1. Introduction
With the speeding up of the industrial process based on the plastic technology, a large amount of plastic waste is directly discharged into the nature, inducing the accumulation of large amounts of toxic organic compounds in our daily lives and bringing with it an enormous threat to human health [
1]. Most of the plastic based organic pollutants are burned for disposal, producing toxic gases. Recycling is applied to some plastics, but the recycling cost is high and requires high technology equipment [
2]. The degradation of the plastic waste has attracted more attention in recent years and most of the studies have focused mainly on biodegradation. However, certain types of plastic materials are biodegradable under both aerobic and anaerobic conditions and the degradation rate of the most used plastics under natural environmental conditions is too low to apply in practice [
3]. Most of the plastics are very difficult to degrade by the ordinary processing methods [
1]. The solid-phase photocatalytic process is an ideal method to degrade plastic waste by using the inexhaustible solar light energy [
1].
The solid-phase photocatalytic degradation process is based on solar energy corresponding to the band energy of the photocatalyst and the following photo-generated electron transfer. There are many kinds of semiconductor photocatalysts and titanium dioxide (TiO
2) is the most commonly used among them because of its high photostability, non-toxicity, low cost, and high activity [
4]. TiO
2 as a photocatalyst has attracted great attention in various fields like air and waste water treatment, hydrogen fuel production, metal anti-corrosion, antibacterial activity, and self-purification. TiO
2 and other semiconductor atoms possess a valence band, which is occupied with stable energy electrons, and a conduction band, which is empty. The band gap energy, which is the energy difference between the top energy state of the valence band and the bottom energy state of the conduction band in semiconductors, is utilized to emit light inside TiO
2 to induce a redox reaction on its surface, which is known as the photocatalytic reaction [
5]. When photons of sun light with energy greater than the band gap of TiO
2 are absorbed by TiO
2 semiconductor, one electron from the valance band rises into the conduction band, generating a photoinduced electron-hole pair. This electron-hole pair transfers to the photocatalyst surface, where it reacts with the surface-absorbed molecules like water and oxygen to form active radicals. These active radicals can degrade the plastic based organic pollutants through oxidation reactions [
2]. Plastic waste is discharged into nature, where it is directly subjected to sunlight in open air. Therefore, it is noteworthy to study the solid-phase photocatalytic degradation of plastics under the sunlight.
The significance of immobilization of the TiO
2 photocatalyst in polymer matrix for the solid-phase photocatalytic degradation of plastics is highlighted by the recent studies. In literature, the photocatalytic degradation of polystyrene [
6,
7], polyaniline [
8], polyvinyl chloride [
9,
10], low-density polyethylene [
2], polyvinyl alcohol [
3], and poly (methyl methacrylate) [
11] was investigated in the presence of TiO
2 nanoparticles. The solid-phase photocatalytic degradation rate of plastics including TiO
2 nanoparticles was much faster than the simple photolysis of pure plastics. Polyvinyl borate is a synthetic polymer prepared through the condensation reaction of polyvinyl alcohol and boric acid. Polyvinyl alcohol is crosslinked with boric acid to improve the thermal and mechanical properties of the polymer. In addition, polyvinyl alcohol is a water-soluble polymer and crosslinking the polymer with boric acid enhances the moisture resistance of the matrix [
12]. A few studies have been performed on crosslinking of polyvinyl alcohol with boric [
4,
12,
13,
14]. In contrast to polyvinyl alcohol, PVB is a non-environmental polymer and it degradation is also significant in terms of human health and the environment. There is no study in the literature on the solid-phase photocatalytic degradation of PVB. In this study, PVB composites were prepared through the condensation reaction of polyvinyl alcohol and boric acid in the presence of TiO
2 nanoparticles. The photocatalytic activity of PVB/TiO
2 composites was evaluated by investigating the solid-phase photocatalytic degradation of the polymer matrix in the ambient air under ultraviolet light irradiation.
2. Results and Discussion
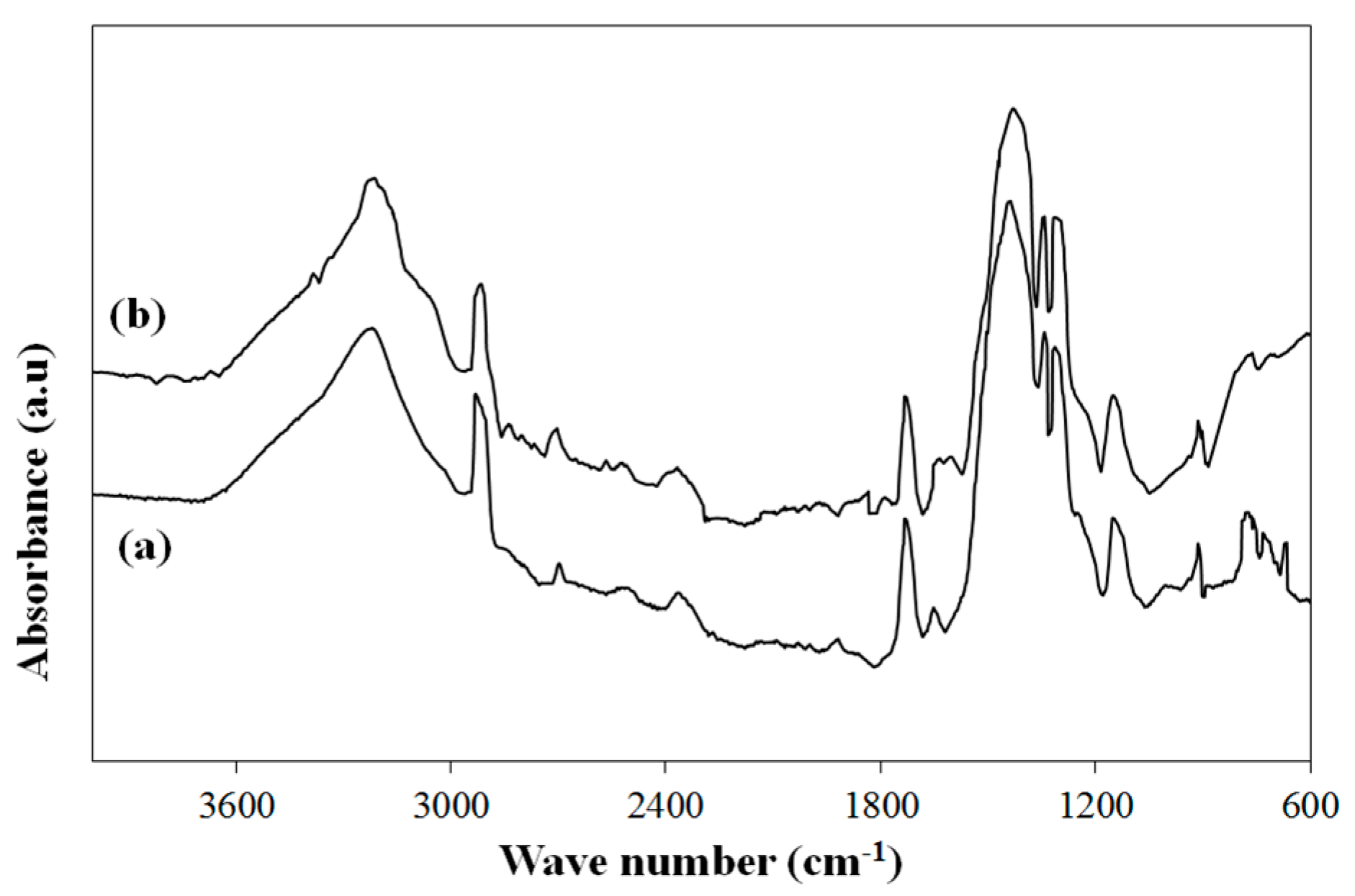
Figure 1 illustrates the FTIR spectra of pure PVA and PVB/TiO
2 composite, including 10 wt.% of the photocatalyst nanoparticles. The broad absorption band around 3200 cm
−1 was related to the O–H group of polyvinyl alcohol, which formed complexes with boron-containing oxyanions during the crosslinking reactions [
13]. On the other hand, this broad band could also be related to the stretching band of O–H, which might be due to unreacted O–H groups of polyvinyl alcohol (
Figure 1a) [
14]. The absorption peaks at 2923 cm
−1 and 1338 cm
−1 are related to the stretching bond of C–H (
Figure 1a) and the peak at 1724 cm
−1 was assigned to the stretching bond of C=O (
Figure 1a) [
14]. The absorption peaks at 1299 cm
−1 and 1133 cm
−1 were assigned to the stretching vibrations of B–O–C bonds. This absorption peak provided strong evidence for the condensation reaction between polyvinyl alcohol and boric acid to synthesize PVB (
Figure 1a) [
14]. In addition, the peak at 1430 cm
−1 was attributed to the stretching vibration of the B–O bond, which might be due to the unreacted boric acid (
Figure 1a).
Figure 1b illustrates FTIR spectrum of the composite. According to
Figure 1a,b, the spectrum of the composite matched with that of the pure polymer. Characteristic absorption peaks of PVB, the stretching vibrations of B–O–C bonds, were also observed on the spectrum of the composite (
Figure 1b). Different from the spectrum of pure PVB, there was a broad absorption band between 600 cm
−1 and 900 cm
−1, which was attributed to Ti–O stretching vibrations (
Figure 1b) [
15]. This absorption band proved the presence of TiO
2 nanoparticles in PVB matrix. The small absorption peak of pure PVB, present at around 1600 cm
−1 and related to physically absorbed moisture, could also be seen on the spectrum of the composite (
Figure 1a,b) [
15]. This peak intensity became wider and increased. Hence, TiO
2 contribution might increase the moisture content of the composite.
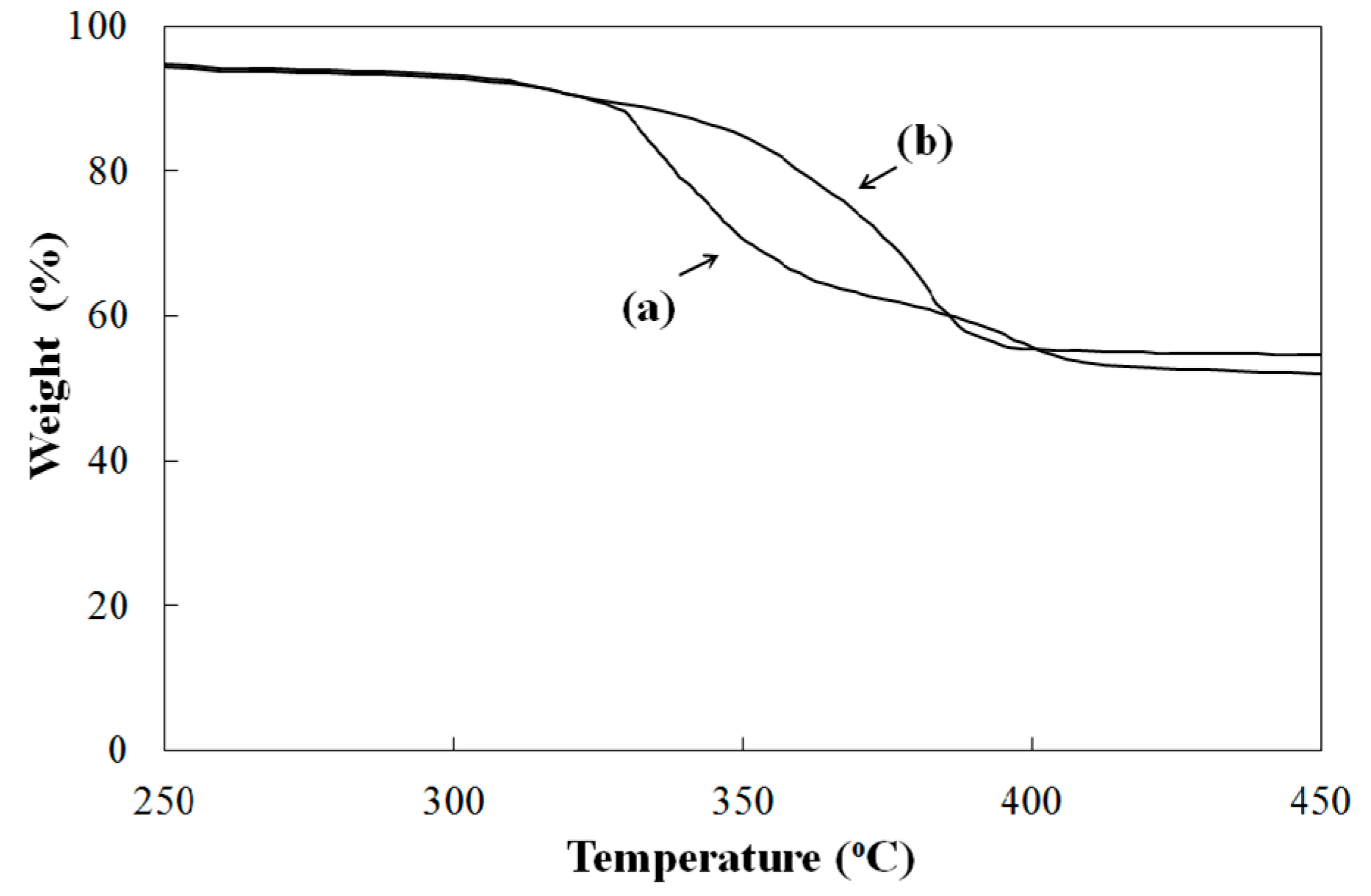
Figure 2 shows TGA curves of pure polymer and the composite, containing 10 wt.% of the photocatalyst nanoparticles. The TGA curve of the pure polymer contains two main degradation steps between 300–400 °C. One of these degradation steps is the large step, corresponding to deacetylation reactions, and the other is the small step, corresponding to chain scission reactions [
12]. TGA curves revealed that the photocatalyst nanoparticles slightly enhanced the thermal stability of PVB. The onset temperature of degradation is higher than that of pure PVB. Pure PVB and PVB/TiO
2 composite exhibited total weight losses of 42% and 39%, respectively, in the temperature range between 250 °C and 450 °C.
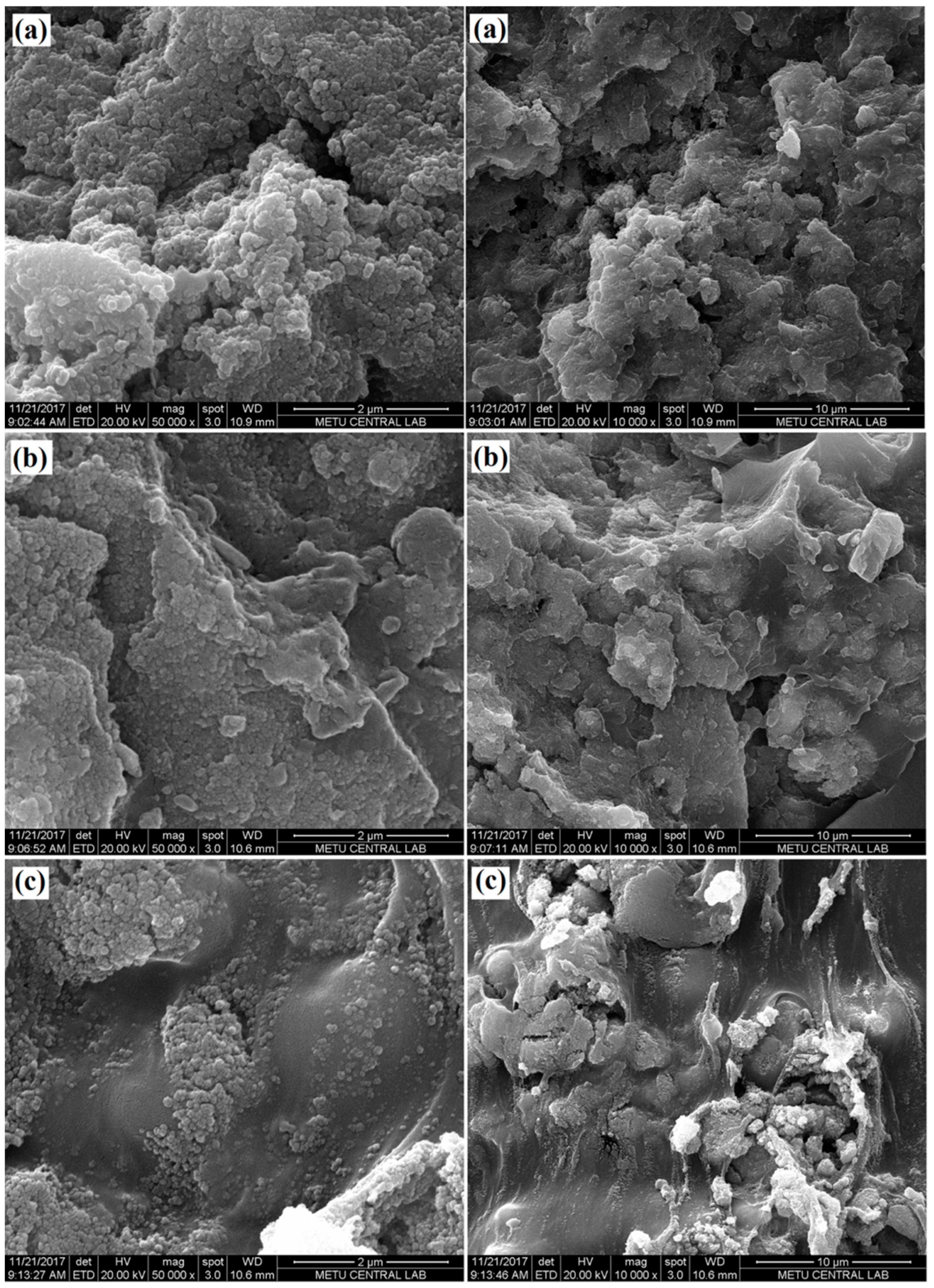
SEM analyses of the composites, containing 5 wt.%, 10 wt.%, and 15 wt.% of TiO
2, respectively, were carried out to visualize the distribution of the photocatalyst nanoparticles in PVB matrix.
Figure 3 illustrates SEM images of the composites. TiO
2 aggregates, which consisted of hundreds of nanoparticles, could be seen on the composite surfaces, which suggested that TiO
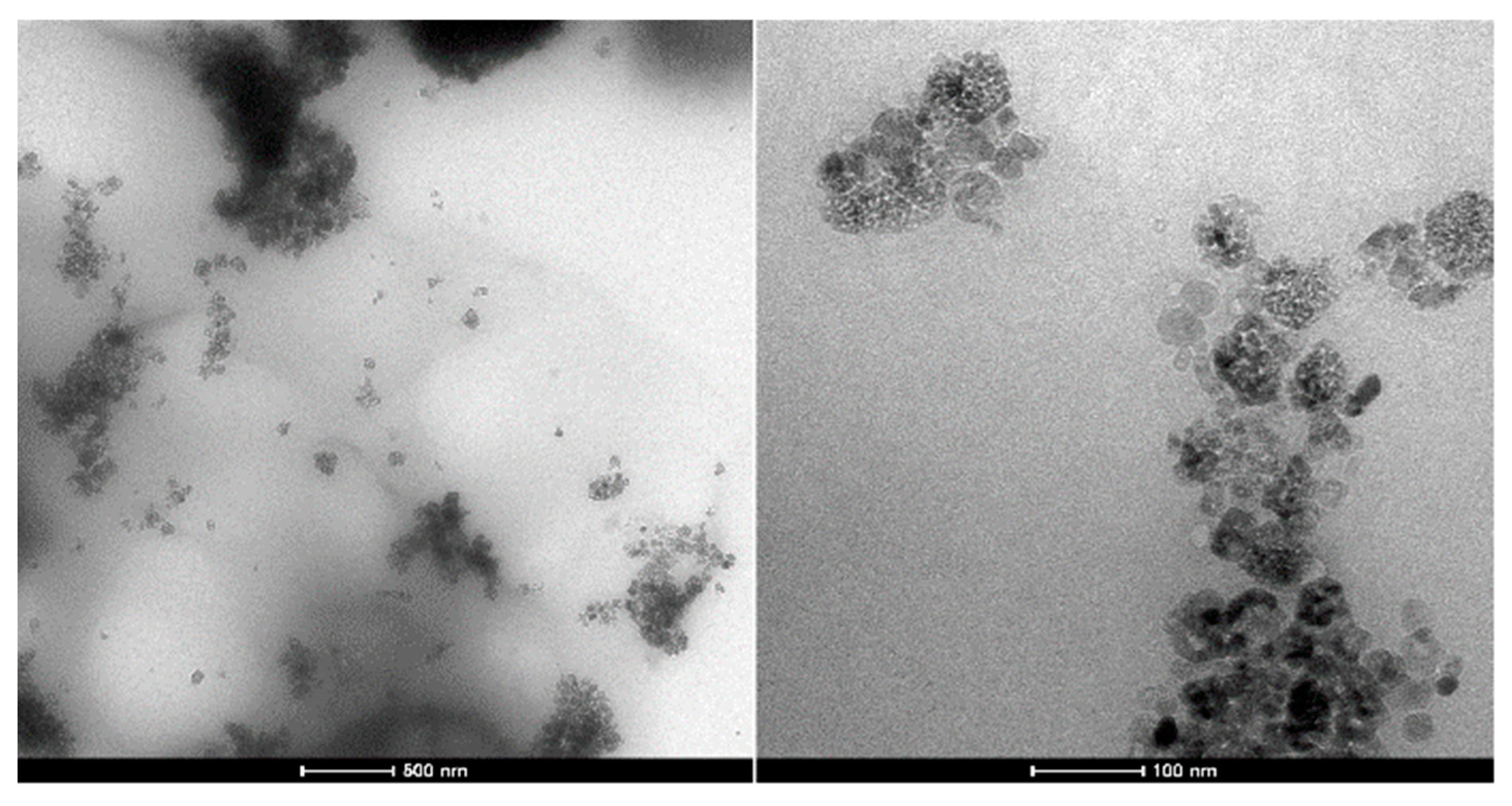
2 nanoparticles tended to agglomerate. During the synthesis of the composite, insufficient mixing might be performed to lead to a partial aggregated morphology. TEM images supported the stated thought (
Figure 4). TiO
2 aggregates were well distributed in the polymer matrix and the size of most aggregates was less than 500 nm. According to SEM and TEM images, the polymer matrix held TiO
2 nanoparticles in intimate contact form, which is also important for enhanced photocatalytic activity.
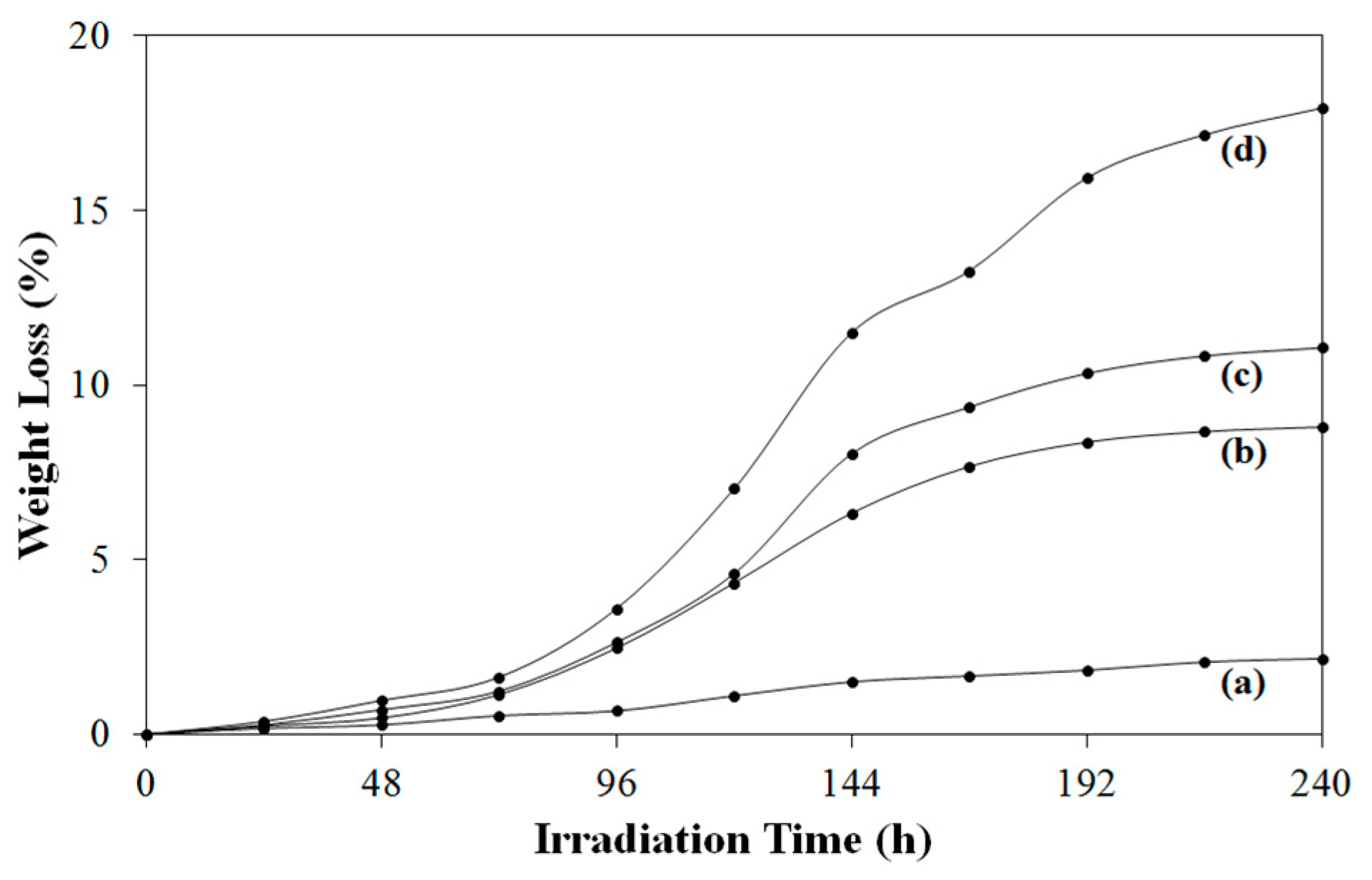
Figure 5 presents the solid-phase photocatalytic degradation tendency of the pure polymer and the composites under air atmosphere. The weight loss rate of the composites was higher than that of PVB. The weight loss of the pure polymer and the composites increased continuously with UV irradiation. Pure PVB resulted in insignificant weight loss, almost 2 wt.%, after 240 h under UV irradiation, which could be ascribed to the boron containing chain structure of the pure polymer [
4]. TGA results also supported the stated thought. Boron containing chain structure might be the reason for enhanced degradation stability and thermal stability. For the composites with 5 wt.%, 10 wt.% and 15 wt.% of TiO
2 nanoparticles, the weight loss values were 8.8 wt.%, 11.2 wt.%, 17.9 wt.%, respectively, after 240 h of UV irradiation. Photoinduced weight loss of the composites increased in parallel with TiO
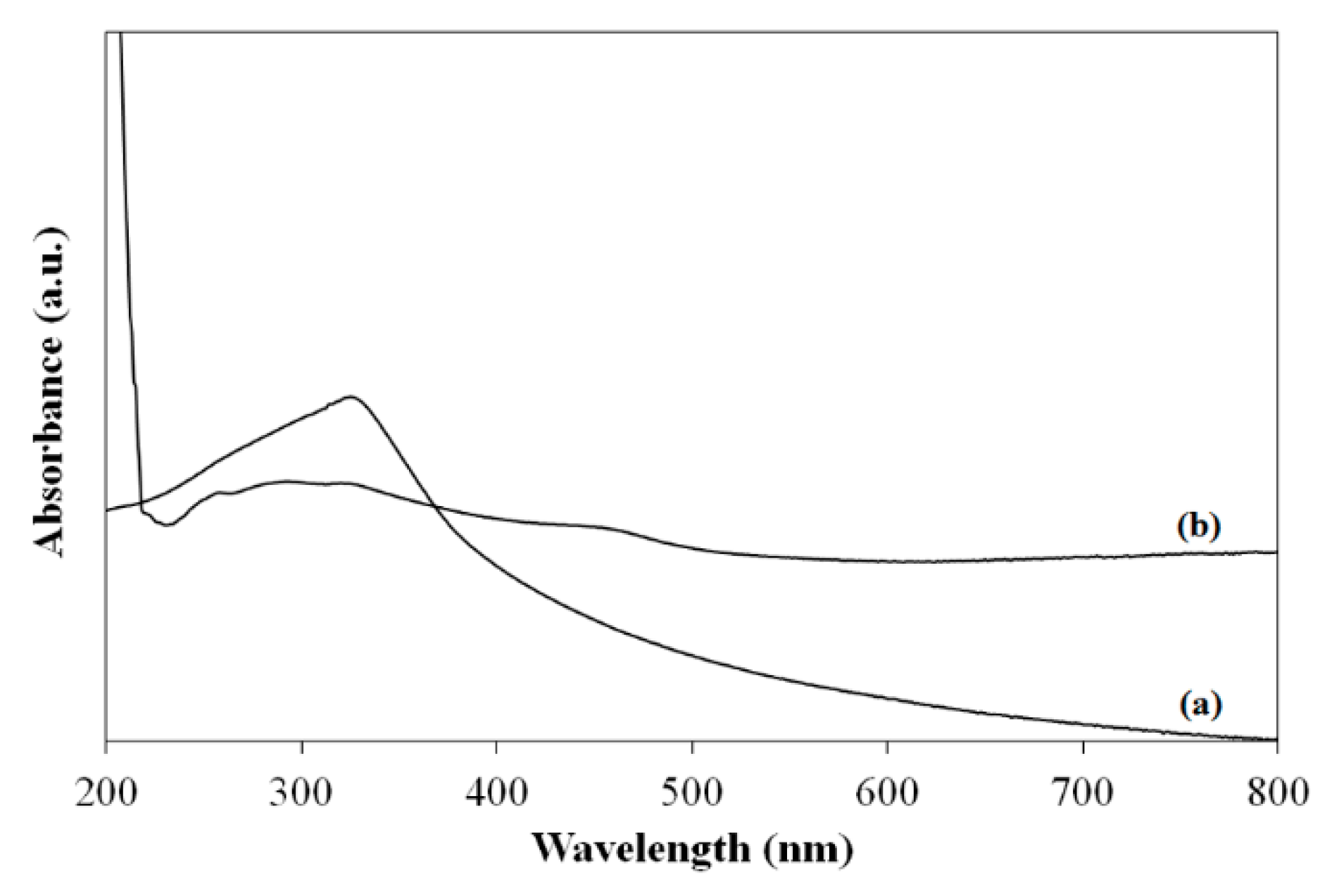
2 content, which demonstrated the effectiveness of the photocatalyst. It was thought that the strong chain structure of PVB lead to the low weight loss values in the composites. UV-Vis spectroscopy was performed to investigate the optical property of TiO
2 and the composite with 10 wt.% of TiO
2 nanoparticles. According to
Figure 6, TiO
2 absorbed the majority of the incoming light between 250 and 350 nm. On the other hand, the composite absorbed the majority of the incoming light below 400 nm. UV-Vis spectrum of pure TiO
2 exhibits a characteristic absorption band at around 322 nm, which was attributed to the characteristic Ti–O–Ti stretching vibrations [
16].
3. Materials and Methods
Polyvinyl alcohol (PVA) with the molecular weight between 89,000 and 98,000 g/mol, boric acid and titanium dioxide (TiO
2, anatase, <25 nm) were obtained from Sigma-Aldrich (Munich, Germany). Pure PVB, which did not contain the photocatalyst nanoparticles, was synthesized through the condensation reaction of PVA and boric acid according to the procedure given in the literature [
4]. In detail, 4.0 g of PVA was dissolved in 100 mL of distilled water and the solution was heated up to 80 °C under stirring. At the same time, 4.0 g of boric acid was dissolved in 100 mL of distilled water and the solution was kept under stirring at room temperature. Afterward, the boric acid solution was fed into the polyvinyl alcohol solution. The mixture was maintained at 80 °C under stirring for half an hour, which resulted in the formation of PVB in gel form. PVB in solid form was obtained after drying the gel polymer in an oven at 120 °C [
4]. PVB/TiO
2 composites, including 5, 10, and 15 wt.% of TiO
2 nanoparticles, were synthesized with the same procedure followed to prepare pure PVB. Different from the given synthesis procedure, the photocatalyst nanoparticles were fed into the PVA solution prior to mixing with the boric acid solution.
Fourier transform infrared (FTIR) spectra of pure polymer and the composite, containing 10 wt.% of TiO2 nanoparticles, were recorded on a Thermo Scientific FTIR Nicolet 380 (Nicolet Thermo Corporation, Edina, MN, USA) in the wavenumber range between 600 and 4000 cm−1. The thermogravimetric analyses (TGA) of the pure polymer and the composite, including 10 wt.% of TiO2, were performed with a Setaram Labsys TGA/DTA thermogravimetric analyzer (Setaram Instrumentation, Ankara, Turkey) under nitrogen atmosphere at the heating rate 5 °C/min. The morphology of the pure polymer and PVB/TiO2 composites, including 5, 10, and 15 wt.% of TiO2, respectively, were studied in a QUANTA 400F model field emission scanning electron microscope (FE-SEM) (Thermo Fisher, Hillsboro, OR, USA). A FEI-Tecnai G2 Spirit Biotwin model conventional transmission electron microscope (CTEM) (Thermo Fisher, Hillsboro, OR, USA) was used for transmission electron microscopy (TEM) analysis of the composite, containing 10 wt.% of TiO2. For this purpose, PVB/TiO2 composite sample was grinded into powder form. Then, the powder sample was dispersed in ethanol and the dispersion was dropped on carbon coated copper grids. The solid-phase photocatalytic degradation of the pure polymer and the composite samples (1.0 g) was carried out in the ambient air using a 30 W UV lamp (254 nm, Philips, Istanbul, Turkey). All samples were weighed before and after UV irradiation to evaluate the weight loss of PVB through the solid-phase photocatalytic degradation. Each photocatalytic degradation experiment used a triplicate set of samples. The UV–Vis absorption spectrum of TiO2 and PVB/TiO2 composite, containing 10 wt.% of TiO2 nanoparticles, were carried out by a Genesys 10S spectrophotometer (Thermo Fisher, Hillsboro, OR, USA) in the wavelength of 200–800 nm.