Abstract
Hydroisomerization of long chain paraffins for the production of branched alkanes has recently been intensively studied due to a large availability of these compounds. The most interesting research topics have been the development of novel bifunctional catalysts to maximize the yield of isomers and to suppress the cracking reactions. Since both of these reactions are catalyzed by Brønsted acid sites, the optimum catalyst exhibits equal amounts of metal and acid sites, and it facilitates rapid mass transfer. Thus, several hierarchical and nano-shaped zeolites have been developed, in addition to composite catalysts containing both micro- and mesoporous phases. In addition to catalyst development, the effect of the reactant structure, optimal reaction conditions, catalyst stability and comparison of batch vs continuous operations have been made.
1. Introduction
Hydroconversion has played an important role in the petroleum industry for decades. It is a well-explored and recognized technology that has been used in crude oil refining. The term hydroconversion generally refers to two typical oil refinery processes, i.e., hydrocracking and hydroisomerization, carried out for different oil fractions in oil refinery. These processes are carried out in the presence of hydrogen, and therefore, known as hydroconversion. In this paper, the hydroisomerization of long-chain hydrocarbons is mainly discussed over different bi-functional catalysts.
Transformations of normal to branched alkanes is carried out mainly by hydroconversion, i.e., hydrocracking and hydroisomerization. In hydrocracking, the feedstock is catalytically converted to lower carbon numbers. For example Mobil, using a ZSM-5 catalyst with strong acidity and a uniform pore size, commercialized selective catalytic cracking of long-chain hydrocarbons in the lube dewaxing (MLDW) process [1]. In hydroisomerization, the properties of the feedstock are improved by transforming normal hydrocarbons to branched ones having the same carbon number. Selective hydroisomerization is a highly desirable reaction in oil refineries, and it is used mainly in two processes; first, for the improvement of the octane number for the gasoline pool (C5–C6), and second, for dewaxing of long-chain hydrocarbons for improvement in their cetane number and cold flow properties. In both of these processes, linear hydrocarbons are transformed to branched alkanes exhibiting the same carbon number [2,3]. Selective isomerization of normal short-chain alkanes is easier to realize than for long-chain hydrocarbons, because of rapid cracking of the latter during hydroisomerization. Improving the selectivity of the bifunctional catalysts for the production of isomerized middle distillates is challenging [4], and several novel catalysts have been developed over recent years. Several reviews on hydroisomerization of long-chain alkanes have been published a rather long time ago [2,5,6]. Recently, Guisnet [7] published a review on the hydroisomerization of alkanes over Pt acid zeolites. Furthermore, Yadav et al. [8] overviewed SAPO materials as catalysts in the hydroisomerization of alkanes and alkenes. Thus, in this work, the main emphasis is put on very recent developments, especially that of tailored catalysts for the hydroisomerization of long chain alkanes.
Recently, long-chain hydrocarbons have attracted a significant amount of interest because of their increased availability. These long-chain hydrocarbons (>C15) make up to 80 wt % of material known as wax, i.e., long straight-chained (normal) hydrocarbons [2]. Extensive research has been carried out to obtain value-added products from a low-grade long-chain waxy feedstock to branched products with improved fuel quality [2]. Such research has been mainly concentrated on catalyst development, in particular micro/mesoporous catalysts, to increase the mass transfer and to optimize the residence time of the molecules with the active sites.
In this review paper, the main emphasis is on understanding the reaction pathways in transformations of long chain hydrocarbons on bifunctional catalysts. The role of different metals and supports will be elucidated especially for bifunctional catalysts. A detailed analysis of possible model compounds used to understand the behavior of long-chain hydrocarbons i.e., octane, n-decane, n-dodecane to n-hecadecane, and their activities, will be given. The review paper will also discuss the properties of fuels that are obtained by the hydroisomerization of long-chain hydrocarbons and the effect of carbon chain length on hydroisomerization activity. A detailed analysis of different catalysts, with their deactivation behavior in hydroisomerization of long-chain hydrocarbons in comparison with the straight chain will be made. The reactor and reactor conditions are discussed in depth for the hydroisomerization of long-chain hydrocarbons. The aim of this paper is to describe the most suitable catalysts that can be used in hydroisomerization of the long-chain hydrocarbons under optimized reaction conditions, providing the best isomerization selectivities with the lowest amount of by-products. In addition, batch and continuous operations will be compared, and the long-term performance of catalysts will be discussed.
2. Fuel Properties of Hydroisomerization of Long-Chain Paraffin Products
The aim in hydroisomerization of long-chain alkanes is to transform the long-chain waxy alkanes to branched products. The long straight chain hydrocarbons on one side improve the thermal stability and viscosity of the fuel, having, however, adverse effects on other fuel properties such as freezing and pour points, and therefore, they should be removed to improve the fuel quality. The branched alkanes have superior cold flow properties, and they do not compromise other properties of the fuel [9]. The specification of the cold flow properties of fuels mainly depends on the geographical region where they are used, and they may vary from −3 °C from tropical regions to −35 °C for cold regions [10].
Hydroisomerization of long-chain alkanes is required to decrease the pour point, and to obtain a high viscosity index improving the cold flow properties of the oil [10]. For example, base oils with good lubrication properties contain large amounts of multibranched isoparaffins [11]. In hydroisomerization of long-chain alkanes several products are formed, such as light hydrocarbons (C1–C4), hydrocarbons in the gasoline range (C5–C8), jet fuels (C9–C14), and diesel (C15–C18). The pour point has been correlated with the product distribution in the hydroisomerization of hexadecane, and presented as a function of conversion (Figure 1) [10]. It has been reported that the pour point increases with an increase in the paraffin molecular weight, and decreases with increasing branching; thus, (multi)branched products are desired in hydroisomerization of long-chain alkanes [12].
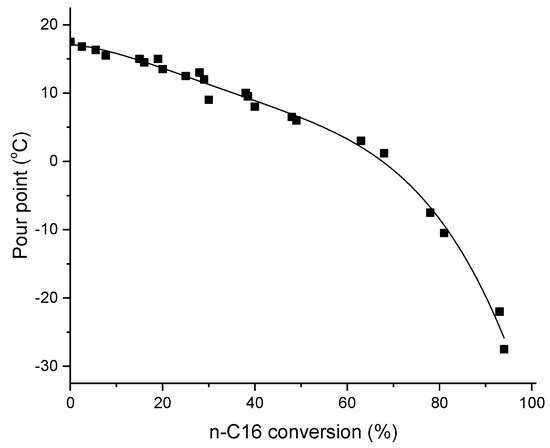
Figure 1.
Pour point as a function of hexadecane conversion: Conditions: temperature range of 260–320 °C and 50–100 bar, respectively using weight hourly space velocity (WHSV) of 1.1–2.8 hr−1 adapted from [10]. Reproduced with permission from Gomes et al., Fuel; published by Elsevier, 2017.
3. Reaction Mechanism in Hydroisomerization of Long-Chain Paraffins
The reaction scheme for hydroisomerization over long-chain paraffins of the bifunctional catalysts was suggested more than five decades ago [9,10]. The role of a metal is to provide the hydrogenation/dehydrogenation function, while the acid sites are responsible for skeletal isomerization. An ideal hydroisomerization catalyst should achieve high isomerization selectivity for long-chain hydrocarbons, resulting in a high liquid yield, avoiding at the same time any parallel reactions resulting in the formation of cracked products. Hydroisomerization occurs consecutively (Figure 2) [13,14,15] when n-alkane is first dehydrogenated on a metal site forming an alkene, and thereafter a carbenium ion, which in turn is isomerized onto an acidic site to an isoolefin through an isocarbocation, finally forming bi- or multibranched alkanes (Figure 3) [13,16]. Alkylcarbenium ions can also further undergo -scission reaction [17], forming cracking products.
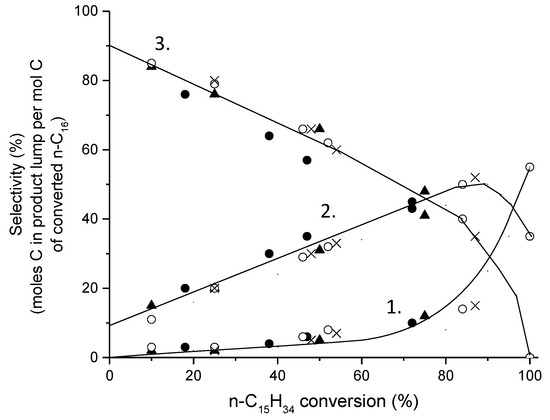
Figure 2.
Selectivities of 1. monobranched, 2. dibranched, and 3. cracking products as a function of hexadecane conversion at 310 °C under 30 bar over different Pd-SiO2-Al2O3 catalysts adapted from [18]. Reproduced with permission from Regali et al., Catal. Today; published by Elsevier, 2014.
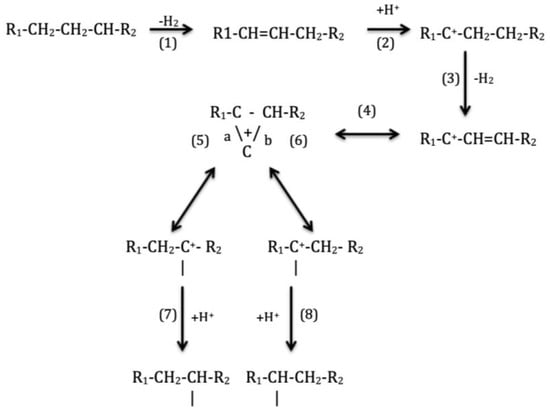
Figure 3.
Reaction network for hydroisomerization of long-chain alkanes adapted from [19]. The numbers denote different reaction steps. Reproduced with permission from Zhang et al., Ind. Eng. Chem. Res.; published by American Chemical Society, 2016.
Different reaction mechanisms have also been proposed for the hydroisomerization of long- chain paraffins, depending on the catalyst acidity [13,16]. For example, for Pt on acidic Beta zeolite, the kinetic results fit well with the reaction mechanism that was composed of both parallel, reversible, and irreversible steps [16]. With decreasing acidity, mono- and bibranched products were still produced, reacting further in parallel to the cracking products, whereas over a mildly acidic Pt-Beta, only a consecutive mechanism was proposed to be operative [16]. These results indicate that a suitable balance between the metal and acid functions is required to provide sufficient activity and high selectivity.
In addition to the formation of isomers, it is interesting to consider from the mechanistic point of view how cracking products are formed over bifunctional catalysts. This has been investigated by Regali et al. [15], who confirmed that there is no correlation between the hydroconversion activity and the Brønsted acidity [15], indicating that hydrogenolysis forming cracking products is metal-catalyzed. This idea was additionally confirmed by selective poisoning of the acid sites, resulting in high hydrogenolysis activity, which originated from a charge transfer from the metal particles to the framework atoms, and not from the acid sites [15].
Realkylation of cracked products can occur also during the hydroisomerization of long-chain alkanes, such as hexadecane [16]. It was reported by these authors that e.g., isobutene can react with isopentene, isohexene, and isoheptene and form C9–C12 isoalkanes. Analogously, isopentene reacted with isohexene and isoheptene forming C11 to C12 isoalkanes. This was also experimentally confirmed via testing of the reactivity of linear n-hex-1-ene and 2-methylpent-1-ene using Pt-H-Beta-26 as a catalyst. The results showed that the linear alkene was unreactive, whereas the alkylation of branched olefin occurred.
4. Effect of the Alkane Carbon Chain Length
It is generally considered that long-chain hydrocarbons are more reactive than short-chain hydrocarbons, and their acidity requirements are inversely proportional to the chain length [10].
Hydroisomerization of a mixture of C15–C18 hydrocarbons over Ni-Mo-SAPO-11 was demonstrated at 350 °C under 30 bar by (Figure 4) [20]. In their work, the isomerization selectivity increased with increasing carbon chain length from C12 to C16. However, at a lower reaction temperature, 310 °C, selectivity was very low for C8 to C15 hydrocarbons, increasing then exponentially up to C18. The highest isomer selectivity with C18 hydrocarbon was 57% over Ni-Mo-SAPO-11 catalyst [20].
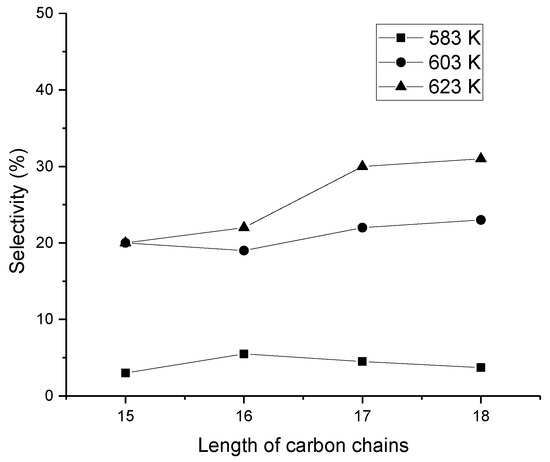
Figure 4.
Effect of carbon chain length in the hydroisomerization using Ni-Mo-SAPO 11 as a catalyst under 30 bar with H2/oil (C15–C18 alkanes) ratio of 800 with liquid hourly space velocity (LHSV) of 1 hr−1 [20]. Reproduced with permission from Xing et al., Catal. Today; published by Elsevier, 2018.
A comparative study for hydroisomerization of C10 and C19 alkanes was performed over Pt-ZSM-22, giving the highest isomer yield for C10 at 230 °C and 84% conversion, whereas a higher yield (89%) was obtained for C19 at 90% conversion and 240 °C [21]. As a conclusion, it can be stated that the isomerization of long-chain hydrocarbons is promoted at rather low reaction temperatures, around 230–240 °C over noble metal catalysts, and slightly above 300 °C with transition metal catalysts.
Due to a lack of comparative studies of carbon chain length effects in hydroisomerization in the recent years, the highest hydroisomerization yields of hexadecane and dodecane were compared by collecting these values obtained over different catalysts under optimum reaction conditions and conversion levels, and plotted as a function of conversion (Figure 5 and Figure 6). It can be observed from these figures that the highest isomer yields for hexadecane were higher than for dodecane, being in line with the results of [21]. A detail discussion of these results using different catalysts is presented below.
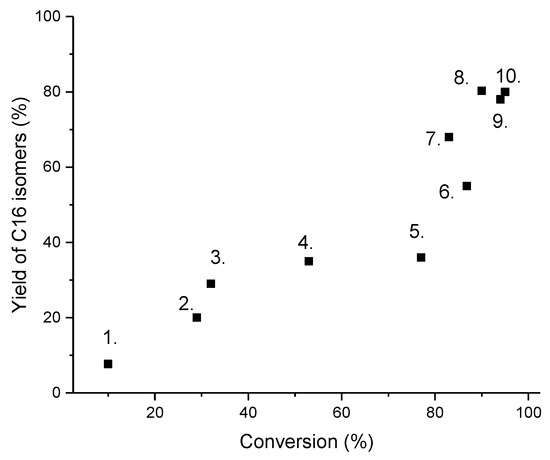
Figure 5.
The yield of C16 isomers as a function of conversion. Notation: 1. Pt-H-Beta, 30 bar, 220 °C [22], 2. Pt-H-ZSM-23 (dual templated), 300 °C, 40 bar, volumetric ratio of H2/feed 600 L/L [23], 3. Pt-H-ZSM-23, 270 °C, 40 bar, volumetric ratio of H2/feed 600 L/L [19], 4. Pt-Al-Si, 310 °C, 30 bar, molar ratio of H2/feed 10 [15], 5. Pt-Al-H-Beta (Si/Al ratio 80), 290 °C, 75 bar, WHSV of 1.3 hr−1 [10], 6. Pt-Al-MCM-41, 200 °C, 20 bar, molar ratio of H2/feed pf 4 [24], 7. Pt-ZSM-23, 260 °C, [13], 8. Ba-Pt-ZSM-12, 295 °C, 60 bar [12], 9. Pt-H-Beta (Si/Al 26) 220 °C, 30 bar, molar ratio of H2/feed 20 [16], 10. Pt-Beta-Al2O3, 220 °C, 30 bar, molar ratio of H2/feed of 20 [9].
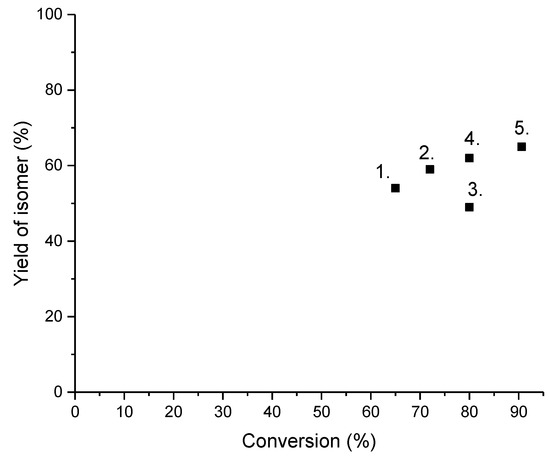
Figure 6.
Yield of isomers as a function of n-dodecane conversion. Notation: 1. Pt-SAPO-11, 260 °C, H2/feed molar ratio of 25 [25], 2. Ni2P-SAPO-11 [26], 350 °C, 20 bar, WHSV 1 hr−1, H2/feed molar ratio of 19, 3. Pt-ZSM-22/ZSM-23, 320 °C, 80 bar, LHSV 1 hr−1, H2/feed volumetric ratio of 500 [27], 4. Pt-ZSM-22 desilicated, 280 °C, 20 bar, H2/feed volumetric ratio of 600, WHSV = 2 hr−1 [28] 5. Pt-Fe-ZF1, 330 °C, 20 bar, H2/feed volumetric ratio of 600, LHSV 2 hr−1 [29].
5. Catalyst Selection
Hydroisomerization of long-chain alkanes has been investigated for decades, but still there is a demand to develop tailored materials to maximize the isomer yield. Hydroisomerization is typically performed over bifunctional catalysts comprising metals on acidic supports (Table 1). These catalysts contain mostly noble metals, e.g., platinum and palladium, providing hydrogenation/dehydrogenation activity and such acidic supports, as ZSM-5, SAPO-11, ZSM-22, Y, and Beta-zeolites and composite materials containing mesoporous MCM-41, MCM-48, and SBA-15 [17]. In addition to Pt and Pd, Ni and Ni-Cu also have been used recently in hydroisomerization of long-chain alkanes [26,30]. The most important properties are the metal nature, amount, and dispersion, as well as the acid strength, location, and concentration [31]. The catalyst texture is also of crucial importance, because isomerization occurs in the micropore and the isomer should rapidly diffuse to the bulk phase prior to consecutive undesired cracking reactions.

Table 1.
The highest yield of isomers in hexadecane and dodecane hydroisomerization over different catalysts at optimum reaction conditions and conversion levels.
During the recent years, several tailored materials with tuned acidity and structural properties have been developed, including shape-selective 10-membered ring zeolites (Table 2) [19], they can facilitate shape selectivity, whereas mesoporous materials can enhance mass transfer. In this section, different types of supports, i.e., zeolites, hierarchical zeolites, and composite materials applied in hydroisomerization will be discussed. A special emphasis will be put on the benefits and limitations of the use of specific supports.

Table 2.
Cavity sizes and topology of zeolites exhibiting shape selectivity in hydroisomerization of long-chain alkanes. MR denotes membered ring channels in zeolites.
5.1. Zeolites in Hydroisomerization of Long-Chain Paraffins
5.1.1. Structure of Zeolites
Different types of zeolites have been used in hydroisomerization including both 8, 10, and 12 membered structures [22]. The following zeolites possessing a one-dimensional structure, i.e., ZSM-22, ZSM-23, ZSM-48 [19], and SAPO-11 exhibit shape selectivity (Table 2), whereas 12 MR zeolites are devoid of shape selectivity. ZSM-35 has a ferrierite (FER) topology and contains 8–10 MR (Table 2).
Shape selectivity is very characteristic for 10 MR zeolites, when a long-chain alkane cannot diffuse into the pores of, for example, Pt-H-ZSM-22 [22]. In this case, the substrate is reacting at the pore entrance giving higher selectivity to monobranched products. This phenomenon is called pore mouth selectivity. Furthermore, the zeolite structure has a major effect on the product distribution in hydroisomerization of long-chain paraffins; for example, an inverse correlation has been shown for the formation of an ethyl-branched C8 isomer vs monobranched C9 in decane isomerization. The highest selectivity to monobranched products was with 10 MR zeolites, exhibiting better performance than 12 MR. The lowest selectivity was obtained for 14 MR zeolites [21].
A comparative work on shape selectivity from 10 MR zeolites (ZSM-22, ZSM-23, ZSM-35, and ZSM-48) was performed in [19] using hexadecane hydroisomerization as a model reaction, showing that the channel system has a large effect on the product distribution (Figure 7). Over Pt-ZSM-35 with a two dimensional channel system, a high conversion was obtained without formation of multibranched products, indicating a pore mouth mechanism. Pt-SAPO-11 has also displayed high selectivity to skeletal isomers in n-dodecane hydroisomerization (Table 1) [33], due to its pore dimensions (Table 2). Platinum was loaded into this catalyst via the incipient wetness method, resulting in a highly reducible Pt located at the external surface of SAPO-11. This catalyst exhibited also high long-term stability (see Section 8).
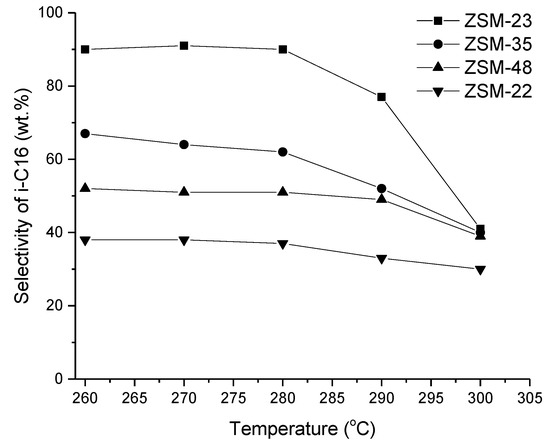
Figure 7.
The selectivity to isohexadecane as a function of reaction temperature under 40 bar over different Pt-ZSM catalysts [19]. Reproduced with permission from Zhang et al., Ind. Eng. Chem. Res.; published by American Chemical Society, 2013.
Siliceous Pt-H-ZSM-22 is an excellent catalyst for hydroisomerization of n-dodecane, giving 96% selectivity to isomers, however, only at 29% conversion at 290 °C under 40 bar using H2/feed volume ratio of 750 [37]. High selectivity was obtained at relatively low conversion, over siliceous Pt-H-ZSM-22 is due to its low acid site density, the presence of acidic silanol groups, and an overall good balance between the metal and acidic functions. It was also stated in [37] that Pt particles in this catalyst are located on the zeolite external surface. Note that ZSM-22 exhibits shape selectivity, which is not present in zeolites with a similar pore size, but containing a three-dimensional pore structure; e.g., ZSM-5 and MCM-22 [38].
A novel zeolite, IZM-2, with both 10 MR and 12 MR rings, facilitated formation of multibranched isomers, giving an analogous product distribution as 10-MR zeolites. The yield of dibranched C10 is shown as a function of monobranched C10 in n-decane hydroisomerization (Figure 8) [39]. This catalyst was prepared in the presence of ethanol as a solvent, leading to formation of intergrowth zeolite nanocrystals mostly containing two types of pores. Such structure in addition to surface area of 201 m2/g and a large external surface can explain high isomer selectivity [39].
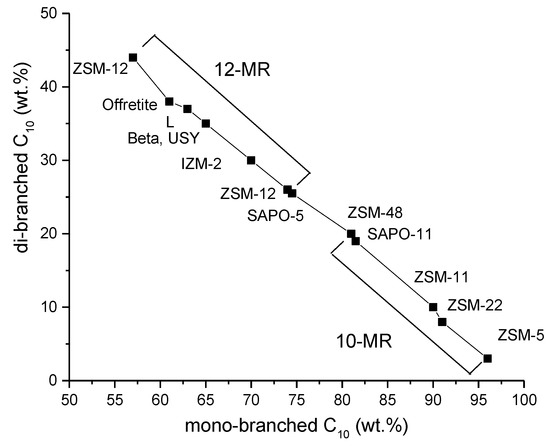
Figure 8.
The yield of di-branched C10 as a function of monobranched C10 in n-decane hydro-isomerization at 5% isomerization yield. Conditions: 4.5 bar, 300 °C, H2/n-C10 molar ratio of 214, WHSV of 0.37 hr−1 [39]. Reproduced with permission from Mota et al., J. Catal.; published by Elsevier, 2013.
On the contrary, 12 MR zeolites, such as Beta and USY (USY denotes ultra stable Y zeolite) produce mono-, di, and multibranched compounds, as well as cracking products (Figure 9) [21]. One Pt-H-Beta zeolite catalyst with optimized acidity (Pt-H-Beta with Si/Al ratio of 26) was giving a 76% yield of isomers at 94% conversion and 220 °C under 30 bar with the molar feed ratio of H2/feed of 20 [16]. However, over Pt-H-Beta with Si/Al molar ratio of 15 the yield of isomerization products in the hydroisomerization of hexadecane exhibited a maximum, 44 wt % at 60% conversion, thereafter, more cracking products were formed [22]. This result was explained by ability of reactant to diffuse into large zeolite pores and further cracking reactions of the formed products. The formed monobranched products exhibited high cracking rate. An enhanced formation of C1, C2, and C14 compound was confirmed to occur via hydrogenolysis on Pt sites present in the Pt-H-Beta catalyst [22]. One conclusion of that study was that acidity of Pt-H-Beta is too high, leading to the undesired cracking products.
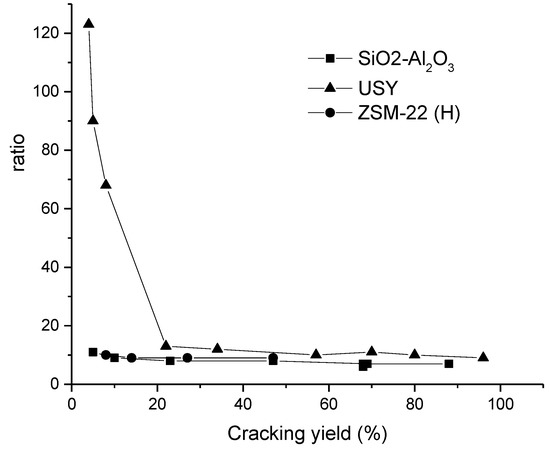
Figure 9.
The ratio between mono- to dibranched isomers as a function of cracking in pristane hydroisomerization over Pt-supported silica alumina, USY, and hierarchical H-ZSM-22 catalysts [21]. Reproduced with permission from Martens et al., Catal. Today; published by Elsevier, 2013.
Zeolite nanosheets have shown high selectivity to branched isomers, when isomerization occurs intensively also on the external catalyst surface [40]. When increasing the zeolite sheet thickness, the isomer yield decreased from 50% with 2 nm thick sheets, to 22% when using 300 nm sheets [40]. It was, however, interesting that no correlation was found between acidity and selectivity to isoheptane in heptane hydroisomerization when using thin zeolite nanosheets (2 nm) [40]. It was concluded in [40] that due to diffusion limitations, bulk zeolites are not efficient catalysts for the hydroisomerization of long-chain paraffins.
5.1.2. Effect of Acidity and Metal Dispersion in Bifunctional Metal Supported Zeolites
Two main parameters determining activity and selectivity in hydroisomerization of long chain alkanes are acidity and metal dispersion. It has been reported that the selectivity of bifunctional catalysts in isomerization is expected to depend on the balance between the metal and acidic functions [41]. Furthermore, typically acidity of zeolites is too high for highly selective hydroisomerization, and several methods have been used to suppress it.
A correlation has been found between the metal and acid site concentration ratios and the formation of different products, such as isomers and cracking products [9]. It has been stated that an optimum hydroisomerization catalyst exhibits this ratio that is close to one, i.e., the acid catalyzed reaction becomes rate limiting, and the number of acid sites between two metal sites is low enough. When either metal or acid functions are too dominant, more hydrogenolysis on metal sites or cracking on acid sites occurs, respectively. It has been stated that the maximum yield of isomers with an ideal catalyst can be obtained when cracking and isomerization are rate-determining steps, (de)/hydrogenation steps are in quasi-equilibrium, and the transport of molecules between different sites is very rapid [41]. This was also confirmed in hexadecane hydroisomerization over 1 wt % Pt-Beta-(26), for which the isomer yield was independent on reaction pressure and temperature, increasing with increasing conversion level [16]. Zeolites with different geometrical constrains and an optimum acidity exhibit the following order of the isomer yield: Pt-H-ZSM-22 > Pt-H-USY > Pt-H-ZSM-5, showing clearly that Pt-H-ZSM-22 exhibits shape selectivity, which is absent in large pore zeolites. The three dimensional zeolite is the least selective [4]. On the other hand, the yield of isodecane over Pt-H-ZSM-5 was only 19% at an 83% conversion. It was concluded that Pt-ZSM-22 facilitated pore-mouth catalysis being more selective towards isodecane.
The challenge in acidity optimization for hydroisomerization of long-chain alkanes is linked to occurrence of both hydroisomerization and hydrocracking reactions on Brønsted acid sites. After isomerization, the reaction can also proceed with the cracking reaction. More acidic catalysts are prone to excessive cracking resulting in the yield loss, while less acidic catalysts do not catalyze the desired reaction, or allow only weak isomerization, resulting in the formation of low octane number long-branched products with low volatility. The acidity of the catalyst determines the extent of isomerization/cracking reactions, with a decrease in acidity decreasing cracking [4,31,42]. The acidity of zeolites can be tuned via changing the silica-to-alumina ratio. In addition, the acidity of zeolites can be suppressed by the addition of basic elements into the structure; e.g., Ba [12], Mg [13], Fe [29], and coating zeolite with alumina [38]. The location of aluminum and its acidity is also crucial, namely if it is present on external and internal zeolite surfaces [36].
One example of using different Si/Al ratios in Pt-H-Beta catalysts was demonstrated in hexadecane hydroisomerization at 220 °C under 30 bar hydrogen [16]. In that work, three Si/Al ratios (11, 16, and 26) were used in Pt-H-Beta catalysts. The isomer yield decreased at 70% conversion from 52% over 1 wt % Pt-H-Beta (hydroisomerization) to 32% for 1 wt % Pt-H-Beta-(11) in the hydroisomerization of hexadecane at 220 °C under 30 bar hydrogen. The best results were obtained for 1.9 wt % Pt-H-Beta (26), giving 72% yield of isomers at 90% conversion [16].
Modification of zeolites with basic (Ba2+, Mg2+, Na+) and acidic (Fe2+) elements, aiming at a decrease/modification of acidity and a higher metal dispersion has been investigated in hydroisomerization of long-chain alkanes [12,13,22,29]. Ba-modification of ZSM-12 favored hydroisomerization over cracking in hexadecane hydroisomerization [12], due to an increase of Pt dispersion, and a lower Brønsted acidity in comparison with the parent zeolite. The highest lumped yield of isomers, i.e., mono- and multibranched products (weight ratio) was obtained over 0.43 wt % Pt-25 wt % Ba-ZSM-12 at 295 °C under 60 bar [12]. It was stated that the role of Ba is to stabilize Pt dispersion and tailor the catalyst acidity. The main products with 0.43 wt % Pt-25 wt % Ba-ZSM-12 catalyst were multibranched isomers. Another example is Mg modified Pt-H-ZSM-23 catalyst being a medium pore size MTT type zeolite. When using trace amounts of Mg 55% yield of isomers at 65% conversion level at 260 °C under 30 bar with H2/HD ratio of 6 and WHSV of 1 hr−1 [13] was obtained. This performance was explained by the fact that because the radius of Mg2+ cation is smaller than the pore diameter of ZSM-23, it can also enter the zeolite pores. A slight decrease of BET surface area was observed when Mg2+ was added into Pt-ZSM-23. Simultaneously, Mg2+ increased the Lewis acidity and decreased the Brønsted acidity [13]. Furthermore, sodium modified Pt-Beta zeolite was highly selective to multibranched isomers in hexadecane hydroisomerization at 220 °C under 30 bar, in comparison with Pt-H-Beta [22].
Isomorphous iron substitution decreases the catalyst acidity, and thus iron-modified Pt-H-ZSM-22 exhibits higher selectivity towards formation of dibranched isomers, compared to the parent catalyst [29]. This result was explained by the presence of Si-OH-Fe bridge hydroxyl, whereas the unmodified zeolite contains Si-OH-Al [29].
5.2. Composite Materials and Hierarchical Zeolites in Hydroisomerization of Long-Chain Alkanes
Composite materials and hierarchical zeolites have been prepared in order to increase the mass transfer inside zeolites. Several methods exist for preparation of hierarchical zeolites, such as dual templating [25,36], desilication via alkali treatment [35], dealumination via acid treatment [25], and synthesis of composite materials such as intergrowth micro/mesoporous ZSM-22/ZSM-23 (Table 3) [27]. Agglomeration of zeolites can be suppressed via their alumination, which was performed in [9,10]. Several composites, including mesoporous materials, have been developed and tested in hydroisomerization, such as Pt-Y-MCM-41 [45], ZSM-5/SBA-15 [46], and aluminated MCM-48 [24].

Table 3.
Different method used for tailoring the catalyst acidity and structure.
Solvent-free synthesis method was used for SAPO-11 in order to enhance its acidity, and simultaneously, Pt metal dispersion in comparison with a conventional SAPO-11 [14]. This method promotes the formation of many Si species in the SAPO region, enhancing its acid site density and the amount of Brønsted acid sites. The amount of mesoporous was lower than in hydrothermally prepared SAPO-11. This material was present in hexadecane isomerization at a relatively low reaction temperature, 290 °C, with an 80% yield of isomerization products under 60 bar with WHSV of 1 hr−1 and H2/feed molar ratio of 15, whereas the hydrothermally synthesized Pt-SAPO-11 gave only a 62% maximum isomer yield at 355 °C. More multibranched isomers were formed over Pt-SAPO-11 prepared by the solvent free method, due to a relatively low Pt dispersion [14]. The reaction mechanism was proposed to be of the lock-and-key type mechanism over this catalyst, i.e., the two ends of an alkane penetrated into different pore mouths, favoring the formation of the multibranched products.
Desilication of zeolites by using an alkali enhances the molecular diffusion inside zeolites [24]. Alkali treatment is also more selective for removing Si, and realumination occurs on the zeolite external surface (Figure 10). Alkali-treated Pt-ZSM-22 exhibited lower amounts of Brønsted acid sites with increasing NaOH concentration. Over this alkali-leached Pt-SAPO-11 catalyst, a 70% yield of isomers was obtained at 80% conversion in the hydroisomerization of n-dodecane at 280 °C under 20 bar using the volumetric ratio of H2/feed of 600 and a WHSV of 2 hr−1 [28]. Alkali treatment can rearrange the acid distribution, and at the same time it increases the Brønsted acid sites in zeolites [28]. For example hierarchical zeolite ZSM-22 contained less acid sites inside the zeolite framework, and an increased amount of acid sites on its external surface [21], when acidity was determined using pyridine and 2,6-lutidine, respectively, as probe molecules.
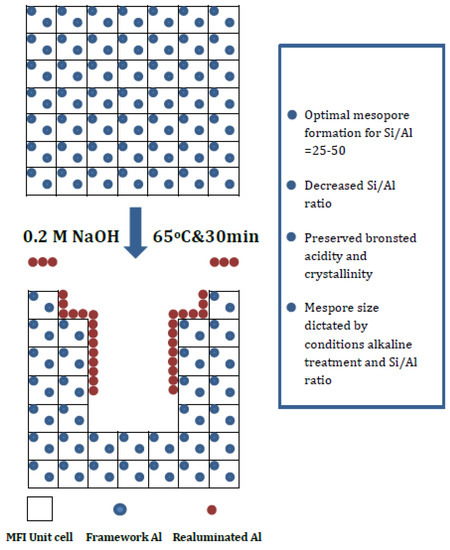
Figure 10.
A schematic picture of desilication of MFI zeolite [47]. Reproduced with permission from Groen et al., J. Mater. Chem.; published by Royal Society of Chemistry, 2006.
A comparative study of ZSM-22 desilication vs in situ alkaline treatment in the presence of cetyl triammonium bromide (CTAB) for preparation of ZSM-22/MCM-41 composite material was made in [35]. In the desilicated ZSM-22, no destruction of zeolite was visible, while in the presence of alkali and CTAB, the formation of MCM-41 phase was observed in 29Si magic angle spinning nuclear magnetic resonance spectroscopy (29Si MAS NMR). This study showed that a lower Brønsted acidity determined by pyridine Fourier-transform infrared spectroscopy (FTIR) was obtained for ZSM-22/MCM-41, in comparison with the desilicated material. Furthermore, this desilicated material exhibited a slightly higher amount of external Brønsted acid sites, as determined by 2,6-dimethylpyridine-FTIR [35]. Especially with an optimum amount of NaOH added in the synthesis of micro/mesoporous hierarchical zeolite, ZSM-22/MCM-41, high selectivity to monobranched isomer was obtained in dodecane hydroisomerization [35].
Another option to enhance the aluminum presence on the external zeolite (ZSM-22) surface, is to use ethanol as a co-solvent in hydrothermal zeolite synthesis [36], leading to the formation of ZSM-22 nano-bundles. These particles exhibited, according to 27Al 2D 3Q MAS NMR, more aluminum that was located on the T1, T2, and T3 sites than a conventional ZSM-22. This result was interpreted by the oriented fusion of nanorods, as promoted by the presence of ethanol and formation of new micropores. This Pt-ZSM-22 nanorod catalyst was tested in n-dodecane hydroisomerization, giving 52% selectivity at 96% conversion at 300 °C under 20 bar using LHSV of 2 hr−1 and H2/feed volumetric ratio of 600. Another method for producing nanorods is to separate them during desilication [48] leading to formation of mesopores between the nanorods. This type of catalyst loaded with Pt was successfully used in hydroisomerization of decane, nonadecane, and pristane (2,6,10,14-tetramethylpentadecane) [21]. The results showed that the hierarchical Pt-ZSM-22 exhibited a much higher ratio of mono- and dibranched isomers to cracked products in comparison with Pt-USY and Pt on alumosilicate catalysts (Figure 9) [21]. It was further concluded that catalyst washing after desilication with a mild acid is essential to remove the Al species blocking the pore mouths.
Dealumination via an acid treatment is a method to prepare hierarchical zeolites containing mesopores. It is typically performed by stirring zeolite in the acid solution at a low temperature; e.g., 40 °C, followed by washing, drying and calcination at a high temperature (550 °C) [25]. Dealumination of SAPO-11 was investigated using either HCl or citric acid treatment, and facilitating the formation of mesopores [25]. The HCl-treated catalyst exhibited mesopores mainly on the crystal surface, whereas citric acid treatment formed mesopores both inside and outside the crystals. With HCl treatment, Lewis acid sites were formed. The latter catalyst, treated with citric acid and containing Pt as an active metal, was more selective in the hydroisomerization of n-dodecane at 280 °C under 1 bar giving the isomer yield of 74%. This catalyst was prepared with an optimum amount of citric acid, as high amounts of citric acid resulted in a lower crystallinity. It is also noteworthy that the ratio between mono- to multibranched isomers was elevated using citric acid-modified Pt-SAPO-11 as a catalyst compared to commercial SAPO-11, especially at high reaction temperatures [25]. Since the dealuminated hierarchical zeolites, such as ZSM-22, can have Al on the external surface of the zeolite, blocking the micropores, it has to be washed away. This catalyst exhibited a higher selectivity to isomerization products [21].
Dealumination of zeolites is beneficial to enhance isomer yields in hydroisomerization of long-chain alkanes [24]. An acid-leached treated Pt-HY with Si/Al ratio of 24.8 containing both micro- and mesopores has also been investigated in hexadecane hydroisomerization, giving the isomer yield of 53% at 67% conversion [24] which was higher than the corresponding values for Pt-HY (Si/Al = 28.4), namely 42% at a 62% conversion. The Pt-HY catalyst intracrystalline diffusion limitations were revealed in the Arrhenius plots, whereas for acid-leached catalyst, noted as Pt-HY-A, this was absent and the activation energy for hydroisomerization of n-hexadecane was 140 kJ/mol [24]. For hierarchical zeolites exhibiting both meso- and micropores, such as dealuminated HY-30 with 30 nm mesopores, an efficient mass transfer was obtained while the reaction occurs in micropores enhancing selectivity towards isomers [49]. It is also important to note that an acid-leached catalyst (Pt-HY-A) gives a bell shaped cracking products distribution, whereas microporous Pt-HY facilitates the formation of shorter hydrocarbons (Figure 11) [24]. When the Si/Al ratio was changed in Pt-HY zeolites applied for hexadecane hydroisomerization, a bell-shaped product distribution was obtained for Pt-HY with Si/Al of 5, whereas a flatter shape for the cracking product distribution was obtained for Pt-HY (Si/Al 100) [17].
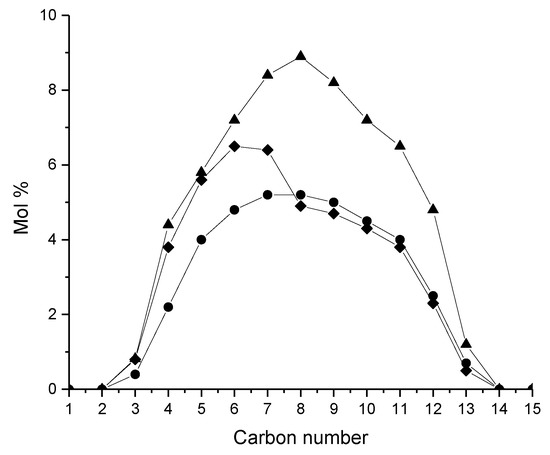
Figure 11.
The distribution of cracked products in hexadecane hydroisomerization over Pt-HY-30 (diamond), Pt-Al-MCM-48 (cirle, and Pt-HY-acid leached at 45% yield of cracking products [24]. Reproduced with permission from Kenmogne et al., J. Catal.; published by Elsevier, 2015.
Mesoporosity in zeolites can be created by using structure-directing agents [34]. Carbon black has been used for this purpose to create mesoporosity in Pt-SAPO-11 [34]. This catalyst exhibited high hydroisomerization selectivity due to diminished external acidity, as determined by the adsorption of 2,6-ditertbutylpyridine.
Furthermore, in [34] an effort was made to quantify the accessibility of the catalyst pores; thus adsorption studies were made with 2,5-dimethylhexane for SAPO-11 catalysts prepared using different templates. Thereafter, the diffusion constant of this molecule (D) was calculated and divided by the calculated diffusion length (L) of this material using the formula: D/L2. This value was correlated with the isomer yield revealing a linear correlation between diffusion characteristics and this yield [34].
A dual templating method was used to tune the zeolite properties, such as acidity and external surface area. This has been successfully demonstrated in [23], in which a dual templated Pt-ZSM-23 with SiO2/Al2O3 ratio of 155 exhibited a lower microporous surface area than the material for which only isopropyl amine was used as a template. In the dual template Pt-ZSM-23 zeolite, pyrrolidine and isoprolylamine were used as templates, resulted in a rapid crystallization [50]. The isomer yield in hydroisomerization of hexadecane was 20% at a 22% conversion (Figure 5) [23]. Notably, with this dual templated catalyst, the external surface area was high, 9 m2/gcat, and the amount of strong acidic sites was low, promoting high isomer formation and inhibiting cracking.
Mesoporous materials exhibit excellent properties for mass transfer possessing at the same time low acidity. It has, however, been demonstrated that Pt-Al/SBA-15 has suitable acidity, predominantly Lewis acidity, and a pore structure favoring the formation of isomers [46]. In decane isomerization at 325 °C under 40 bar using a H2/feed molar ratio 9 and a WHSV of 5.2 hr−1 over Pt-Al-SBA-15, 68% selectivity towards C6 and C7 hydrocarbons was obtained at a 62% conversion [46]. It was hypothesized that for hexadecane hydroisomerization, with this mesoporous support, the product selectivity was determined only by the pore size, which was determining the optimum adsorption strength. In the case of microporous zeolites, the adsorption strength of molecules was too high, being detrimental for product selectivity towards isomers [24]. One example of a mesoporous composite catalyst is aluminated Pt-MCM-48 exhibiting a narrow pore size distribution with 3.2 nm pores. The acidity of MCM-48 has been enhanced by alumination using Al(O-sec-C4H9)3 in suspension with MCM-48 [24]. It was stated that the surface acidity of the resulting Al-MCM-48 material resembles that of zeolites with the surface containing large amounts of Lewis acid sites. This material exhibited a higher acid density than directly in situ aluminated MCM-48. For Pt-modified catalyst [24], the yield of isomers was 70% at 82% conversion in hexadecane transformation at 240 °C under 20 bar using H2/feed molar ratio of 4. Noteworthy is also the preferential cracking during hydroconversion of hexadecane in the center of a reactant favoring the formation of C8 products (Figure 11) when using the mesoporous catalyst [24].
A catalyst made from a commercial Y zeolite dispersed with CTAB; followed by synthesis of MCM-41, resulted in the formation of a composite material exhibiting both faujasite and MCM-41 phases [45]. This catalyst exhibited much lower acidity than the corresponding Pt-Y, but on the other hand, its metal dispersion was 1.8 fold that of Pt-Y. The composite Pt-Y-MCM-41 catalyst afforded 91% selectivity to isomers, while Pt-Y gave only 76% selectivity at a 30% conversion rate in hexadecane isomerization at 270 °C under 30 bar [19]. It was concluded that the microporous-mesoporous composite with a mesoporous shell structure is superior, due to a rapid diffusion of the formed isomers, while Pt-Y has interior zeolite crystals in which secondary reactions occur. One example of a micro-mesoporous composite material that is not efficient in hydroisomerization was Pt-ZSM-5/SBA-15, which mainly favored cracking in n-decane hydroisomerization, further emphasizing the effect of Brønsted acidity in this reaction [46].
A composite catalyst comprising -Al2O3 particles coated with Pt-H-Beta (Si/Al ratio of 11) having zeolite loading of 13 wt % was investigated as a catalyst in hexadecane hydroisomerization at 220 °C under 30 bar using a H2/feed molar ratio of 20 [9]. The formed catalyst exhibited lower agglomeration of the catalyst particles than the parent Pt-H-Beta zeolite, facilitating high activity and selectivity towards isomers. The Pt particle size in this catalyst was 2.6 nm. The total isomer yield was 80 wt % at a 95% conversion level, while the parent Pt-H-Beta gave a 28 wt % isomer yield at 78% conversion (Table 1, Figure 12). The ratio between mono- to bibranched isomers was 0.2, indicating that the yield of dibranched isomers was high at high conversion. It was concluded that the -Al2O3-Pt-H-Beta-11 composite catalyst with isolated nanocrystals suppressed the secondary reactions of the formed isomers, due to a short residence time of the isomers inside the zeolite framework. As a comparison, a composite catalyst Pt--Al2O3–H-Beta (with a high Si/Al ratio of 150) was investigated in the hydroisomerization of hexadecane [10]. When the Al2O3-to-zeolite ratio was varied, it was observed that the rate was proportional to the amount of Brønsted acid sites, while distribution of the isomerization and cracking products was independent on the zeolite content. A yield of isomers (35.5 wt %) was obtained at a 36.5 wt % conversion rate, with Pt-Al2O3-H-Beta (80% zeolite) at 75 bar and 290 °C using WHSV of 1.3 hr−1.
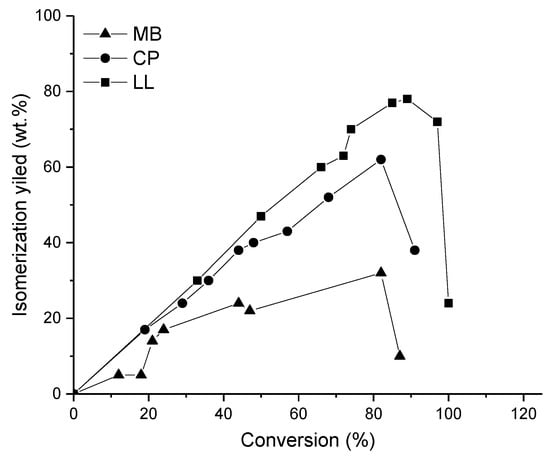
Figure 12.
The yield of isomerization products as a function of conversion in hexadecane hydroisomerization over Pt-Beta, low loading Al2O3-H-Beta (LL) and high loading Al2O3-H-Beta [38]. Reproduced with permission from Batalha et al., Microporous Mesoporous Mater.; published by Elsevier, 2013.
Intergrowth of ZSM-22/ZSM-23, loading of Pt by impregnation resulted in the formation of micro-mesoporous catalyst with such specific properties as lower acidity, needle-shaped particles, which were agglomerating, forming spherical 10 µm particles [27]. Although this catalyst had the lowest activity, it exhibited the highest selectivity towards the hydroisomerization of dodecane, with a 68% yield of isododecane at 320 °C, in comparison with the corresponding Pt-H-ZSM-22 and Pt-H-ZSM-23 catalysts.
Pt-loaded zeolite nanosheets with the zeolite framework type MFI structure exhibited a much higher yield-to-isomer products in n-decane hydroisomerization than the parent Pt-ZSM-5. This catalyst exhibited a lower micropore volume, lower acidity, higher external surface area, and high crystallinity, compared to Pt-ZSM-5, yielding an 80% conversion in dodecane isomerization with a 42% yield of isomers, whereas the corresponding yield with Pt-ZSM-5 was 32% [44]. It was concluded that this type of nanosheet Pt-ZSM-5 catalyst exhibited short channels, suppressing the formation of ethyl- and propyl-branching among the products, in line with the transition state shape selectivity concept.
5.3. Effect of Metal
The majority of studies on hydroisomerization of long-chain alkanes has been made with Pt-supported catalysts, since this noble metal facilitates hydrogenation/dehydrogenation reaction at lower temperatures compared to transition metal catalysts. Moreover, a lower reaction temperature is beneficial for maximizing the selectivity towards isomers. Pt catalysts used in hydroisomerization have been supported on H-Beta [9], HY [17], MCM-41 [45], MCM-48 [24], ZSM-12 [12], ZSM-23 [19,23,27], ZSM-22 [19,21,22,27,28,32,35,36], ZSM-35 [19], ZSM-48 [19], intergrown ZSM-22/ZSM-23 [27], IZM-2 [39], sulphated ZrO2 [50], -Al2O3 [51], Pt--Al2O3-H-Beta [10], SAPO-11 [14,25,33,34], MFI nanosheet [44]. There are only a few publications applying other metals than Pt, such as Pd supported on SiO2-Al2O3 [18], Fe-ZSM-22 [29], Ni2P-SAPO-11 [26], Ni-SAPO-11 [26], Ni-Mo-SAPO-11 [20], Ni-Cu-SAPO-11 [30].
Loading of a noble metal (Pt and Pd) in hydroisomerization catalysts is typically very low. The drawback in using noble metals is their high price; therefore, several efforts have recently been made to perform hydroisomerization over cheap transition metals, such as Ni [30] and Ni2P-SAPO-11 [26]. It has, however, been observed that Ni-SAPO-11 gave selectivity towards hydroisomerization of octane lower than a bimetallic Ni-Cu-SAPO-11, which allowed lower hydrogenolysis activity. Ni2P-SAPO-11 catalyst was investigated in hydroisomerization of n-dodecane [26]. The results showed that the optimum metal loading was 3 wt % and the highest isododecane yield was 65% obtained at 350 °C under 20 bar using a WHSV of 3 hr−1 at 73% conversion [26], which is a promising result in hydroisomerization of long-chain alkanes in comparison to noble metal catalysts.
5.4. Effect of Metal Dispersion and Loading
High metal dispersion facilitates selective hydroisomerization [32]. Several factors determine the metal dispersion, such as the metal loading, precursor type and the catalyst preparation method [32,33]. In addition, a very important parameter is the metal location, i.e., whether the metal is on the pore mouth, on the internal or external surface of the catalyst. The metal location can also be tuned with a proper selection of the catalyst preparation method [52].
The effect of metal loading and the catalyst preparation method was investigated in a comparative work, using different metal loading methods for SAPO-11 [33]. It was confirmed that the reducibility of Pt in Pt-SAPO-11 prepared by the incipient wetness method was very good, because Pt was mainly located on the outer surface of SAPO-11. On the other hand, when ion exchange and atomic layer epitaxy methods were used for catalyst preparation, Pt was mainly located inside of SAPO-11, making it difficult to reduce. Out of these three Pt-SAPO catalysts, the best behavior was shown be Pt-SAPO-11 prepared by the incipient wetness method [33]. For Pt sites located outside SAPO-11 close to the pore mouth, when the catalyst was prepared by the incipient wetness method, the yield of 58% to multibranched isomers was obtained at an 88% conversion rate in the hydroisomerization of n-dodecane at 290 °C under 80 bar, using a LHSV of 2 hydroisomerization hr−1, and a H2/feed molar ratio of 22.6 [52].
The catalyst precursor can have a substantial effect on the metal dispersion; for example, (NH4)2PtCl4 could produce Pt particles on ZSM-22 surface that were smaller than Pt(NH3)4Cl2 as a precursor [32]. It was also concluded [32] that Pt-ZSM-22 catalysts prepared from anionic precursors exhibited smaller particle sizes than those prepared from cationic precursors (PtCl4, Pt(NH3)4Cl2), promoting selective hydroisomerization of n-hexadecane [32].
6. Effect of Reaction Conditions in the Hydroisomerization of Long-Chain Paraffins
The effect of reaction conditions is compared in this review for continuous operation, since majority of the recent studies have been made in fixed bed reactors. The effect of reaction temperature has been very intensively investigated, while variations in the contact time have received less attention, and only a few studies have report data with varying WHSV [19,23,26]. Temperature and the contact time are the most important parameters in the hydroisomerization of long-chain paraffins, due to their high reactivity towards undesired cracking products. In addition, other parameters such as pressure and the ratio between hydrogen to feed are discussed below.
6.1. Effect of Temperature and Pressure
The optimum temperature and pressure for the hydroisomerization of hexadecane over Pt-supported bifunctional catalysts are typically in the range of 220–310 °C and 20–60 bar, respectively [9,12,14,15,19,33] in the fixed bed reactor (Table 1). However, much higher pressures are typically required in the batch reactor due to different H2/feed ratios. It is also known that a high hydrogen pressure inhibit catalyst deactivation [53]. Activation energy has been determined for the isomerization of hexadecane, being ca. 140 kJ/mol, which is a typical value for alkane hydroconversion in the absence of mass transport limitations [24].
In dodecane hydroisomerization over Pt-supported bifunctional catalysts, the optimum temperatures and pressures are 260–330 °C and 20 bar, respectively [14,28,29], although the reaction has also been performed at an ambient pressure at 330 °C [25]. When using a non-noble metal catalyst, Ni2P-SAPO-11, a high temperature of 350 °C and 20 bar gave the highest isomer yield (Figure 13b).
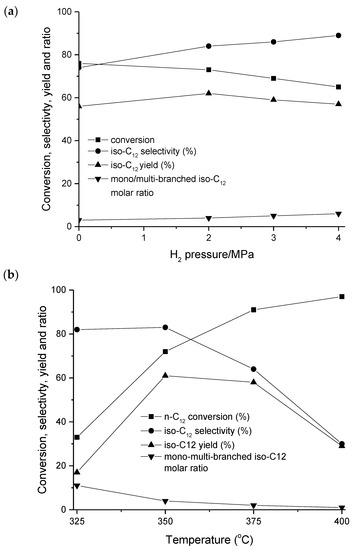
Figure 13.
Conversion, selectivity, yield of isomer, and the ratio between mono/multibranched isomers in dodecane hydroisomerization over NixPy-SAPO-11 as a function of (a) hydrogen pressure and (b) H2/feed molar ratio [26]. Reproduced with permission from Tian et al., Fuel Process. Technol.; published by Elsevier, 2014.
The reaction order for hydrogen in the hydroisomerization of hexadecane was equal to −0.42, whereas when the catalyst was poisoned by pyridine, the reaction order with respect to hydrogen was zero [15]. Analogous results were obtained in the work of [33], in which both the activity and the selectivity for the formation of isomers decreased with increasing hydrogen partial pressure and increasing conversion over Pt-ZSM-23.
6.2. Effect of H2/Feed Molar Ratio
The effect of the molar ratio H2/feed has been investigated in several publications [12,14,15,22,24,26,33]. This ratio for hexadecane hydroisomerization is typically varied, from 4 to 20 [9,12,14,15,16,22,24,33], while for a mixture of C15–C18, this ratio was 30 [17]. An optimum molar ratio of H2/feed of 10 was found, giving a stable time-on-stream conversion in the hydroisomerization of hexadecane over Pt on a sulphated zirconia catalyst at 225 °C under 20 bar, with a WHSV of 18.4 hr−1 [50]. It was also stated that a high hydrogen pressure is required, since long-chain paraffins give rise to form heavier coke precursors compared to short chain feedstock molecules, for which the optimum ratio of H2/feedstock is 6.
In the hydroisomerization of n-dodecane, the H2/feed ratio was varying, in the range of 15–25 [25,26,33,46], while for n-decane, the H2/feed ratio was 9 over Pt-Al-SBA-15 catalyst at 250–350 °C, using a WHSV of 5.1 hr−1 [46]. This illustrates that the H2/feed ratio is slightly lower for shorter alkanes. The optimum H2/feed molar ratio was, however, 18 over N2P/SAPO-11 (Figure 14) [26] in the hydroisomerization of dodecane, showing that the catalyst type also has an effect on the optimum reaction conditions.
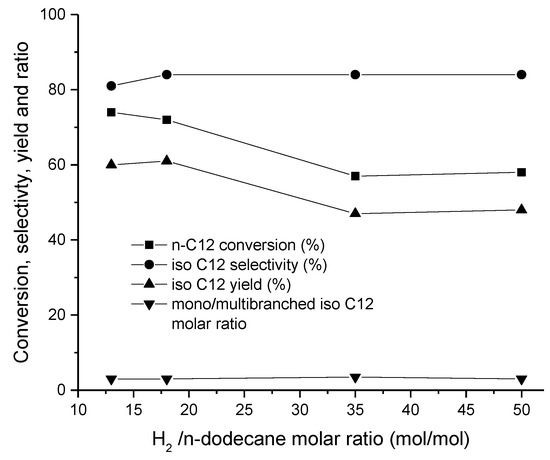
Figure 14.
Conversion, selectivity, yield of isomer, and ratio between mono/multibranched isomers in dodecane hydroisomerization over NixPy-SAPO-11 as a function of H2/feed molar ratio [26]. Reproduced with permission from Tian et al., Fuel Process. Technol.; published by Elsevier, 2014.
6.3. Effect of WHSV in Continuous Operation
Hydroisomerization of long-chain alkanes is a complex reaction, composed of consecutive and parallel reaction routes. For an ideal catalyst with optimized properties, the reaction is mainly consecutive (Figure 2) [18], and thus, typically relatively short residence times and low WHSV (LHSV) are required [19,23,26]. The optimum WHSV are typically quite short, being 1.5 hr−1 for n-dodecane hydroisomerization at 350 °C under 20 bar over Ni2P-SAPO-11 [26]. At longer WHSV, isomers can react further to cracking products. In hexadecane, hydroisomerization over Pt-ZSM-23/35 at 300 °C under 40 bar the optimum contact time was ca. 0.7 s [19].
For C15–C18 alkanes over Pt-HY (Si/Al ratio of 100) at 310 °C under 31 bar, the effect of LHSV was clearly demonstrated (Figure 15), namely that for high conversion, the selectivity to isomers was low and vice versa; i.e., at a LHSV of 1.0 hr−1 conversion, and selectivity were 90% and 47%, while an increase of LHSV to 4.0 hr−1 resulted in a conversion of 52%, and a selectivity of 60% [17].
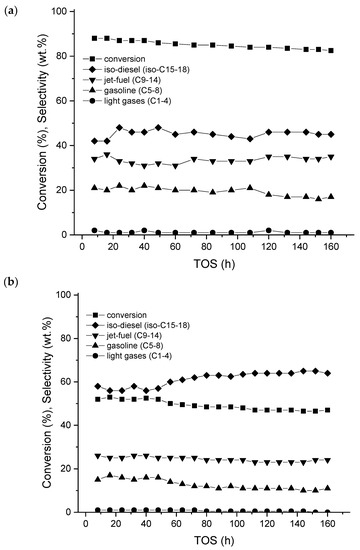
Figure 15.
The effect of LHSV: of (a) 1.0 hr−1 and (b) 4.0 hr−1, at 310 °C under 31 bar using H2/BDH molar ratio of 30 in the hydroisomerization of C15–C18 alkanes over Pt-HY-100 (a Si/Al ratio of 100), conversion and selectivity as a function of time-on-stream adapted from [17]. Reproduced with permission from Hengsawad, et al., Ind. Eng. Chem. Res.; published by American Chemical Society, 2018.
7. Effect of Reactor Selection in Hydroisomerization of Long-Chain Paraffins
Comparison of Continuous and Batch Reactors
Typically, hydroisomerization of long-chain alkanes has been performed in fixed bed reactors [9,12,15,18,19,25], with few exceptions when batch reactors were used [1,45]. The reason for this is that a higher hydrogen-to-reactant ratio in a continuous mode, facilitating a higher conversion in comparison with a batch mode.
Hydroisomerization of hexadecane was performed in batch reactors with bifunctional catalysts containing 0.5 wt % platinum with ZSM-5, ZSM-22, SAPO-11, Al-MCM-41, H-Y, or H-β as supports [1]. The same reaction was used with decane as a substrate over a Pt-Al-MCM-41, Pt-Y, or Pt-Y-MCM-41 composite, and a mechanical mixture of Pt-Y-MCM-41 [45]. A high conversion of n-hexadecane was achieved in a batch reactor at very harsh reaction conditions, i.e., at 350 °C under 103 bar over Pt-ZSM-22 catalyst in 2 hr [1]. On the other hand, only a 38% of conversion of hexadecane was obtained over Pt-SAPO-11 in a batch reactor under these harsh conditions after 14 hr. At the same time, 96% conversion of n-hexadecane was obtained at 60 bar, 325 °C and WHSV of 1 hr−1 using the molar ratio of H2/n-hexadecane of 15 over Pt-SAPO [14], clearly illustrating that a batch operation requires higher temperatures and pressures to achieve comparative performance with the fixed bed operation.
The highest selectivity to isomers was obtained at 350 °C under 103 bar in a batch reactor at conversion levels between 37–45% over 0.5 wt % platinum supported on ZSM-5, ZSM-22, SAPO-11, Al-MCM-41, H-Y, and H-Beta [1]. Pt-Al-MCM-41 displayed the best hydroisomerization as well as the highest selectivity to iso-hexadecanes (89 wt. %) at a 44% conversion. These results can be explained by the mild acidity and a metal dispersion of Pt-MCM-41, which is higher than in other catalysts. Pt-SAPO-11 also showed comparative conversion, but exhibited a lower selectivity towards the branched products than Pt-H-MCM-41. It is important to mention that no alkenes were found in products, which is due to a high hydrogen pressure, allowing for the hydrogenation of all alkenes to respective alkanes [1].
The residence time is of crucial importance for the selective hydroisomerization of long-chain alkanes in continuous operation. It is beneficial to shorten the residence time in order to diminish the diffusion path of alkenes from one metal site to another and at the same time decrease the number of acid sites the alkene is in contact with [16,23]. For example in hydroisomerization of hexadecane a high selectivity to isomer was obtained with a mildly acidic dual template, Pt-ZSM-23, using contact times in the range of 1–2.5 s at 300 °C under 40 bar when conversion varied in the range of 24–60% [23]. This result indicates clearly that in a continuous reactor, a high yield of isomers can be obtained only with tailored catalysts (Figure 5 and Figure 6) [9,12,38], whereas with e.g., conventional Pt-supported zeolites, short contact times are preferred [22]. On the other hand, a shortened diffusion path can also be obtained by modifying the catalyst structure e.g., via dealumination, desilication, using nano-structured zeolites and applying composite catalysts (see Section 5).
8. Catalyst Deactivation and Stability in Hydroisomerization of Long-Chain Paraffins
Catalyst stability is very important from an industrial viewpoint. Several highly stable catalysts have been identified in hydroisomerization, including Pt-HY [17], Pt-SAPO-11 [33], Pt-ZSM-22 [17], and Pt-SAPO-11 [19].
Long term stability (ca. 200 hr) of siliceous Pt-ZSM-22 was demonstrated in the hydroisomerization of hexadecane at 343 °C under 40 bar with H2/feed volumetric ratio of 750 using LHSV of 1.2 hr−1 [37]. Analogously Pt-HY(Si/Al ratio of 100) exhibited a very high stability in hydroisomerization of biohydrogenated diesel composed of C15- to C18 alkanes at 310 °C using 31 bar and a molar ratio of H2/feed of 30 [17]. This catalyst exhibited a relatively low acidity and a high Pt dispersion (56%) [17]. A stable performance was also obtained for Pt-SAPO-11 in dodecane hydroisomerization at 320 °C under atmospheric pressure. In addition, 10 MR ring zeolites can have high stability and resist coke formation in hydroisomerization [19]. Analogously, 0.5 wt % Pt-SAPO-11 was a stable catalyst in hydroisomerization of n-dodecane at 300 °C under 1 bar using WHSV of 2 hr−1 for 120 min [33]. This catalyst was prepared by an incipient wetness method and reducibility of Pt was high for this catalyst, as Pt was locating onto the external surface of the catalyst (Section 5). An overall conclusion is that deactivation is not an issue when cracking is not too extensive.
9. Conclusions
The hydroisomerization of long-chain paraffins for the production of isomers with better fuel properties has recently been intensively investigated. Main efforts have been devoted to developing active and selective bifunctional catalysts, such as different 10 MR zeolites, giving shape selectivity, hierarchical zeolites, and composite materials. The main drawback in zeolites is that their acidity is too high, whereas mesoporous catalyst generally have too low an acidity. The acidity of zeolites has been decreased via coating the zeolite with alumina, or by adding basic or acidic elements to the zeolite. This modification can decrease acidity and enhance metal dispersion at the same time. On the other hand, the acidity of mesoporous materials has been increased via alumination, or by making zeolite-mesoporous composite catalysts.
Hierarchical zeolites with optimized acidity and high metal dispersion also facilitate a high mass transfer, promoting a high selectivity to the formation of isomers. These materials have been produced via desilication and dealumination, using either alkaline or acidic treatments, respectively. The hierarchical zeolites typically contain both micro- and mesopores, and lower amounts of acid sites, compared to parent zeolites. The texture of desilicated alkali-treated zeolites is changed so that they contain short channels and a high external surface area. Furthermore, the acid sites can also locate on the external surface.
Another method to promote mass transfer in zeolites is to use metal-modified nanoshaped zeolites, i.e., nanosheets and nanobundles also facilitating a rapid reaction and a short residence time for the formed alkene to react further to cracking products.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Park, K.; Ihm, S. Comparison of Pt/zeolite catalysts for n-hexadecane hydroisomerization. Appl. Catal. A Gen. 2000, 203, 201–209. [Google Scholar] [CrossRef]
- Deldari, H. Suitable catalysts for hydroisomerization of long-chain normal paraffins. Appl. Catal. A Gen. 2005, 293, 1–10. [Google Scholar] [CrossRef]
- Mériaudeau, P.; Tuan, V.; Nghiem, V.; Lai, S.; Hung, L.; Naccache, C. SAPO-11, SAPO-31, and SAPO-41 Molecular sieves: Synthesis, characterization, and catalytic properties in octane hydroisomerization. J. Catal. 1997, 169, 55–66. [Google Scholar] [CrossRef]
- Martens, J.; Parton, R.; Uytterhoeven, L.; Jacobs, P.; Froment, G. Selective conversion of decane into branched isomers: A comparison of platinum/ZSM-22, platinum/ZSM-5 and platinum/USY zeolite catalysts. Appl. Catal. 1991, 76, 95–116. [Google Scholar] [CrossRef]
- Akhmedov, V.M.; Al-Khowaiter, S.H. Recent advances and future aspects in the selective hydroisomerization of high n-alkanes. Catal. Rev. 2007, 49, 33–139. [Google Scholar] [CrossRef]
- Bouchy, C.; Hastoy, G.; Guillon, E.; Martens, J.A. Fischer-Tropsch waxes upgrading via hydrocracking and selective hydroisomerization. Oil Gas Sci. Technol. 2009, 64, 91–112. [Google Scholar] [CrossRef]
- Guisnet, M. “Ideal” bifunctional catalysis over Pt-acid zeolite. Catal. Today 2013, 218–219, 123–134. [Google Scholar] [CrossRef]
- Yadav, R.; Sakthivel, A. Silicoaluminophosphate molecular sieves as potential catalysts for hydroisomerization of alkanes and alkenes. Appl. Catal A Gen. 2014, 481, 143–160. [Google Scholar] [CrossRef]
- Batalha, N.; Pimnard, L.; Bouchy, C.; Guilolon, E.; Guisnet, M. n-Hexadecane hydroisomerization over Pt-HBEA catalysts. Quantification and effect of the intimacy between metal and protonic sites. J. Catal. 2013, 307, 122–131. [Google Scholar] [CrossRef]
- Gomes, L.C.; de Oliveiro Rosas, D.; Chistone, R.C.; Zotin, F.M.Z.; de Araujo, L.R.R.; Zotin, J.L. Hydroisomerization of n-hexadecane using Pt/alumina-Beta zeolite catalysts for producing renewable diesel with low pour point. Fuel 2017, 209, 521–528. [Google Scholar] [CrossRef]
- Polzmann, G.; Baladincz, J.; Hanchok, J. Investigation of producing modern base oils. Hung. J. Ind. Chem. 2008, 36, 107–112. [Google Scholar]
- Parmar, S.; Pant, K.K.; John, M.; Kumar, K.; Pai, S.M.; Gupta, M.; Newalkar, B.L. Hydroisomerization of n-hexadecane over Brønsted acid site tailored Pt/ZSM-12. J. Porous Mater. 2014, 21, 849–857. [Google Scholar] [CrossRef]
- Lee, S.-W.; Ihm, S.-K. Characteristics of magnesium-promoted Pt/ZSM-23 catalyst for the hydroisomerization of hexadecane. Ind. Eng. Chem. Res. 2013, 52, 15359–15365. [Google Scholar] [CrossRef]
- Du, Y.; Feng, B.; Jiang, Y.; Yuan, L.; Huang, K.; Li, J. Solvent-free synthesis and n-hexadecane hydroisomerization performance of SAPO-11 catalyst. Eur. J. Inorg. Chem. 2018, 2599–2606. [Google Scholar] [CrossRef]
- Regali, F.; Liotta, L.F.; Venezia, A.M.; Boutonnet, M.; Järås, S. Hydroconversion of n-hexadecane on Pt/silica catalysts: Effect of metal loading and support acidity on bifunctional and hydrogenolytic activity. Appl. Catal. A Gen. 2014, 469, 328–339. [Google Scholar] [CrossRef]
- Batalha, N.; Astafan, J.; Dos Reis, C.; Pouilloux, Y.; Bouchy, C.; Guillon, E.; Pinard, L. Hydroisomerization of n-hexadecane over bifunctional Pt-HBEA catalysts. Influence of Si/Al ratio on activity selectivity. React. Kinet. Mech. Catal. 2015, 114, 661–673. [Google Scholar] [CrossRef]
- Hengsawad, T.; Srimingkwanchai, C.; Butnark, S.; Resasco, D.E.; Jongpatiwut, S. Effect of metal-acid balance on hydroprocessed renewable jet fuel synthesis form hydrocracking and hydroisomerization of biohydrogenated diesel over Pt-supported catalysts. Ind. Eng. Chem. Res. 2018, 57, 1429–1440. [Google Scholar] [CrossRef]
- Regali, F.; Liotta, L.F.; Venezia, A.M.; Montes, V.; Boutonnet, M.; Järås, S. Effect of metal loading on activity, selectivty and deactivation behavior of Pd/silica-alumina catalysts in the hydroconversion of n-hexadecane. Catal. Today 2014, 223, 87–96. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Zhang, Q.; Tsang, C.-W.; Liang, C. Shape selectivity in hydroisomerization of hexadecane over Pt supported on 10-ring zeolites: ZSM-22, ZSM-23, ZSM-35, and ZSM-48. Ind. Eng. Chem. Res. 2016, 55, 6069–6078. [Google Scholar] [CrossRef]
- Xing, G.; Liu, S.; Guan, Q.; Li, W. Investigation on hydroisomerization and hydrocracking of C15-C18 n-alkanes utilizing a hollow tubular Ni-Mo/SAPO-11 catalyst with high selectivity of jet fuel. Catal. Today 2018. [Google Scholar] [CrossRef]
- Martens, J.A.; Verboekend, D.; Thomas, K.; Vanbutsele, G.; Pérez-Ramirez, J.; Gilson, J.P. Hydroisomerization and hydrocracking of linear and multibranched long model alkanes on hierarchical Pt/ZSM-22 zeolite. Catal. Today 2013, 219, 135–142. [Google Scholar] [CrossRef]
- Merabti, R.; Pinardi, L.; Lemberton, J.L.; Magnoux, P.; Barama, A.; Moljord, K. Effect of Na exchange of a HBEA zeolite on the activity and the selectivity of a bifunctional Pt-HBEA catalyst for n-hexadecane hydroisomerization. Comparison with a Pt-HZSM-22 catalyst. React. Kinet. Mech. Cat. 2010, 100, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Chen, X.; Liu, Y.; Liang, C. Synthesis of ZSM-23 zeolite with dual structure directing agents for hydroisomerization of n-hexadecane. Microporous Mesoporous Mater. 2018, 268, 216–224. [Google Scholar] [CrossRef]
- Kenmogne, R.; Finiels, A.; Cammarano, C.; Hulea, V.; Fajula, F. Hydroconversion of n-hexadecane over bifunctional microporous and mesoporous catalysts. Influence of pore architecture on selectivity. J. Catal. 2015, 329, 348–354. [Google Scholar] [CrossRef]
- Song, H.; Liu, Z.; Xing, W.; Ma, Z.; Yan, Z.; Zhao, L.; Zhang, Z.; Gao, X. Preparation of hierarchical SAPO-11 molecular sieve and its application for n-dodecane isomerization. Appl. Petrochem. Res. 2014, 4, 401–407. [Google Scholar] [CrossRef]
- Tian, S.; Chen, J. Hydroisomerization of n-dodecane on a new kind of bifunctional catalyst: Nickel phosphide supported on SAPO-11 molecular sieve. Fuel Process. Technol. 2014, 122, 120–128. [Google Scholar] [CrossRef]
- Chi, K.; Zhao, Z.; Tian, Z.; Hu, S.; Yan, L.; Li, T.; Wang, B.; Meng, X.; Gao, S.; et al. Hydroisomerization performance of platinum supported on ZSM-22/ZSM-23 growth zeolite catalyst. Pet. Sci. 2013, 10, 242–250. [Google Scholar] [CrossRef]
- Wu, X.; Qiu, M.; Chen, X.; Yu, G.; Yu, X.; Yang, C.; Sun, J.; Liu, Z.; Sun, Y. Enhanced n-dodecane hydroisomerization performance by tailoring acid sites on bifunctional Pt/ZSM-22 via alkaline treatment. New J. Chem. 2018, 42, 111–117. [Google Scholar] [CrossRef]
- Liu, S.; Ren, J.; Zhu, S.; Zhang, H.; Lv, E.; Xu, J.; Li, Y.-W. Synthesis and charascterization of the Fe-substituted ZSM-22 zeolite catalyst with high n-dodecane isomerization performance. J. Catal. 2015, 330, 485–496. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Liu, D.; Meng, X.; Liu, C. Hydroisomerization of n-octane over bimetallic Ni-Cu/SAPO-11 catalysts. Appl. Catal. A Gen. 2017, 543, 274–282. [Google Scholar] [CrossRef]
- Soualah, A.; Lemberton, J.; Pinard, L.; Chater, M.; Magnoux, P.; Moljord, K. Hydroisomerization of long-chain n-alkanes on bifunctional Pt/zeolite catalysts: Effect of the zeolite structure on the product selectivity and on the reaction mechanism. Appl. Catal. A Gen. 2008, 336, 23–28. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, Z.; Wu, B.; Xu, J.; Huo, C.; Li, K.; Chen, H.; Yang, Y.; Li, Y. Effect of metal precursors on the performance of Pt/ZSM-22 catalysts for n-hexadecane hydroisomerization. J. Catal. 2015, 322, 1–13. [Google Scholar] [CrossRef]
- Lee, E.; Yun, S.; Park, Y.-K.; Jeong, S.-Y.; Han, J.; Jeon, J.-K. Selective hydroisomreization of n-dodecane over platinum supported on SAPO-11. J. Ind. Eng. Chem. 2014, 20, 775–780. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, K.; Choi, M. Cooperative effects of secondary mesoporosity and acid site location in Pt/SAPO-11 on n-dodecane hydroisomerization selectivity. J. Catal. 2014, 319, 232–238. [Google Scholar] [CrossRef]
- Liu, S.Y.; Ren, J.; Zhang, H.K.; Lv, J.L.; Yang, Y.; Li, Y.W. Synthesis, characterization and isomerization performance of micro/mesoporous materials based on H-ZSM-22 zeolite. J. Catal. 2015, 335, 11–23.36. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Wang, H.; Ning, Q.; Zhang, H.; Yun, Y.; Ren, J.; Li, Y.-W. Synthesis and characterization of bundle-shaped ZSM-22 zeolite via the oriented fusion of nanorods and its enhanced isomerization performance. J. Catal. 2018, 361, 177–185. [Google Scholar] [CrossRef]
- Niu, P.; Xi, H.; Lin, M.; Wang, Q.; Jia, L.; Hou, B.; Li, D. High selectivity fro n-dodecane hydroisomerization over highly siliceous ZSM-22 with low Pt loading. Catal. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Batalha, N.; Morisset, S.; Pinard, L.; Maupin, I.; Lemberton, J.L.; Lemos, F.; Pouilloux, Y. BEA zeolite nanocrystals dispersed over alumina for n-hexadecane hydroisomerization. Microporous Mesoporous Mater. 2013, 166, 161–166. [Google Scholar] [CrossRef]
- Mota, F.M.; Bouchy, C.; Guillon, E.; Fécant, A.; Bats, N.; Martens, J.A. IZM-2: A promising new zeolite for the selective hydroisomerization of long-chain n-alkanes. J. Catal. 2013, 301, 20–29. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Seo, Y.; Kim, J.-C.; Ryoo, R. n-heptane isomerization over Pt/MFI zeolite nanosheets: Effects of zeolite crystal thickness and platinum location. J. Catal. 2013, 301, 187–197. [Google Scholar] [CrossRef]
- Choudhary, J.R.; Hayasaka, K.; Thybaut, J.W.; Narasimhan, C.S.L.; Denayer, J.F.; Martens, J.A.; Marin, G.B. Pt/H-ZSM-22 hydroisomerization catalysts optimization guided by Single-Event Microkinetic modeling. J. Catal. 2012, 290, 165–176. [Google Scholar] [CrossRef]
- Chao, K.; Wu, H.; Leu, L. Hydroisomerization of light normal paraffins over series of platinum-loaded mordenite and beta catalysts. Appl. Catal. A Gen. 1996, 143, 223–243. [Google Scholar] [CrossRef]
- Claude, M.C.; Martens, J.A. Monomethyl-branching of long n-Alkanes in the range from decane to tetracosane on Pt/H-ZSM-22 bifunctional catalyst. J. Catal. 2000, 190, 39–48. [Google Scholar] [CrossRef]
- Verheyen, E.; Changbum, J.; Kurttepeli, M.; Vanbutsele, G.; Gobechiya, E.; Koranyi, T.I.; Bals, S.; Van tendeloo, G.; Ryoo, R.; Kirxscchock, C.E.A.; et al. Molecular shape-selectivity on MFI zeolite nanosheets in n-decane isomerization and hydrocracking. J. Catal. 2013, 300, 70–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Lou, B.; Yu, R.; Men, Z.; Li, M.; Li, Z. Hydroisomerization of n-decane over micro/mesoporous Pt-containing bifunctional catalysts: Effects of the MCM-41 incorporation with Y zeolite. Fuel 2018, 226, 204–212. [Google Scholar] [CrossRef]
- Huyen, P.T.; Nam, L.T.H.; Vinh, T.Q.; Martinez, C.; Parvulescu, V.I. ZSM-5/SBA-15 versus Al-SBA-15 as supports for the hydrocracking/hydroisomerization of alkanes. Catal. Today 2018, 308, 121–127. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Mouilijn, J.A.; Pérez-Ramirez, J. Desilication: On the controlled generation of mesoporosity in MFI zeolites. J. Mater. Chem. 2006, 16, 2121–2131. [Google Scholar] [CrossRef]
- Martens, J.A.; Verboekend, D.; Thomas, K.; Vanbutsele, G.; Gilson, J.-P.; Perez-Ramirez, J. Hydroisomerization of emerging renewable hydrocarbons using hierarchical Pt/H-ZSM-22 catalyst. ChemSusChem 2013, 6, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Keogh, R.A.; Sparks, D.; Hu, J.; Wender, I.; Tiernye, J.W.; Wang, W.; Davis, B.H. Hydroisomerization and hydrocracking of n-hexadecane over a platinum promoted sulfated zirconia catalyst. Energy Fuels 1994, 8, 755–762. [Google Scholar] [CrossRef]
- Busto, M.; Vera, C.R.; Grau, J.M. Optimal processing conditions for the isomerization-cracking of long-chain n-paraffins to high octane isomerizate gasoline over Pt/SO42−-ZrO2 catalysts. Fuel Process. Technol. 2011, 92, 1675–1684. [Google Scholar] [CrossRef]
- Dhar, A.; Vekariya, R.L.; Sharma, P. Kinetics and mechanistic study of n-alkane hydroisomerization reaction on Pt-loaded γ-alumina catalyst. Petroleum 2017, 3, 489–495. [Google Scholar] [CrossRef]
- Lv, G.; Wang, C.; Chi, K.; Liu, H.; Wang, P.; Ma, H.; Qu, W.; Tian, Z. Effects of Pt site distributions on the catalytic performance of Pt/SAPO-11 for n-dodecane hydroisomerization. Catal. Today 2018, 316, 43–50. [Google Scholar] [CrossRef]
- Comelli, R.A.; Finelli, Z.R.; Vaudagna, S.R.; Figoli, N.S. Hydroisomerization of n-hexane on Pt/SO42-/ZrO2: Effect of total and hydrogen partial pressure. Catal. Lett. 1997, 45, 227–231. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).