Effect of H2O and O2 on the Adsorption and Degradation of Acetaldehyde on Anatase Surfaces—An In Situ ATR-FTIR Study
Abstract
1. Introduction
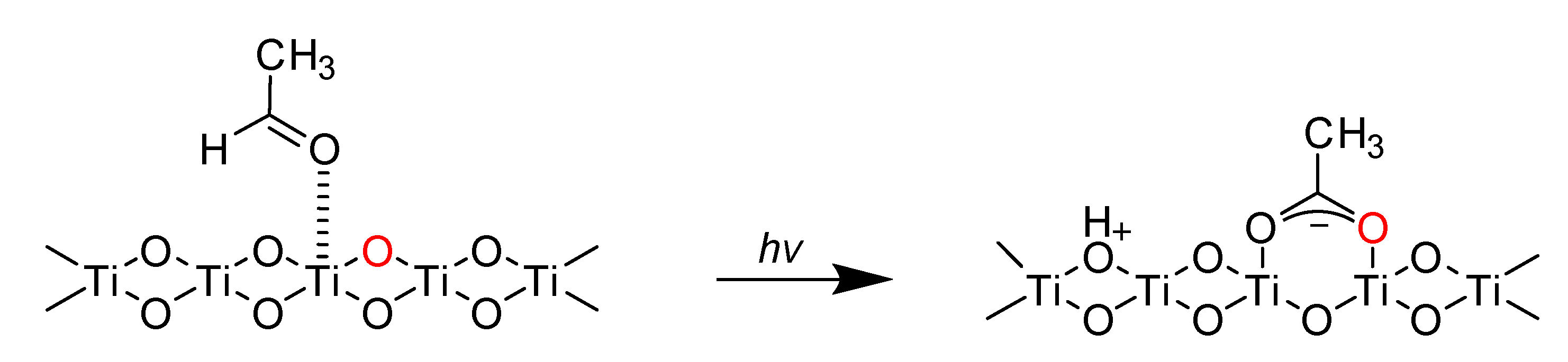
2. Results and Discussion
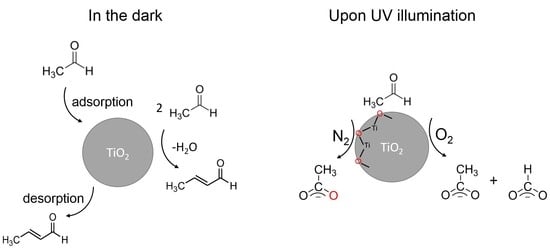
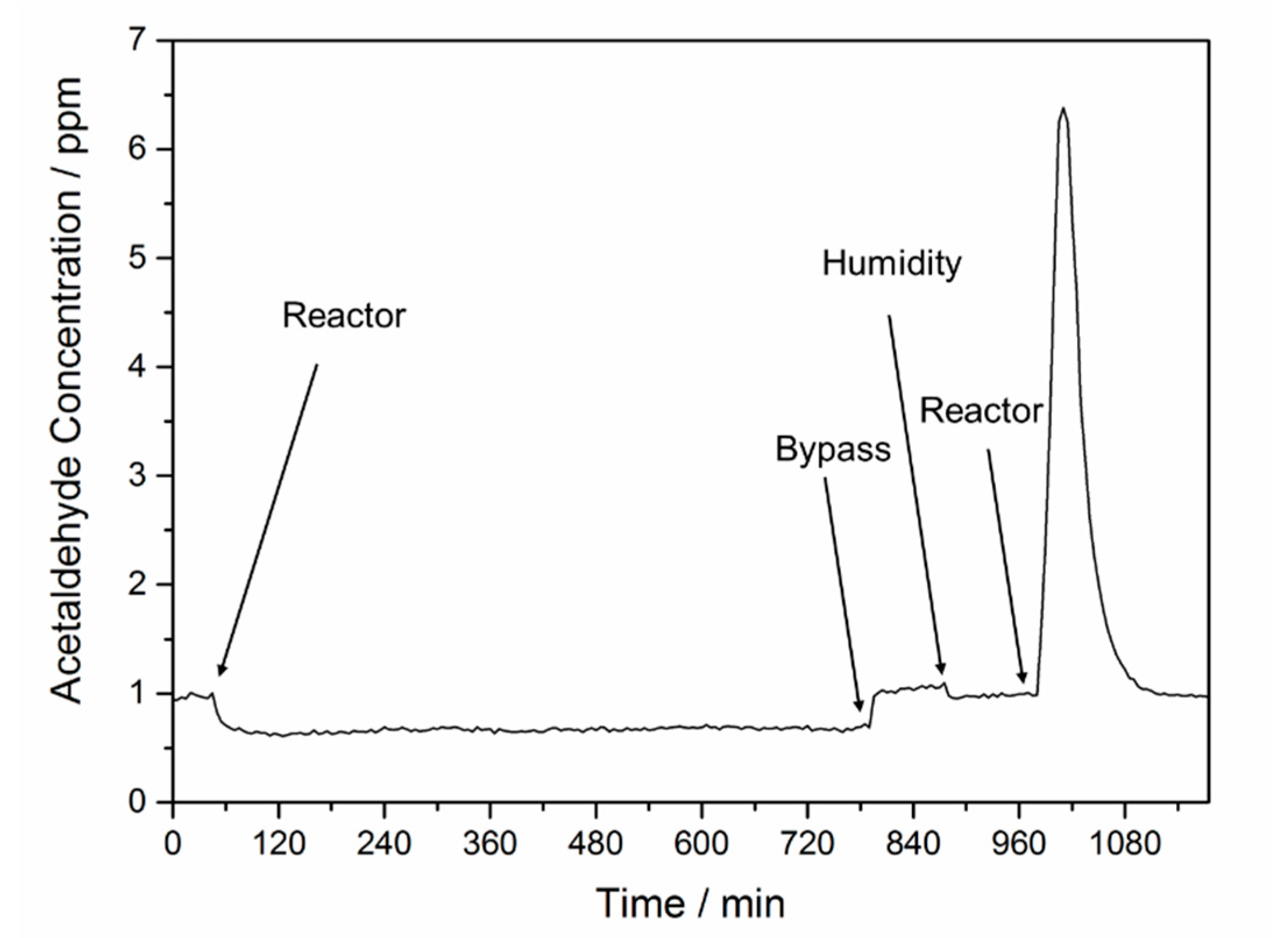
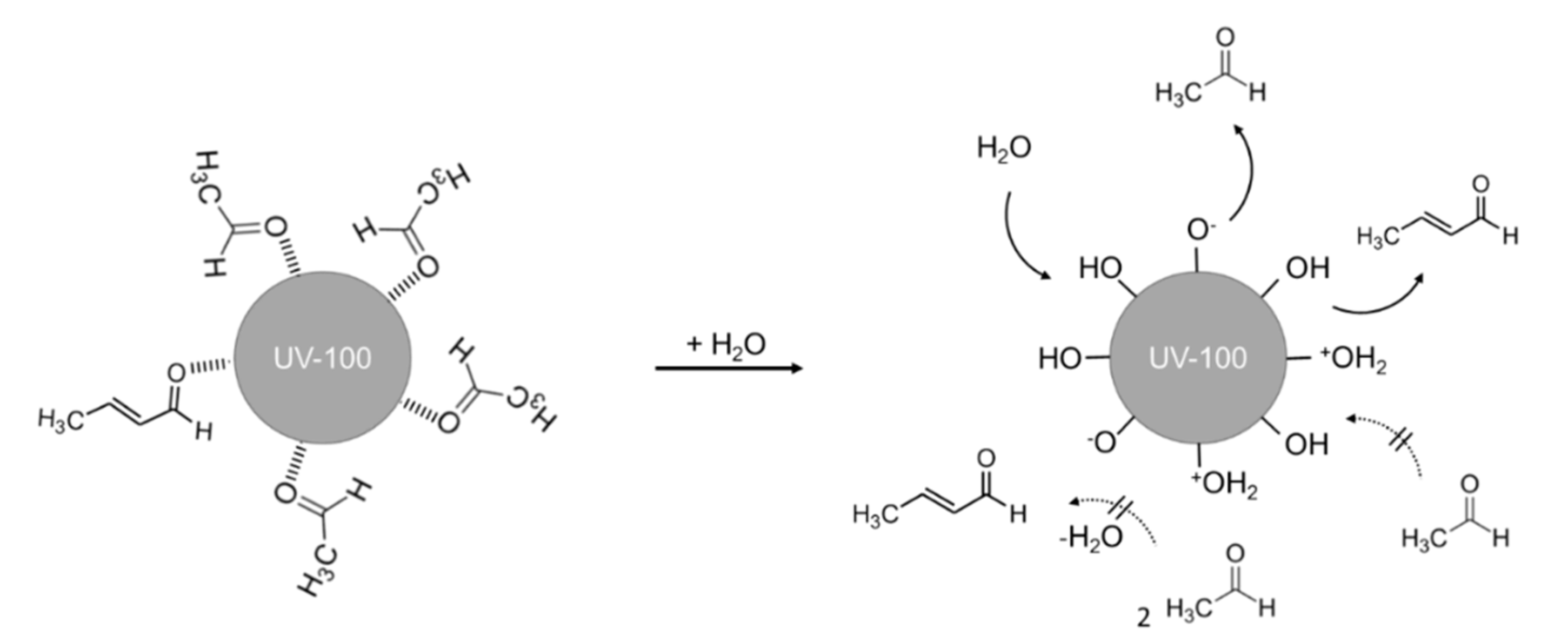
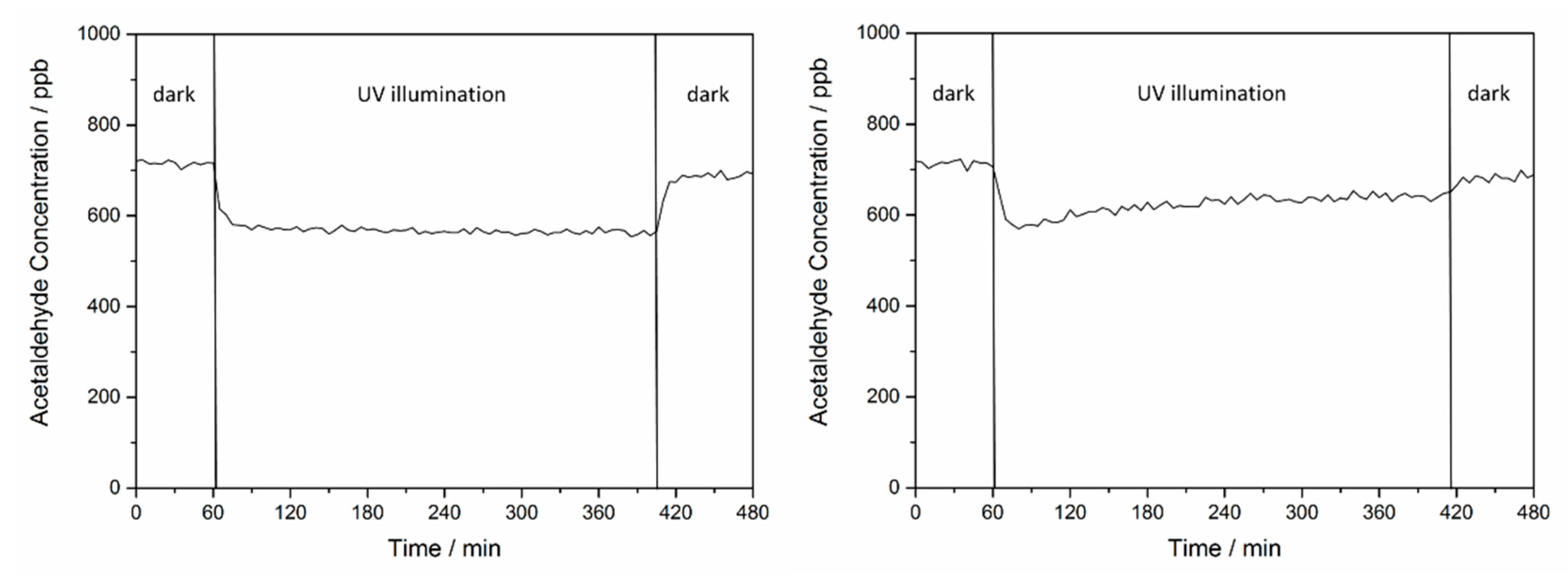
2.1. Effect of H2O on the Acetaldehyde Adsorption
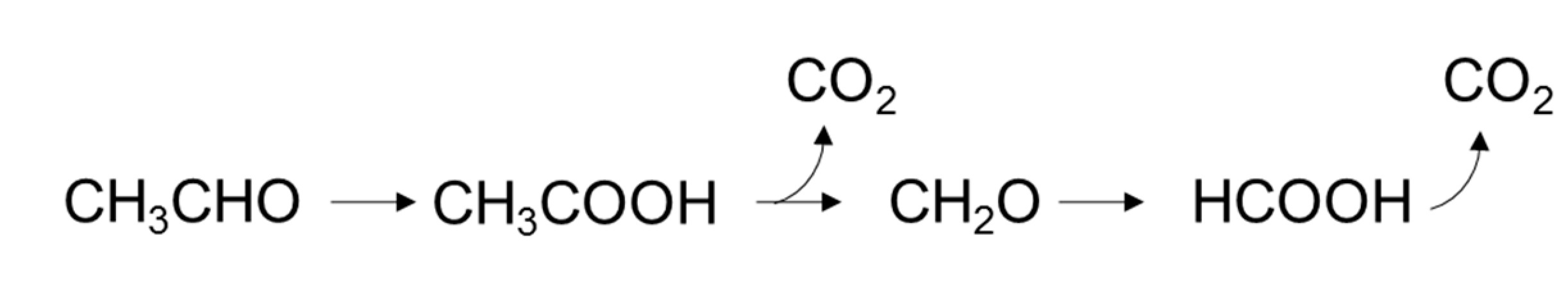
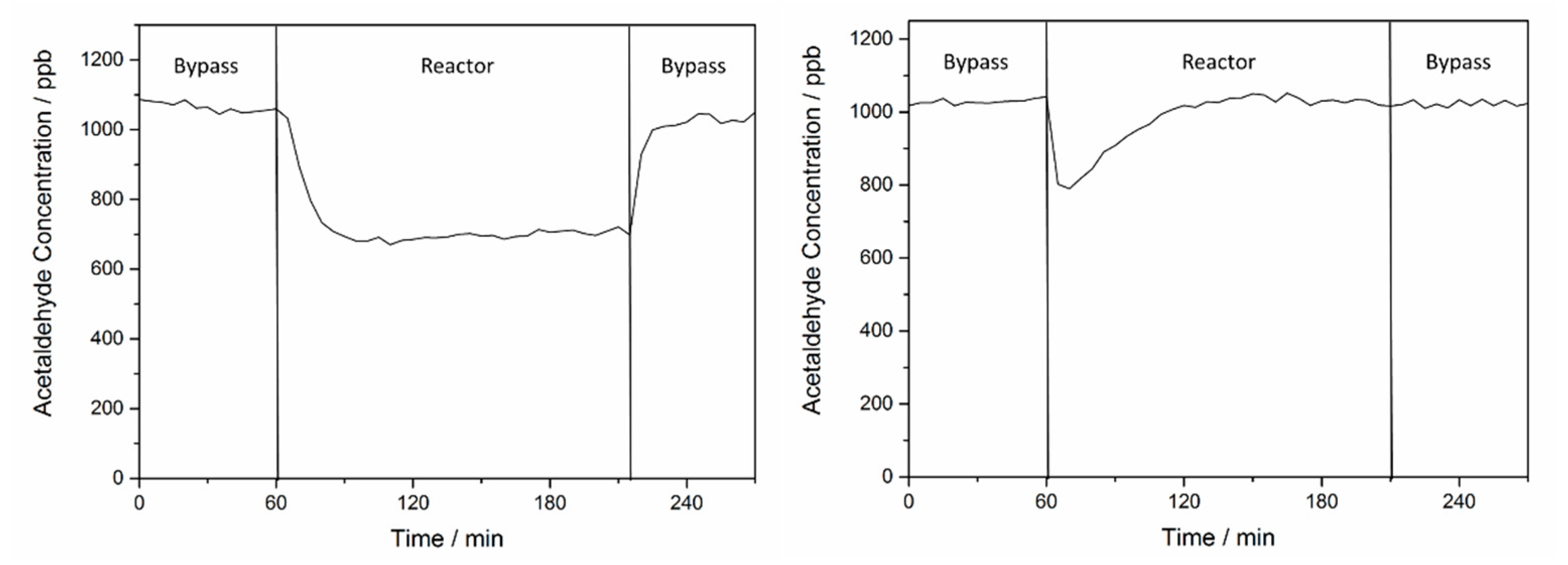
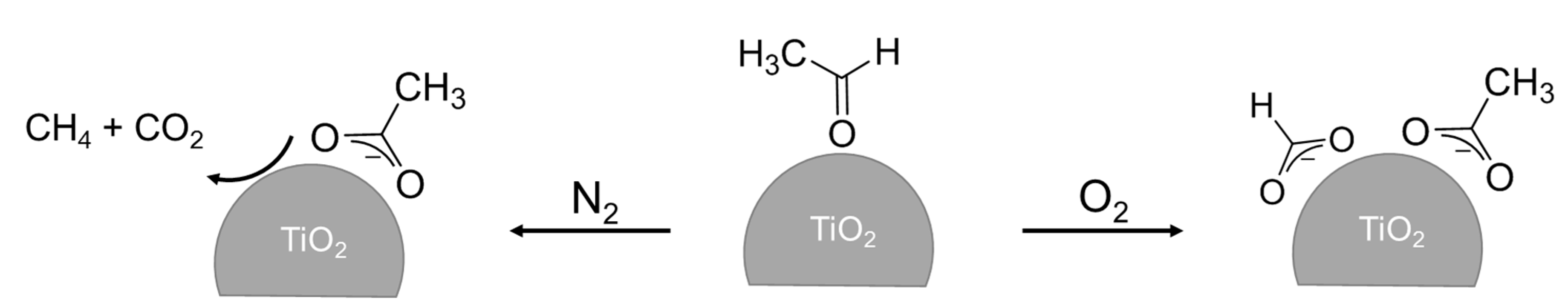
2.2. Effect of O2 on the Adsorption and Degradation of Acetaldehyde
3. Materials and Methods
3.1. Materials
3.2. TiO2 Film Preparation
3.3. ATR-FTIR Spectroscopy
3.4. Acetaldehyde Degradation
3.5. Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Altshuller, A.P. Production of aldehydes as primary emissions and from secondary atmospheric reactions of alkenes and alkanes during the night and early morning hours. Atmos. Environ. Part A Gen. Top. 1993, 27, 21–32. [Google Scholar] [CrossRef]
- European Agency for Safety and Health at Work. European Risk Observatory Report. Exploratory Survey of Occupational Exposure Limits for Carcinogens, Mutagens and Reprotoxic substances at EU Member States Level. 2009. Available online: https://osha.europa.eu/en/publications/reports/548OELs/view (accessed on 1 July 2017).
- Occupational Safety and Health Administration (OSHA). Permissible Exposure Limits, Occupational Safety and Health Administration; OSHA: Washington, DC, USA, 2005. [Google Scholar]
- Sopyan, I.; Watanabe, M.; Murasawa, S.; Hashimoto, K.; Fujishima, A. An efficient TiO2 thin-film photocatalyst: Photocatalytic properties in gas-phase acetaldehyde degradation. J. Photochem. Photobiol. A Chem. 1996, 98, 79–86. [Google Scholar] [CrossRef]
- Atitar, M.F.; Belhadj, H.; Dillert, R.; Bahnemann, D.W. The Relevance of ATR-FTIR Spectroscopy in Semiconductor Photocatalysis. In Emerging Pollutants in the Environment—Current and Further Implications; Larramendy, M.L., Ed.; InTech: London, UK, 2015; pp. 203–229. [Google Scholar]
- Mendive, C.B.; Bredow, T.; Blesa, M.A.; Bahnemann, D.W. ATR-FTIR measurements and quantum chemical calculations concerning the adsorption and photoreaction of oxalic acid on TiO2. Phys. Chem. Chem. Phys. 2006, 8, 3232–3247. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.; Barteau, M.A. Selectivity and mechanism shifts in the reactions of acetaldehyde on oxidized and reduced TiO2(001) surfaces. Catal. Lett. 1996, 40, 147–153. [Google Scholar] [CrossRef]
- Singh, M.; Zhou, N.; Paul, D.K.; Klabunde, K.J. IR spectral evidence of aldol condensation: Acetaldehyde adsorption over TiO2 surface. J. Catal. 2008, 260, 371–379. [Google Scholar] [CrossRef]
- Hauchecorne, B.; Terrens, D.; Verbruggen, S.; Martens, J.A.; Van Langenhove, H.; Demeestere, K.; Lenaerts, S. Elucidating the photocatalytic degradation pathway of acetaldehyde: An FTIR in situ study under atmospheric conditions. Appl. Catal. B Environ. 2011, 106, 630–638. [Google Scholar] [CrossRef]
- Batault, F.; Thevenet, F.; Hequet, V.; Rillard, C.; Le Coq, L.; Locoge, N. Acetaldehyde and acetic acid adsorption on TiO2 under dry and humid conditions. Chem. Eng. J. 2015, 264, 197–210. [Google Scholar] [CrossRef]
- Ohko, Y.; Tryk, D.A.; Hashimoto, K.; Fujishima, A. Autoxidation of Acetaldehyde Initiated by TiO2 Photocatalysis under Weak UV Illumination. J. Phys. Chem. B 1998, 102, 2699–2704. [Google Scholar] [CrossRef]
- Muggli, D.S.; McCue, J.T.; Falconer, J.L. Mechanism of the Photocatalytic Oxidation of Ethanol on TiO2. J. Catal. 1998, 173, 470–483. [Google Scholar] [CrossRef]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair; Schreck, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Topalian, Z.; Stefanov, B.I.; Granqvist, C.G.; Österlund, L. Adsorption and photo-oxidation of acetaldehyde on TiO2 and sulfate-modified TiO2: Studies by in situ FTIR spectroscopy and micro-kinetic modeling. J. Catal. 2013, 307, 265–274. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Topalian, Z.; Granqvist, C.G.; Österlund, L. Acetaldehyde adsorption and condensation on anatase TiO2: Influence of acetaldehyde dimerization. J. Mol. Catal. A Chem. 2014, 381, 77–88. [Google Scholar] [CrossRef]
- János, R.; Kiss, J. Adsorption and surface reactions of acetaldehyde on TiO2, CeO2 and Al2O3. Appl. Catal. A Gen. 2005, 287, 252–260. [Google Scholar]
- Rekoske, J.E.; Barteau, M.A. Competition between Acetaldehyde and Crotonaldehyde during Adsorption and Reaction on Anatase and Rutile Titanium Dioxide. Langmuir 1999, 15, 2061–2070. [Google Scholar] [CrossRef]
- Wu, W.-C.; Yang, S.-J.; Ho, C.-H.; Lin, Y.-S.; Liao, L.-F.; Lin, J.-L. Crotonaldehyde Formation from Decomposition of ICH2CH2OH on Powdered TiO2. J. Phys. Chem. B 2006, 110, 9627–9631. [Google Scholar] [CrossRef] [PubMed]
- Coronado, J.M.; Zorn, M.E.; Tejedor-Tejedor, I.; Anderson, M.A. Photocatalytic oxidation of ketones in the gas phase over TiO2 thin films: A kinetic study on the influence of water vapor. Appl. Catal. B Environ. 2003, 43, 329–344. [Google Scholar] [CrossRef]
- El-Maazawi, M.; Finken, A.N.; Nair, A.B.; Grassian, V.H. Adsorption and Photocatalytic Oxidation of Acetone on TiO2: An in Situ Transmission FT-IR Study. J. Catal. 2000, 191, 138–146. [Google Scholar] [CrossRef]
- Liao, L.-F.; Lien, C.-F.; Lin, J.-L. FTIR study of adsorption and photoreactions of acetic acid on TiO2. Phys. Chem. Chem. Phys. 2001, 3, 3831–3837. [Google Scholar] [CrossRef]
- Busca, G. Infrared studies of the reactive adsorption of organic molecules over metal oxides and of the mechanisms of their heterogeneously-catalyzed oxidation. Catal. Today 1996, 27, 457–496. [Google Scholar] [CrossRef]
- Liao, L.-F.; Wu, W.-C.; Chen, C.-Y.; Lin, J.-L. Photooxidation of Formic Acid vs Formate and Ethanol vs Ethoxy on TiO2 and Effect of Adsorbed Water on the Rates of Formate and Formic Acid Photooxidation. J. Phys. Chem. B 2001, 105, 7678–7685. [Google Scholar] [CrossRef]
- Backes, M.J.; Lukaski, A.C.; Muggli, D.S. Active sites and effects of H2O and temperature on the photocatalytic oxidation of 13C-acetic acid on TiO2. Appl. Catal. B Environ. 2005, 61, 21–35. [Google Scholar] [CrossRef]
- Belhadj, H.; Hakki, A.; Robertson, P.K.J.; Bahnemann, D.W. In situ ATR-FTIR study of H2O and D2O adsorption on TiO2 under UV irradiation. Phys. Chem. Chem. Phys. 2015, 17, 22940–22946. [Google Scholar] [CrossRef] [PubMed]
- Szanyi, J.; Kwak, J.H. Photo-catalytic oxidation of acetone on a TiO2 powder: An in situ FTIR investigation. J. Mol. Catal. A Chem. 2015, 406, 213–223. [Google Scholar] [CrossRef]
- Montoya, J.F.; Ivanova, I.; Dillert, R.; Bahnemann, D.W.; Salvador, P.; Peral, J. Catalytic Role of Surface Oxygens in TiO2 Photooxidation Reactions: Aqueous Benzene Photooxidation with Ti18O2 under Anaerobic Conditions. J. Phys. Chem. Lett. 2013, 4, 1415–1422. [Google Scholar] [CrossRef]
- Hug, S.J.; Sulzberger, B. In situ Fourier Transform Infrared Spectroscopy Evidence for the Formation of Several Different Surface Complexes of Oxalate on TiO2 in the Aqueous Phase. Langmuir 1994, 10, 3587–3597. [Google Scholar] [CrossRef]
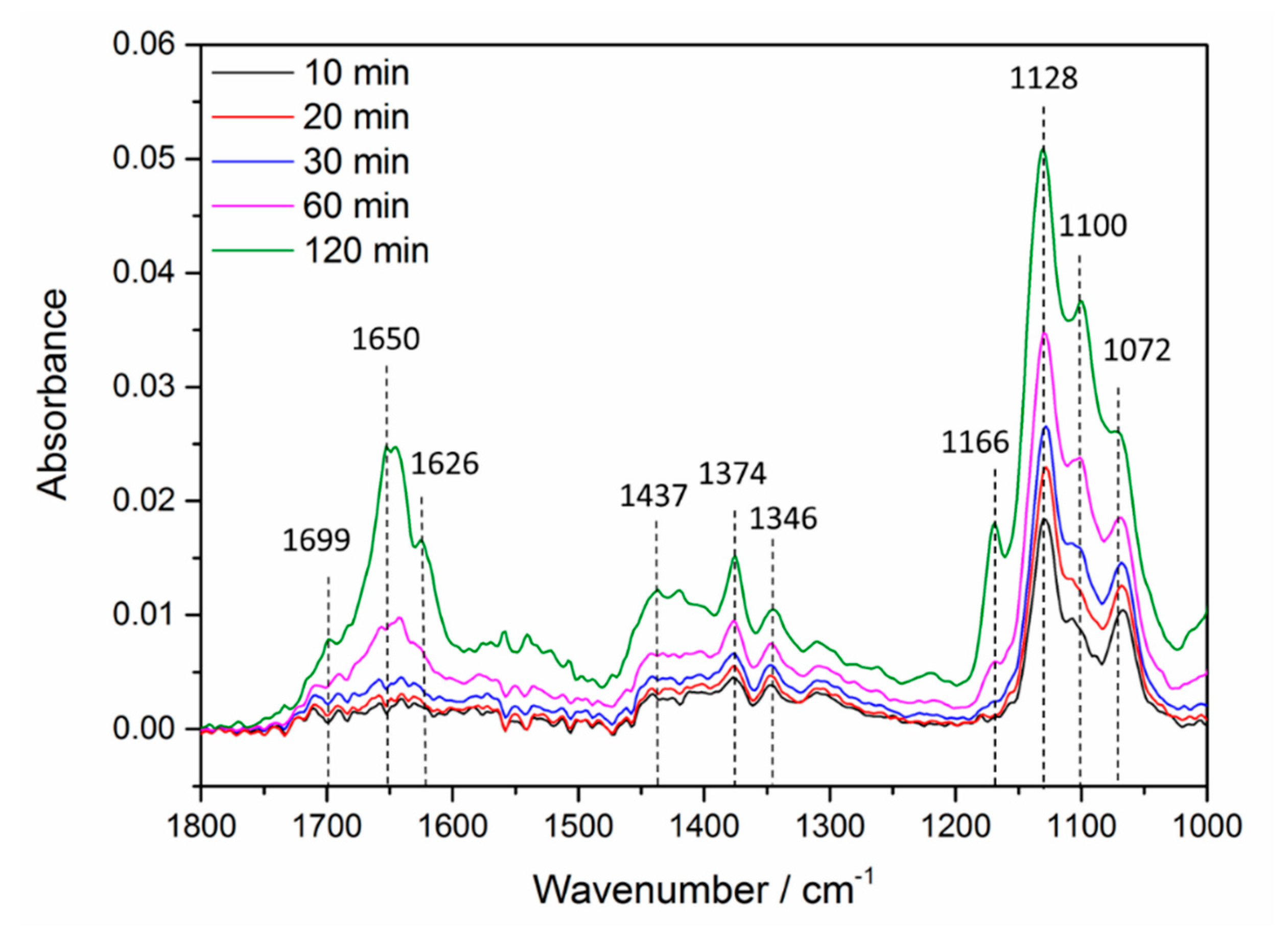



| Position/cm−1 | Assignment [8,17,18] |
|---|---|
| 1072 | ρ(CH3) Ti-O=CHCH3 |
| 1100 | ρ(CH3) Ti-O=CH(CH)2CH3 |
| 1128 | ν(C-C) Ti-O=CHCH3 |
| 1166 | ν(C-C) Ti-O=CH(CH)2CH3 |
| 1346 | δ(CH3) Ti-O=CHCH3 |
| 1374 | δ(CH) Ti-O=CHCH3 |
| 1437 | δ(CH3) Ti-O=CH(CH)2CH3 |
| 1626 | ν(C=C) Ti-O=CH(CH)2CH3 |
| 1650 | ν(C=O) Ti-O=CH(CH)2CH3 |
| 1699 | ν(C=O) Ti-O=CHCH3 |
| Position/cm−1 | Assignment [21,22,23,24,25] |
|---|---|
| 1025 | ρ(CH3) CH3COO− |
| 1051 | ν(C-C) CH3COO− |
| 1340 | νs(COO) HCOO− |
| 1377 | ν(C-H) HCOO− |
| 1411/1417 | δ(CH3) CH3COO− |
| 1436/1444 | νs(COO) CH3COO− |
| 1558/1563 | νas(COO) CH3COO− |
| 1594 | νas(COO) HCOO− |
| 1646 | δ(H2O) |
| 1699 | ν(C=O) CH3COOH |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchers, S.; Schneider, J.; Emeline, A.V.; Bahnemann, D.W. Effect of H2O and O2 on the Adsorption and Degradation of Acetaldehyde on Anatase Surfaces—An In Situ ATR-FTIR Study. Catalysts 2018, 8, 417. https://doi.org/10.3390/catal8100417
Melchers S, Schneider J, Emeline AV, Bahnemann DW. Effect of H2O and O2 on the Adsorption and Degradation of Acetaldehyde on Anatase Surfaces—An In Situ ATR-FTIR Study. Catalysts. 2018; 8(10):417. https://doi.org/10.3390/catal8100417
Chicago/Turabian StyleMelchers, Stephanie, Jenny Schneider, Alexei V. Emeline, and Detlef W. Bahnemann. 2018. "Effect of H2O and O2 on the Adsorption and Degradation of Acetaldehyde on Anatase Surfaces—An In Situ ATR-FTIR Study" Catalysts 8, no. 10: 417. https://doi.org/10.3390/catal8100417
APA StyleMelchers, S., Schneider, J., Emeline, A. V., & Bahnemann, D. W. (2018). Effect of H2O and O2 on the Adsorption and Degradation of Acetaldehyde on Anatase Surfaces—An In Situ ATR-FTIR Study. Catalysts, 8(10), 417. https://doi.org/10.3390/catal8100417