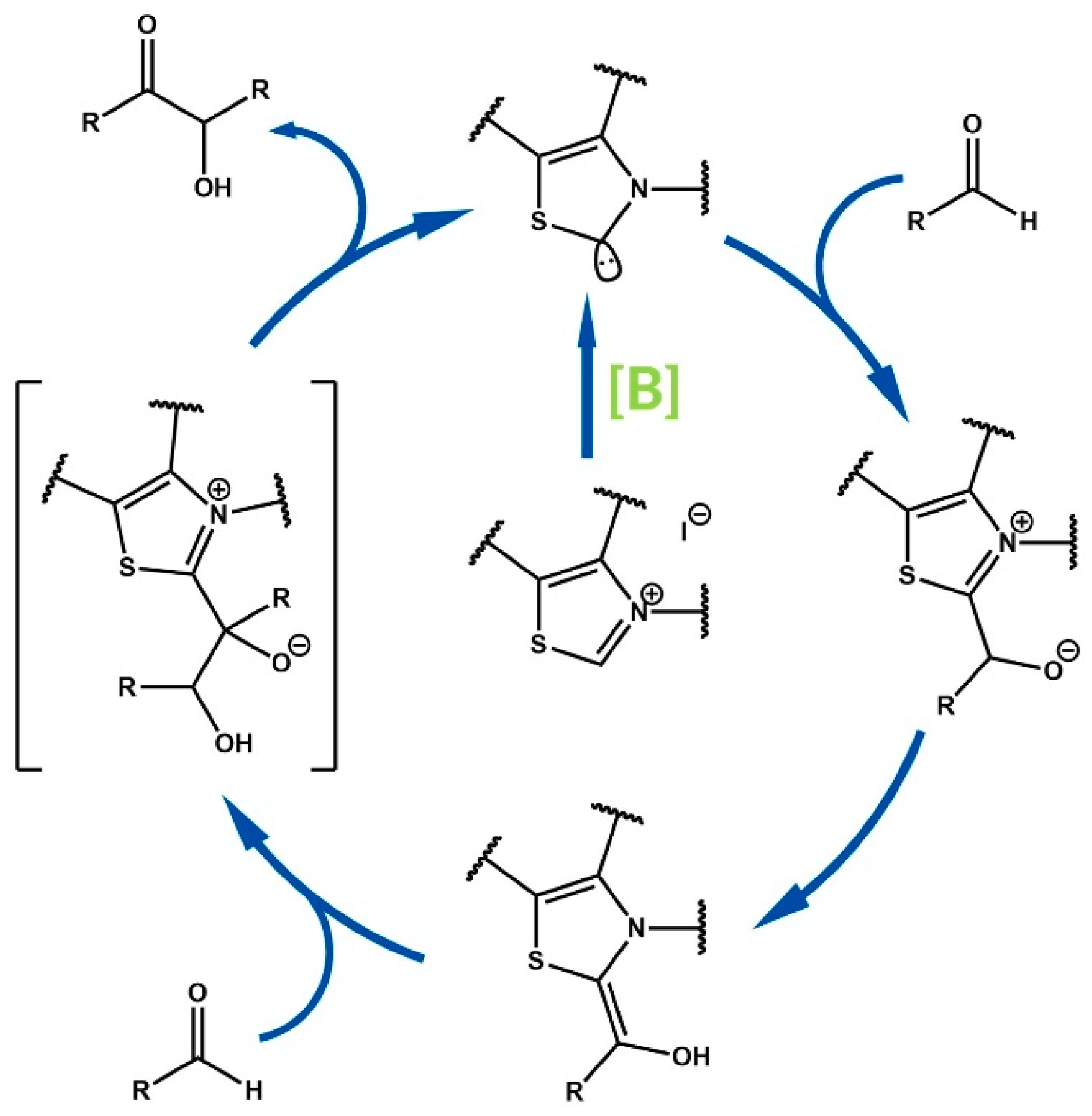
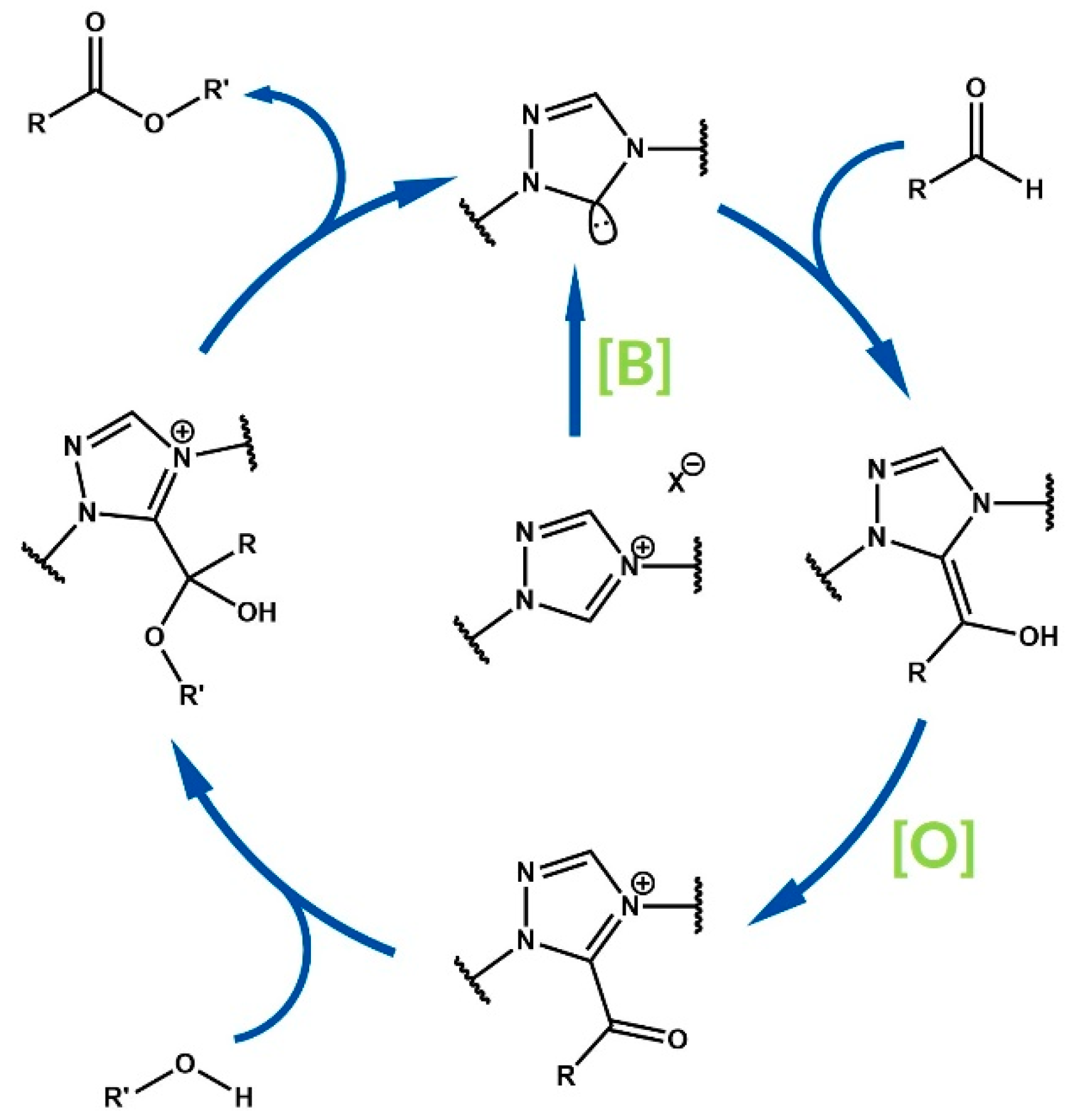
2. Mechanistic and Nomenclature Issues
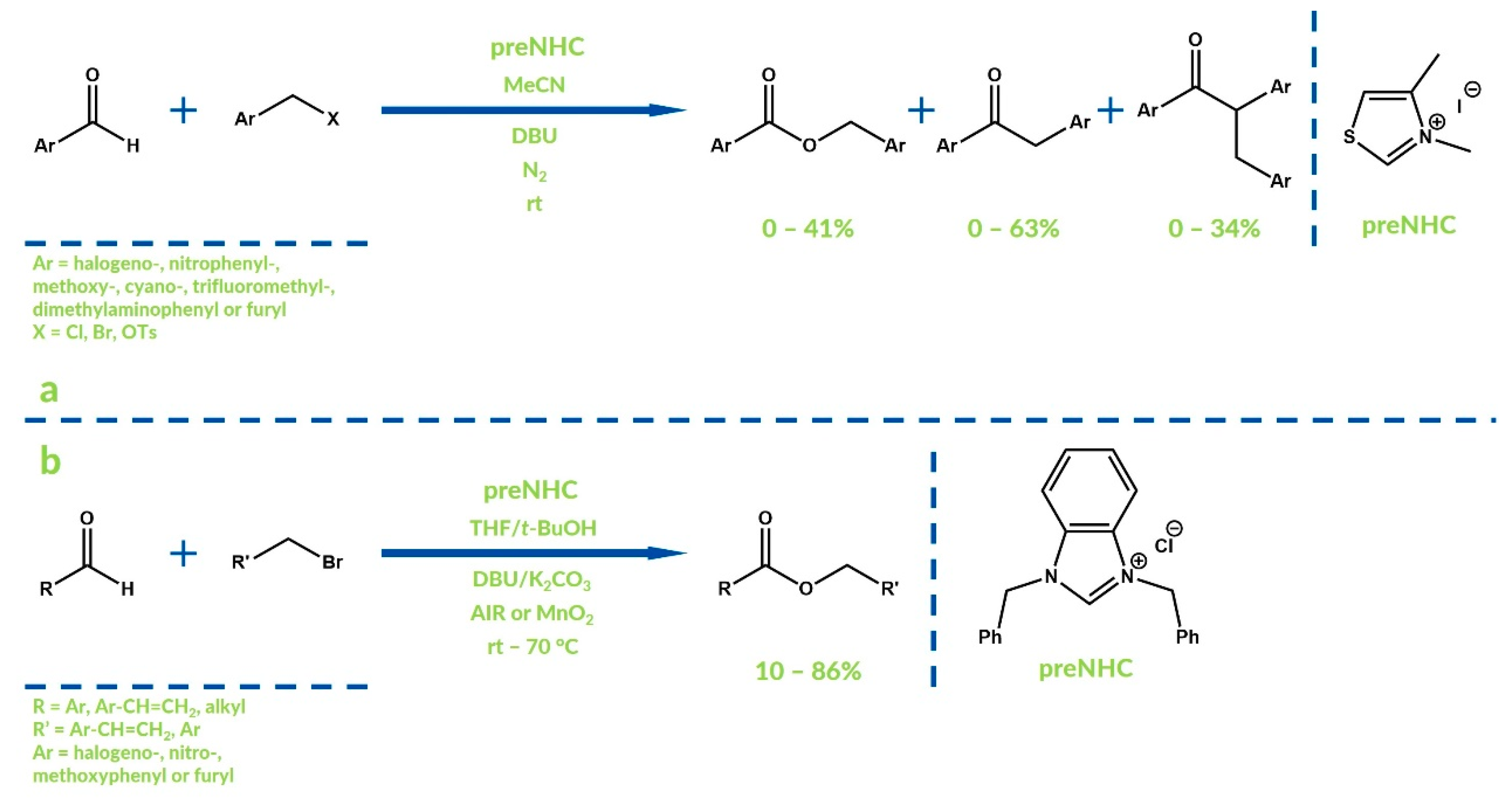
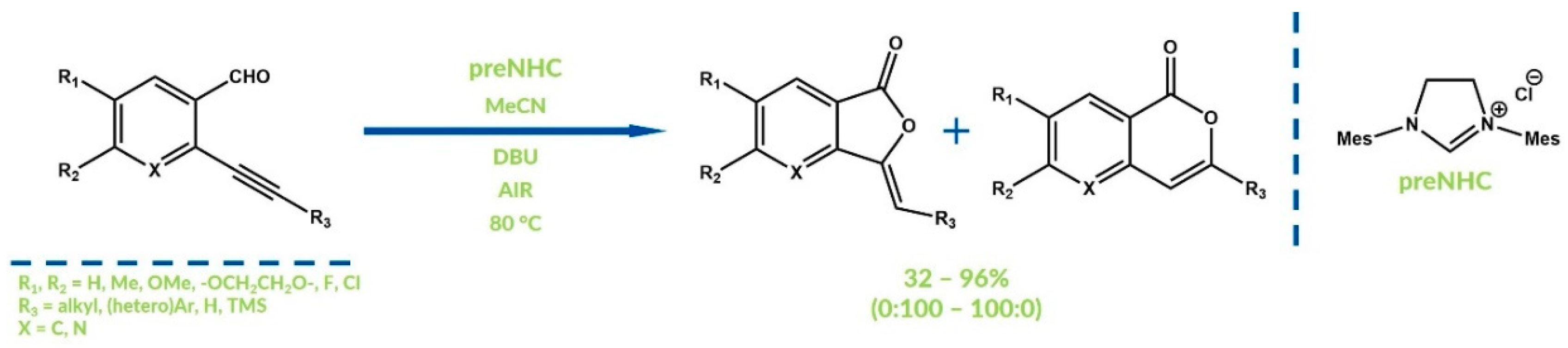
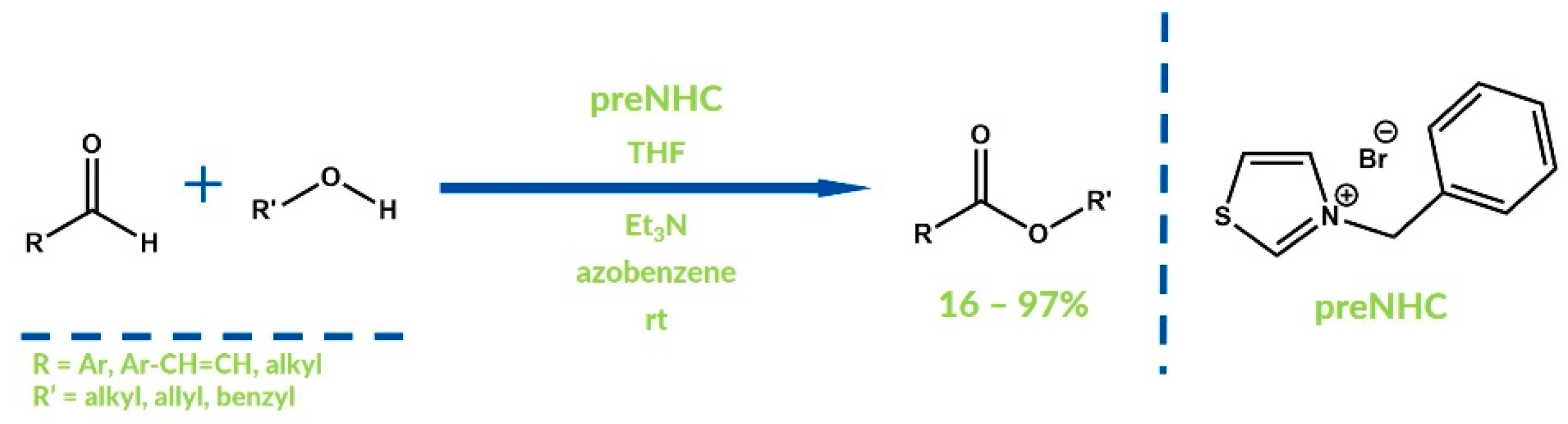
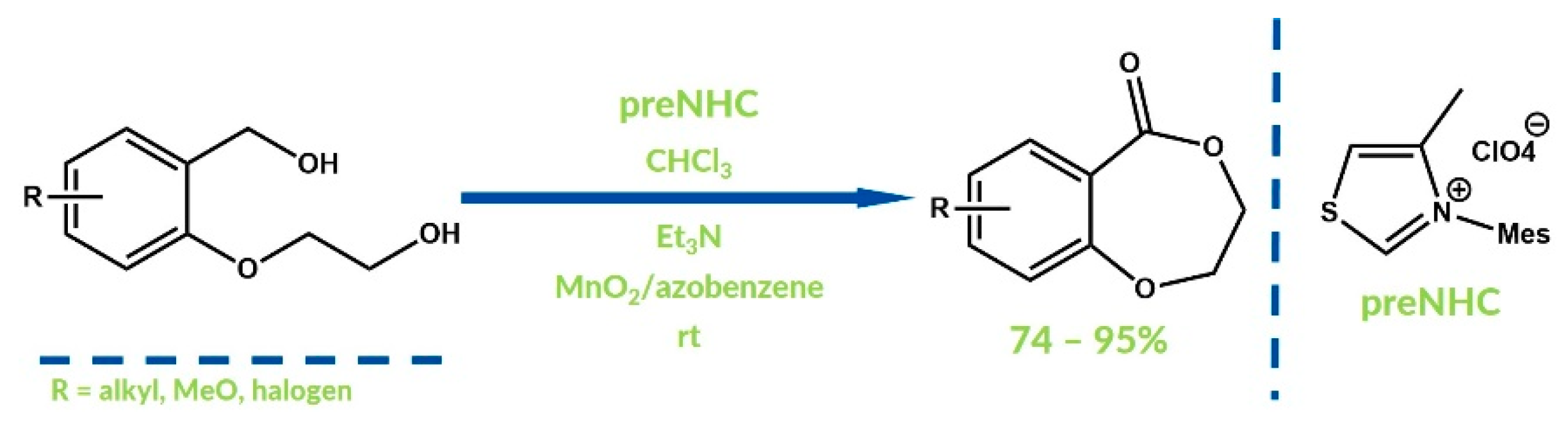
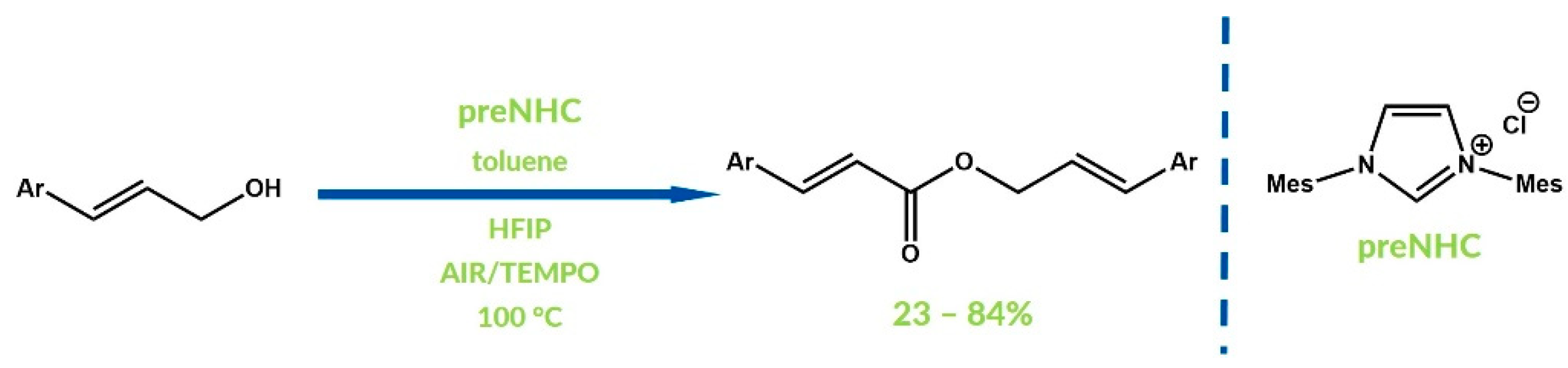
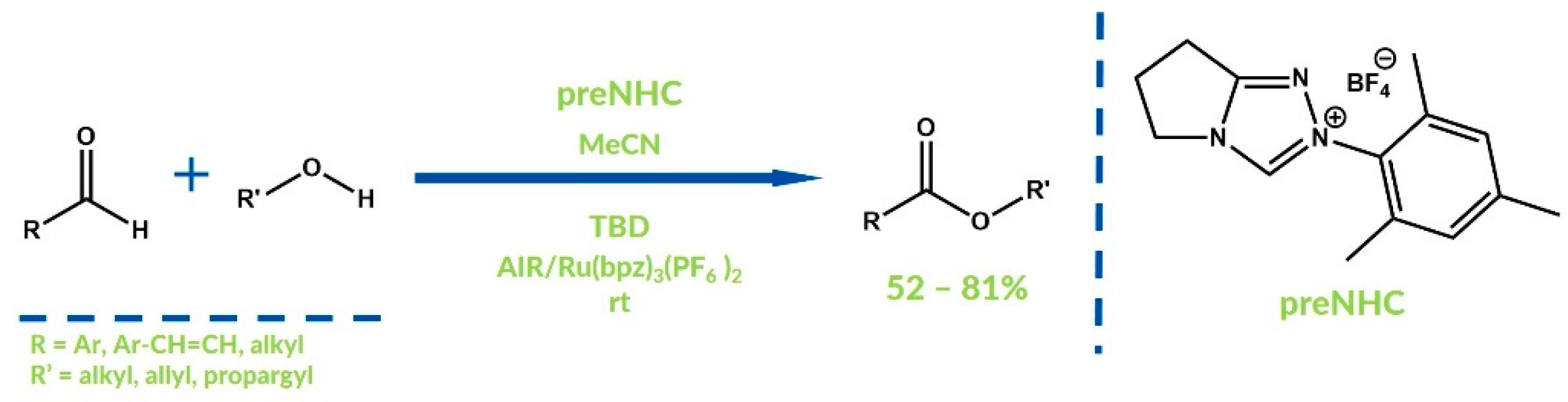
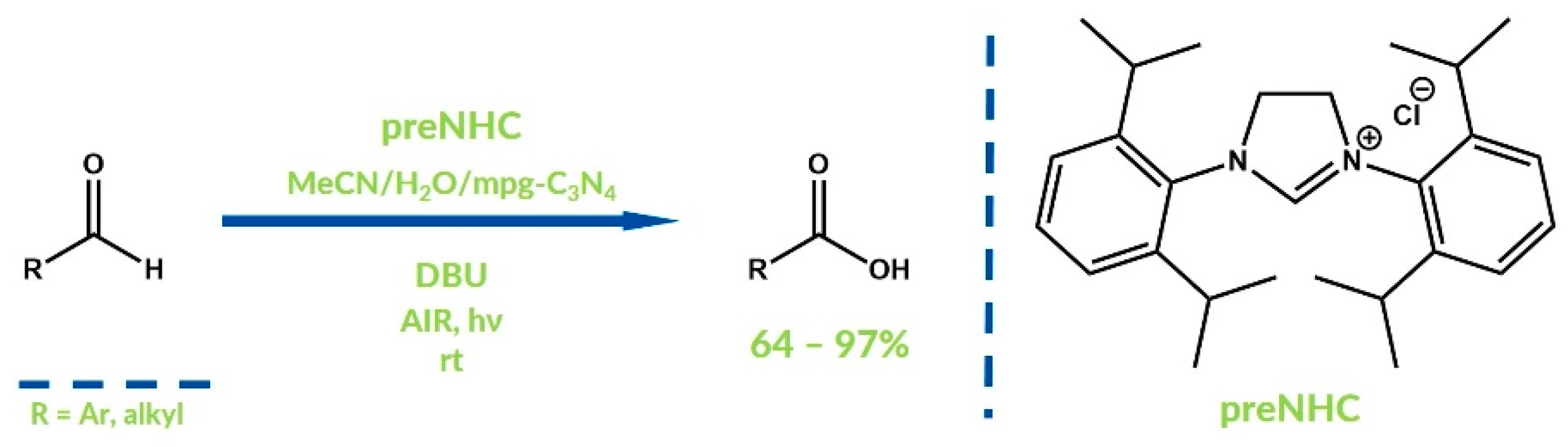
3. Oxygenative NHC Catalysis
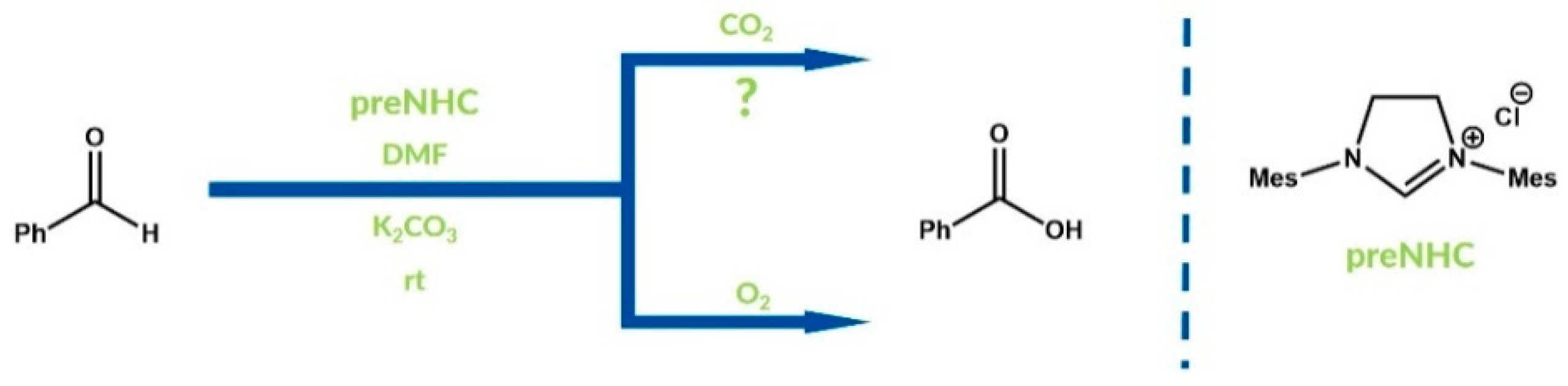
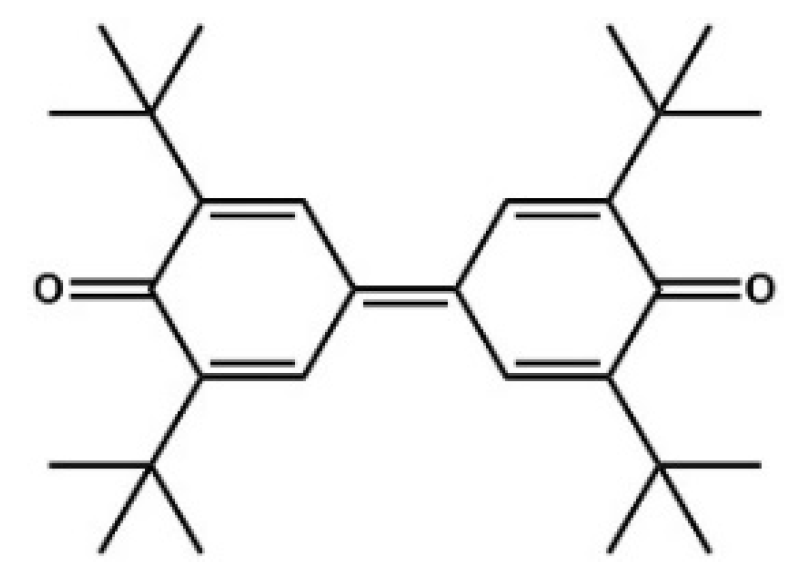
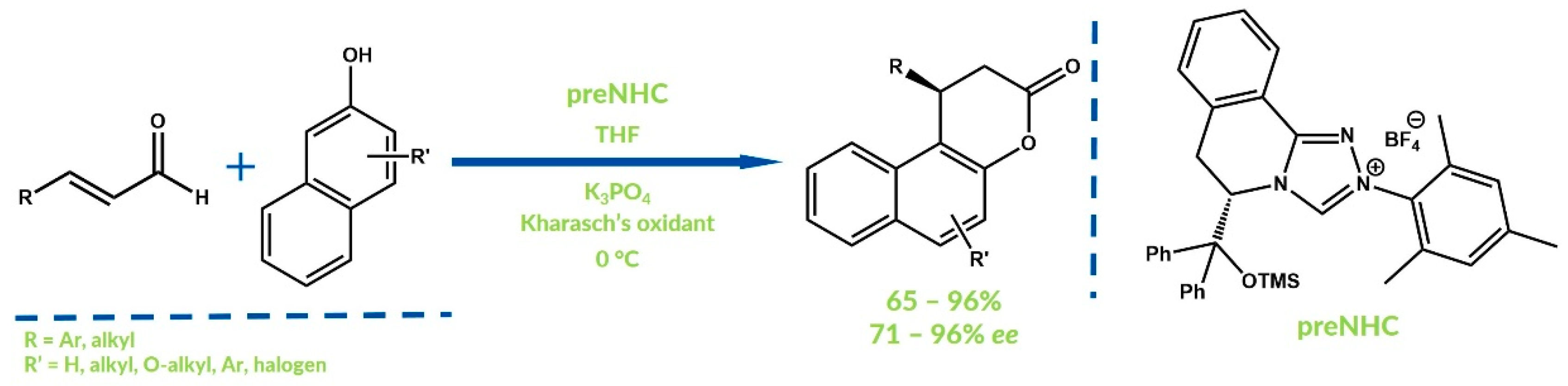
4. Oxidative NHC Catalysis
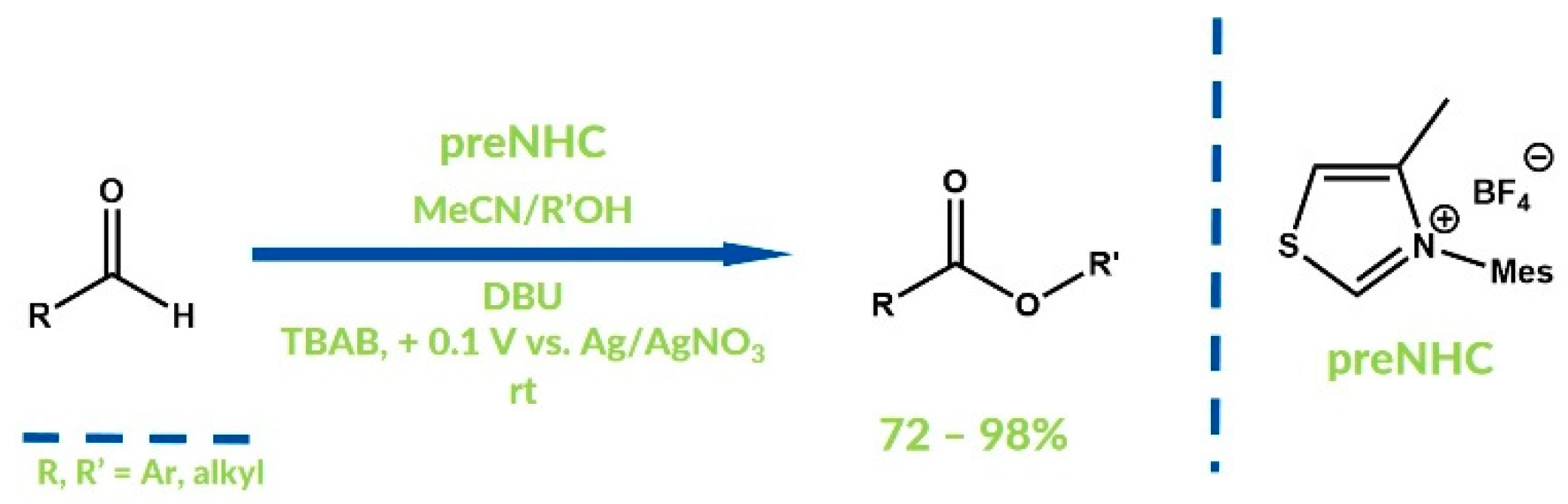
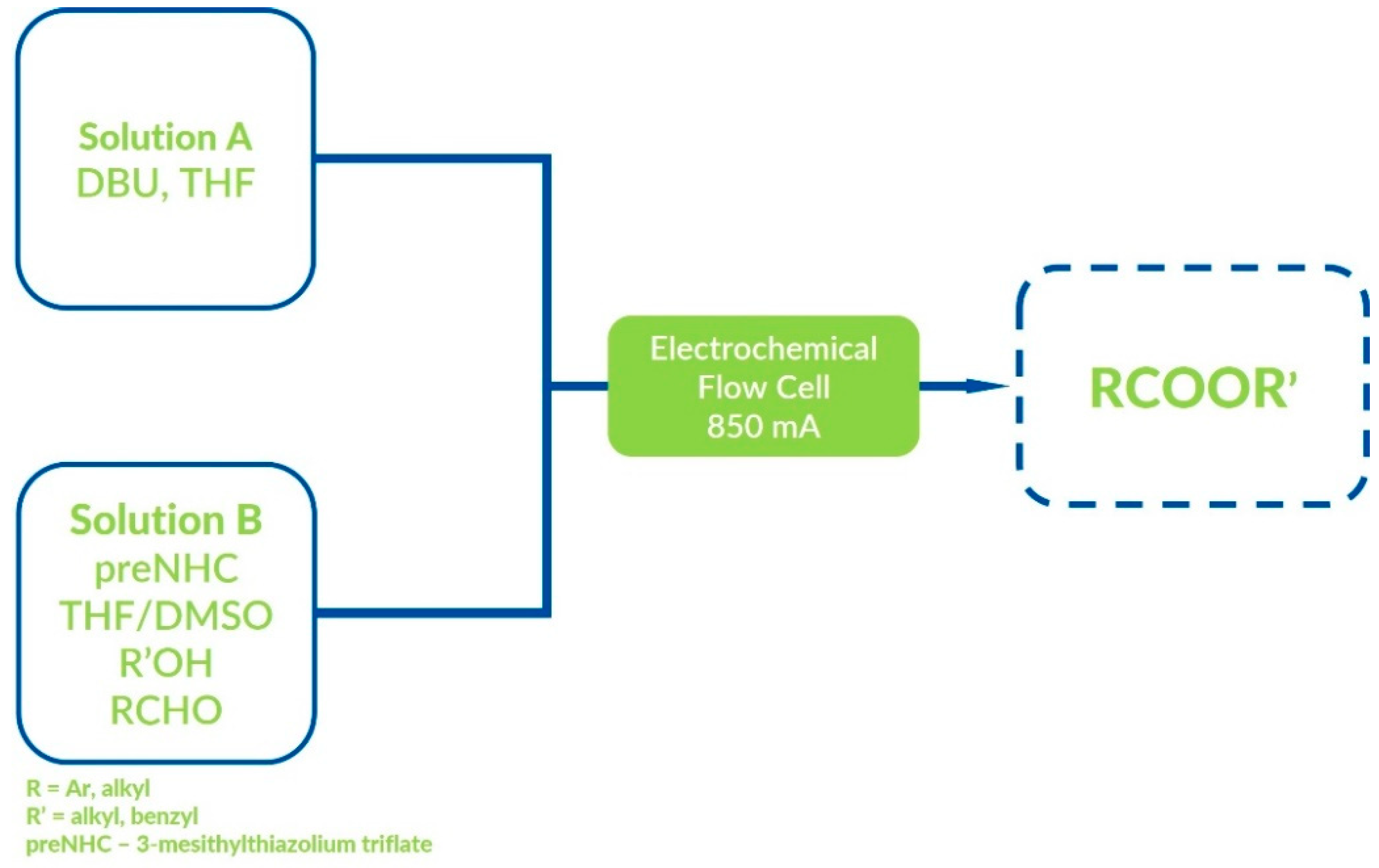
5. Oxidative NHC Catalysis via Electrolysis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.; Churchill, G.; Smith, A.D. NHCs in asymmetric organocatalysis: Recent advances in azolium enolate generation and reactivity. Synthesis 2012, 44, 2295–2309. [Google Scholar] [CrossRef]
- Izquierdo, J.; Hutson, G.E.; Cohen, D.T.; Scheidt, K.A. A continuum of progress: Applications of N-hetereocyclic carbene catalysis in total synthesis. Angew. Chem. Int. Ed. 2012, 51, 11686–11698. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Menon, R.S.; Biju, A.T.; Sinu, C.R.; Paul, R.R.; Jose, A.; Sreekumar, V. Employing homoenolates generated by NHC catalysis in carbon-carbon bond-forming reactions: State of the art. Chem. Soc. Rev. 2011, 40, 5336–5346. [Google Scholar] [CrossRef] [PubMed]
- Rafiński, Z.; Kozakiewicz, A. Enantioselective Synthesis of Chromanones Bearing Quaternary Substituted Stereocenters Catalyzed by (1R)-Camphor-Derived N-Heterocyclic Carbenes. J. Org. Chem. 2015, 80, 7468–7476. [Google Scholar] [CrossRef] [PubMed]
- Rafiński, Z. Enantioselective benzoin condensation catalyzed by spirocyclic terpene-based N-heterocyclic carbenes. Tetrahedron 2016, 72, 1860–1867. [Google Scholar] [CrossRef]
- Rafiński, Z. Novel (−)-β-Pinene-Derived Triazolium Salts: Synthesis and Application in the Asymmetric Stetter Reaction. ChemCatChem 2016, 8, 2599–2603. [Google Scholar] [CrossRef]
- Breslow, R. On the Mechanism of Thiamine Action. IV. Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Menon, R.S.; Biju, A.T.; Nair, V. Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions. Beilstein J. Org. Chem. 2016, 12, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Rehbein, J.; Ruser, S.M.; Phan, J. NHC-catalysed benzoin condensation-is it all down to the Breslow intermediate? Chem. Sci. 2015, 6, 6013–6018. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Candish, L.; Lupton, D.W. N-heterocyclic carbene-catalyzed generation ofα,β-unsaturated acyl imidazoliums: Synthesis of dihydropyranones by their reaction with enolates. J. Am. Chem. Soc. 2009, 131, 14176–14177. [Google Scholar] [CrossRef] [PubMed]
- Candish, L.; Lupton, D.W. The Total Synthesis of Carbene Catalyzed Rearrangement of α,β-Unsaturated Enol Esters. Org. Lett. 2010, 12, 4836–4839. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Stasch, A.; Paddon-Row, M.N.; Lupton, D.W. Synthetic and Quantum Mechanical Studies into the N-Heterocyclic Carbene Catalyzed (4 + 2) Cycloaddition. J. Org. Chem. 2012, 77, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J.; Schimler, S.D.; Bland, D.C.; Sanford, M.S. Acyl azolium fluorides for room temperature nucleophilic aromatic fluorination of chloro- and nitroarenes. Org. Lett. 2015, 17, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Gillard, R.M.; Fernando, J.E.M.; Lupton, D.W. Enantioselective N-Heterocyclic Carbene Catalysis via the Dienyl Acyl Azolium. Angew. Chem. Int. Ed. 2018, 57, 4712–4716. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, K. Stereoselective synthesis of (E)-α,β-unsaturated esters via carbene-catalyzed redox esterification. Org. Lett. 2006, 8, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Q.; Xiao, J.C. N-heterocyclic carbene-catalyzed reaction of alkynyl aldehydes with 1,3-keto esters or 1,3-diketones. Adv. Synth. Catal. 2010, 352, 2455–2458. [Google Scholar] [CrossRef]
- Kaeobamrung, J.; Mahatthananchai, J.; Zheng, P.; Bode, J.W. An enantioselective claisen rearrangement catalyzed by N-heterocyclic carbenes. J. Am. Chem. Soc. 2010, 132, 8810–8812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, D.; Munkerup, K.; Huang, K.W.; Li, F.; Wang, J. Enantioselective [3 + 3] atroposelective annulation catalyzed by N-heterocyclic carbenes. Nat. Commun. 2018, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wang, D.; Lu, J.; Li, T.; Jiao, W.; Yu, C. N-Heterocyclic Carbene Catalyzed Reactions of α-Bromo-α,β-unsaturated Aldehydes/α,β-Dibromoaldehydes with 1,3-Dinucleophilic Reagents. Chem. A Eur. J. 2012, 18, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Feng, P.; Cui, Y.; Jiao, N. NHC-catalyzed C-O or C-N bond formation: Efficient approaches to α,β-unsaturated esters and amides. Chem. Commun. 2012, 48, 7280–7282. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Zou, X.L.; Du, G.F.; Liu, Z.Y.; Dai, B. Nucleophilic carbene-catalyzed redox-esterification reaction of α-halo-α,β-unsaturated aldehyde. Tetrahedron 2012, 68, 6498–6503. [Google Scholar] [CrossRef]
- Lang, M.; Wang, J. N-Heterocyclic Carbene-Catalyzed Enantioselective β-Amination of α-Bromoenals Enabled by a Proton-Shuttling Strategy. Eur. J. Org. Chem. 2018, 2018, 2958–2962. [Google Scholar] [CrossRef]
- Mahatthananchai, J.; Zheng, P.; Bode, J.W. α,β-Unsaturated acyl azoliums from N-heterocyclic carbene catalyzed reactions: Observation and mechanistic investigation. Angew. Chem. Int. Ed. 2011, 50, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Samanta, R.C.; Maji, B.; De Sarkar, S.; Bergander, K.; Fröhlich, R.; Mück-Lichtenfeld, C.; Mayr, H.; Studer, A. Nukleophile Addition von Enolen und Enaminen an α,β-ungesättigte Acylazoliumionen: Mechanistische Studien. Angew. Chem. 2012, 124, 5325–5329. [Google Scholar] [CrossRef]
- Alanthadka, A.; Maheswari, C.U. N-heterocyclic carbene-catalyzed oxidative amidation of aldehydes with amines. Adv. Synth. Catal. 2015, 357, 1199–1203. [Google Scholar] [CrossRef]
- Vellalath, S.; Romo, D. Asymmetric Organocatalysis: The Emerging Utility of α,β-Unsaturated Acylammonium Salts. Angew. Chem. Int. Ed. 2016, 55, 13934–13943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hooper, J.F.; Lupton, D.W. N-Heterocyclic Carbene Catalysis via the α,β-Unsaturated Acyl Azolium. ACS Catal. 2017, 7, 2583–2596. [Google Scholar] [CrossRef]
- Wang, M.H.; Scheidt, K.A. Cooperative Catalysis and Activation with N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2016, 55, 14912–14922. [Google Scholar] [CrossRef] [PubMed]
- Knappke, C.E.I.; Imami, A.; JacobivonWangelin, A. Oxidative N-Heterocyclic Carbene Catalysis. ChemCatChem 2012, 4, 937–941. [Google Scholar] [CrossRef]
- De Sarkar, S.; Biswas, A.; Samanta, R.C.; Studer, A. Catalysis with N-heterocyclic carbenes under oxidative conditions. Chem. A Eur. J. 2013, 19, 4664–4678. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, O.; Chiappe, C.; Fogagnolo, M.; Massi, A.; Pomelli, C.S. Formation, Oxidation, and Fate of the Breslow Intermediate in the N-Heterocyclic Carbene-Catalyzed Aerobic Oxidation of Aldehydes. J. Org. Chem. 2017, 82, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Thorat, S.; Gonnade, R.G.; Biju, A.T. Reaction of N-heterocyclic carbenes with chalcones leading to the synthesis of deoxy-Breslow intermediates in their oxidized form. Chem. Commun. 2015, 51, 13690–13693. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, Y.; Du, W.; Deng, W.P. The NHCs-mediated cross-coupling of aromatic aldehydes with benzyl halides: Synthesis of α-aryl ketones. Tetrahedron Lett. 2010, 51, 3571–3574. [Google Scholar] [CrossRef]
- Maji, B.; Vedachalan, S.; Ge, X.; Cai, S.; Liu, X.W. N-heterocyclic carbene-mediated oxidative esterification of aldehydes: Ester formation and mechanistic studies. J. Org. Chem. 2011, 76, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Bhilare, S.V.; Youn, S.W. NHC-catalyzed oxidative cyclization reactions of 2-alkynylbenzaldehydes under aerobic conditions: Synthesis of O-Heterocycles. Org. Lett. 2011, 13, 2228–2231. [Google Scholar] [CrossRef] [PubMed]
- Arde, P.; Ramanjaneyulu, B.T.; Reddy, V.; Saxena, A.; Anand, R.V. N-Heterocyclic carbene catalysed aerobic oxidation of aromatic aldehydes to aryl esters using boronic acids. Org. Biomol. Chem. 2012, 10, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Soeta, T.; Tabatake, Y.; Fujinami, S.; Ukaji, Y. N-heterocyclic carbene catalyzed oxidative coupling of aldehydes with carbodiimides under aerobic conditions: Efficient synthesis of N-acylureas. Org. Lett. 2013, 15, 2088–2091. [Google Scholar] [CrossRef] [PubMed]
- Guin, J.; De Sarkar, S.; Grimme, S.; Studer, A. Biomimetic carbene-catalyzed oxidations of aldehydes using TEMPO. Angew. Chem. Int. Ed. 2008, 47, 8727–8730. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; Scheldt, K.A. N-heterocyclic carbene-catalyzed oxidation of unactivated aldehydes to esters. Org. Lett. 2008, 10, 4331–4334. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Katagiri, Y.; Zhu, W.B.; Shishido, K. Oxidative carboxylation of arylaldehydes with water by a Sulfoxylalkyl-substituted N-heterocyclic carbene catalyst. Org. Biomol. Chem. 2009, 7, 4062–4066. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; Chan, A.; Phillips, E.M.; Scheidt, K.A. N-Heterocyclic carbene-catalyzed oxidations. Tetrahedron 2009, 65, 3102–3109. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Hazra, A. One-step Direct Conversion of Heterocyclic Aldehydes to Esters. Chem. Lett. 2009, 38, 484–485. [Google Scholar] [CrossRef]
- Rose, C.A.; Zeitler, K. Efficient catalytic, oxidative lactonization for the synthesis of benzodioxepinones using thiazolium-derived carbene catalysts. Org. Lett. 2010, 12, 4552–4555. [Google Scholar] [CrossRef] [PubMed]
- De Sarkar, S.; Grimme, S.; Studer, A. NHC catalyzed oxidations of aldehydes to esters: Chemoselective acylation of alcohols in presence of amines. J. Am. Chem. Soc. 2010, 132, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Desarkar, S.; Biswas, A.; Song, C.H.; Studer, A. Kinetic resolution of secondary alcohols by NHC-catalyzed oxidative esterification. Synthesis 2011, 1974–1983. [Google Scholar] [CrossRef]
- Reddy, R.S.; Rosa, J.N.; Veiros, L.F.; Caddick, S.; Gois, P.M.P. NHC/Iron cooperative catalysis: Aerobic oxidative esterification of aldehydes with phenols. Org. Biomol. Chem. 2011, 9, 3126–3129. [Google Scholar] [CrossRef] [PubMed]
- Iwahana, S.; Iida, H.; Yashima, E. Oxidative esterification, thioesterification, and amidation of aldehydes by a two-component organocatalyst system using a chiral N-heterocyclic carbene and redox-active riboflavin. Chem. A Eur. J. 2011, 17, 8009–8013. [Google Scholar] [CrossRef] [PubMed]
- Noonan, C.; Baragwanath, L.; Connon, S.J. Nucleophilic carbene-catalysed oxidative esterification reactions. Tetrahedron Lett. 2008, 49, 4003–4006. [Google Scholar] [CrossRef]
- Kang, Y.W.; Jang, H.Y. NHC-catalyzed one-pot oxidation and oxidative esterification of allylic alcohols using TEMPO: The effect of alcohol additives. RSC Adv. 2014, 4, 44486–44490. [Google Scholar] [CrossRef]
- Zhao, J.; Mück-Lichtenfeld, C.; Studer, A. Cooperative N-Heterocyclic Carbene (NHC) and Ruthenium Redox Catalysis: Oxidative Esterification of Aldehydes with Air as the Terminal Oxidant. Adv. Synth. Catal. 2013, 355, 1098–1106. [Google Scholar] [CrossRef]
- Axelsson, A.; Hammarvid, E.; Ta, L.; Sundén, H. Asymmetric aerobic oxidative NHC-catalysed synthesis of dihydropyranones utilising a system of electron transfer mediators. Chem. Commun. 2016, 52, 11571–11574. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.; Axelsson, A.; Sundén, H. Attractive aerobic access to the α,β-unsaturated acyl azolium intermediate: Oxidative NHC catalysis via multistep electron transfer. Green Chem. 2016, 18, 686–690. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Zhang, G.; Chen, F.; Cheng, J. Palladium/NHC-catalyzed oxidative esterification of aldehydes with phenols. Tetrahedron Lett. 2011, 52, 2480–2483. [Google Scholar] [CrossRef]
- Möhlmann, L.; Ludwig, S.; Blechert, S. NHC-catalysed highly selective aerobic oxidation of nonactivated aldehydes. Beilstein J. Org. Chem. 2013, 9, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Kiran, I.N.C.; Lalwani, K.; Sudalai, A. N-Heterocyclic carbene catalyzed esterification of aromatic aldehydes with alcohols under aerobic conditions. RSC Adv. 2013, 3, 1695–1698. [Google Scholar] [CrossRef]
- Delany, E.G.; Fagan, C.L.; Gundala, S.; Mari, A.; Broja, T.; Zeitler, K.; Connon, S.J. NHC-catalysed aerobic aldehyde-esterifications with alcohols: No additives or cocatalysts required. Chem. Commun. 2013, 49, 6510–6512. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Varghese, V.; Paul, R.R.; Jose, A.; Sinu, C.R.; Menon, R.S. NHC catalyzed transformation of aromatic aldehydes to acids by carbon dioxide: An unexpected reaction. Org. Lett. 2010, 12, 2653–2655. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhang, Y. Unexpected CO2 Splitting Reactions To Form CO with N-Heterocyclic Carbenes as Organocatalysts and Aromatic Aldehydes as Oxygen Acceptors. J. Am. Chem. Soc. 2010, 132, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.C.; Bode, J.W. On the role of CO2 in NHC-catalyzed oxidation of aldehydes. Org. Lett. 2011, 13, 2422–2425. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; Chan, A.; Phillips, E.M.; Scheidt, K.A. Tandem oxidation of allylic and benzylic alcohols to esters catalyzed by N-heterocyclic carbenes. Org. Lett. 2007, 9, 371–374. [Google Scholar] [CrossRef] [PubMed]
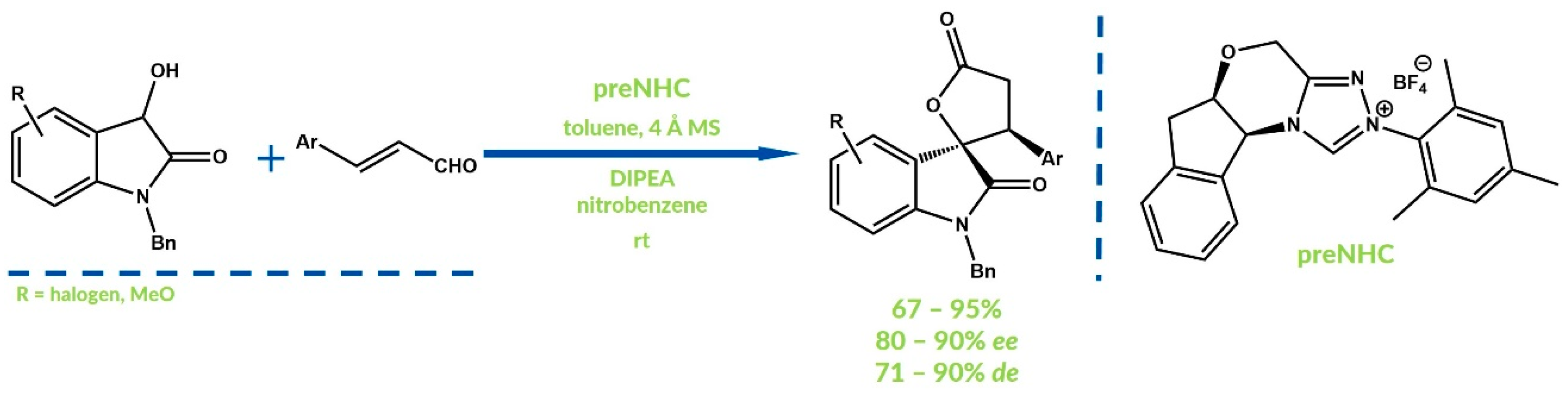
- Li, G.T.; Gu, Q.; You, S.L. Enantioselective annulation of enals with 2-naphthols by triazolium salts derived from L-phenylalanine. Chem. Sci. 2015, 6, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, M.S.; Joshi, B.S. Reactions of Hindered Phenols. II. Base-Catalyzed Oxidations of Hindered Phenols. J. Org. Chem. 1957, 22, 1439–1443. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, T.; Majhi, P.K.; Mou, C.; Chai, H.; Zhang, J.; Zhuo, S.; Chi, Y.R. Carbene-catalyzed enantioselective oxidative coupling of enals and di(hetero)arylmethanes. Chem. Sci. 2018. [Google Scholar] [CrossRef]
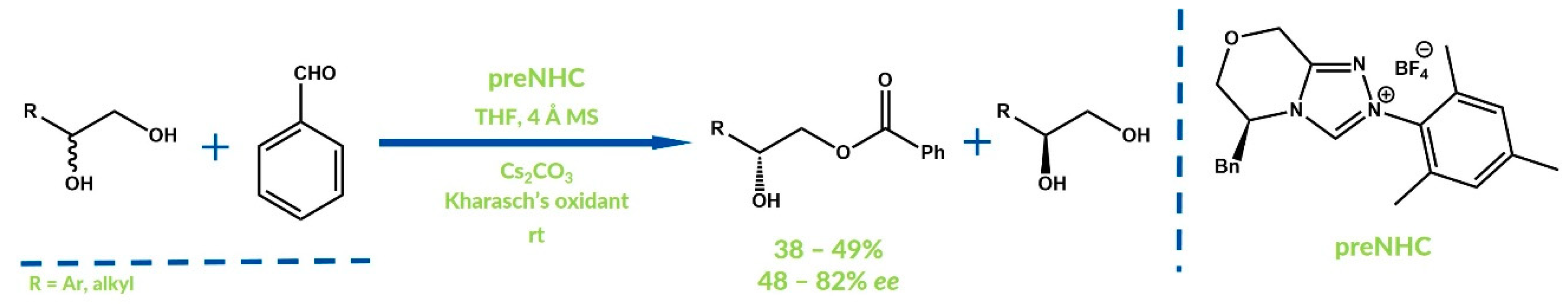
- Liu, B.; Yan, J.; Huang, R.; Wang, W.; Jin, Z.; Zanoni, G.; Zheng, P.; Yang, S.; Chi, Y.R. Kinetic Resolution of 1,2-Diols via NHC-Catalyzed Site-Selective Esterification. Org. Lett. 2018, 20, 3447–3450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, X.; Ma, C. Organocatalytic Direct N-Acylation of Amides with Aldehydes under Oxidative Conditions. J. Org. Chem. 2017, 82, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Wanner, B.; Mahatthananchai, J.; Bode, J.W. Enantioselective synthesis of dihydropyridinones via NHC-Catalyzed Aza-Claisen reaction. Org. Lett. 2011, 13, 5378–5381. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Y.; Li, X.; Shao, Y.; Li, G.; Webster, R.D.; Chi, Y.R. N-Heterocyclic Carbene Organocatalytic Reductive β,β-Coupling Reactions of Nitroalkenes via Radical Intermediates. Org. Lett. 2014. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Chen, K.Q.; Sun, D.Q.; Ye, S. N-Heterocyclic carbene-catalyzed oxidative [3 + 2] annulation of dioxindoles and enals: Cross coupling of homoenolate and enolate. Chem. Sci. 2017, 8, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Chen, K.Q.; Chen, X.Y.; Ye, S. Diastereo- and Enantioselective Synthesis of Spirooxindoles with Contiguous Tetrasubstituted Stereocenters via Catalytic Coupling of Two Tertiary Radicals. J. Org. Chem. 2018, 83, 2966–2970. [Google Scholar] [CrossRef] [PubMed]
- White, N.A.; Rovis, T. Enantioselective N-heterocyclic carbene-catalyzed β-hydroxylation of enals using nitroarenes: An atom transfer reaction that proceeds via single electron transfer. J. Am. Chem. Soc. 2014, 136, 14674–14677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, Y.; Huang, Z.; Xu, J.; Wu, X.; Wang, Y.; Wang, M.; Yang, S.; Webster, R.D.; Chi, Y.R. N-heterocyclic carbene-catalyzed radical reactions for highly enantioselective β-hydroxylation of enals. J. Am. Chem. Soc. 2015, 137, 2416–2419. [Google Scholar] [CrossRef] [PubMed]
- White, N.A.; Rovis, T. Oxidatively initiated NHC-catalyzed enantioselective synthesis of 3,4-disubstituted cyclopentanones from enals. J. Am. Chem. Soc. 2015, 137, 10112–10115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Enders, D. Merging N-Heterocyclic Carbene Catalysis and Single Electron Transfer: A New Strategy for Asymmetric Transformations. Angew. Chem. Int. Ed. 2017, 56, 3754–3756. [Google Scholar] [CrossRef] [PubMed]
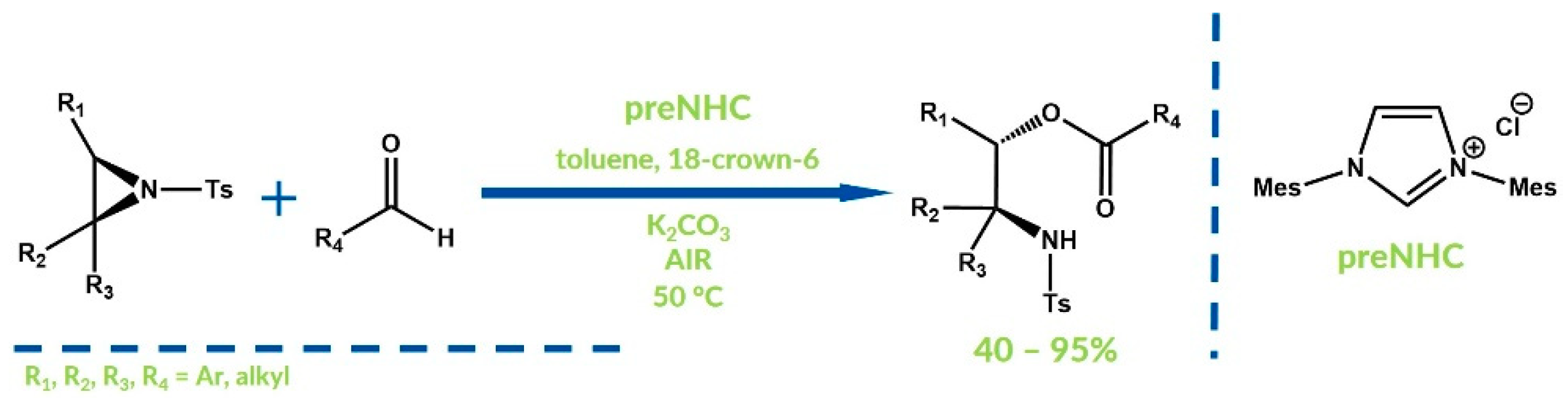
- Liu, Y.K.; Li, R.; Yue, L.; Li, B.J.; Chen, Y.C.; Wu, Y.; Ding, L.S. Unexpected ring-opening reactions of aziridines with aldehydes catalyzed by nucleophilic carbenes under aerobic conditions. Org. Lett. 2006, 8, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Delany, E.G.; Fagan, C.L.; Gundala, S.; Zeitler, K.; Connon, S.J. Aerobic oxidation of NHC-catalysed aldehyde esterifications with alcohols: Benzoin, not the Breslow intermediate, undergoes oxidation. Chem. Commun. 2013, 49, 6513–6515. [Google Scholar] [CrossRef] [PubMed]
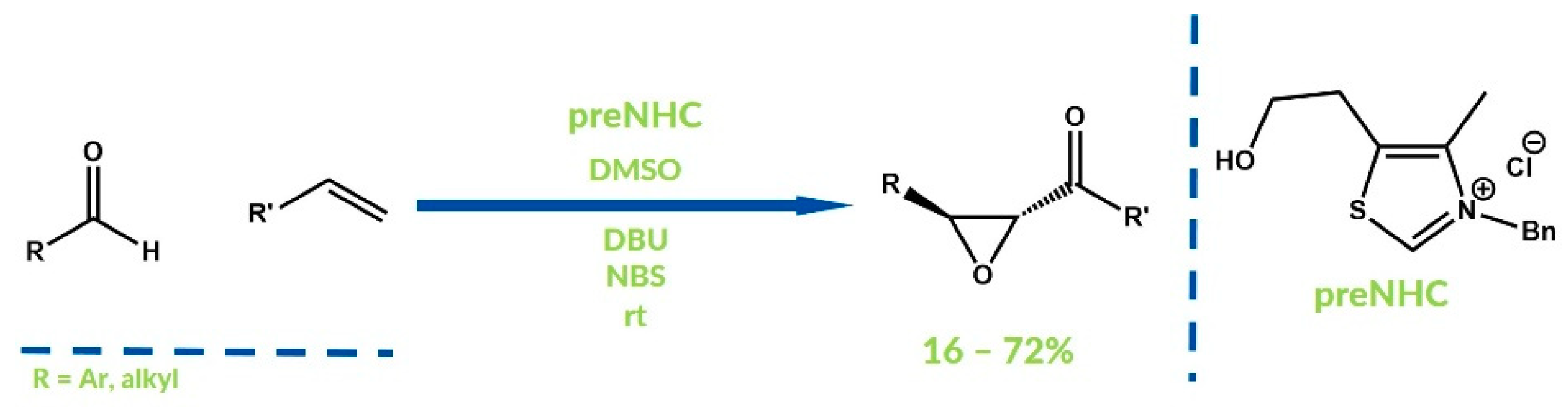
- Reddi, R.N.; Prasad, P.K.; Sudalai, A. N-Heterocyclic Carbene Catalyzed Oxidative Coupling of Alkenes/α-Bromoacetophenones with Aldehydes: A Facile Entry to α,β-Epoxy Ketones. Angew. Chem. Int. Ed. 2015, 54, 14150–14153. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Itoh, S.; Fukuzumi, S. Electron-transfer properties of active aldehydes of thiamin coenzyme models, and mechanism of formation of the reactive intermediates. Chem. A Eur. J. 1999, 5, 2810–2818. [Google Scholar] [CrossRef]
- Finney, E.E.; Ogawa, K.A.; Boydston, A.J. Organocatalyzed Anodic Oxidation of Aldehydes. J. Am. Chem. Soc. 2012, 134, 12374–12377. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Pletcher, D.; Leach, S.G.; Brown, R.C.D. N-Heterocyclic Carbene-Mediated Oxidative Electrosynthesis of Esters in a Microflow Cell. Org. Lett. 2015, 17, 3290–3293. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Pletcher, D.; Leach, S.G.; Brown, R.C.D. N-Heterocyclic Carbene-Mediated Microfluidic Oxidative Electrosynthesis of Amides from Aldehydes. Org. Lett. 2016, 18, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.A.; Boydston, A.J. Organocatalyzed anodic oxidation of aldehydes to thioesters. Org. Lett. 2014, 16, 1928–1931. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzieszkowski, K.; Rafiński, Z. N-Heterocyclic Carbene Catalysis under Oxidizing Conditions. Catalysts 2018, 8, 549. https://doi.org/10.3390/catal8110549
Dzieszkowski K, Rafiński Z. N-Heterocyclic Carbene Catalysis under Oxidizing Conditions. Catalysts. 2018; 8(11):549. https://doi.org/10.3390/catal8110549
Chicago/Turabian StyleDzieszkowski, Krzysztof, and Zbigniew Rafiński. 2018. "N-Heterocyclic Carbene Catalysis under Oxidizing Conditions" Catalysts 8, no. 11: 549. https://doi.org/10.3390/catal8110549
APA StyleDzieszkowski, K., & Rafiński, Z. (2018). N-Heterocyclic Carbene Catalysis under Oxidizing Conditions. Catalysts, 8(11), 549. https://doi.org/10.3390/catal8110549





