Catalytic Activity of Sulfated and Phosphated Catalysts towards the Synthesis of Substituted Coumarin
Abstract
:1. Introduction
2. Results and Discussion
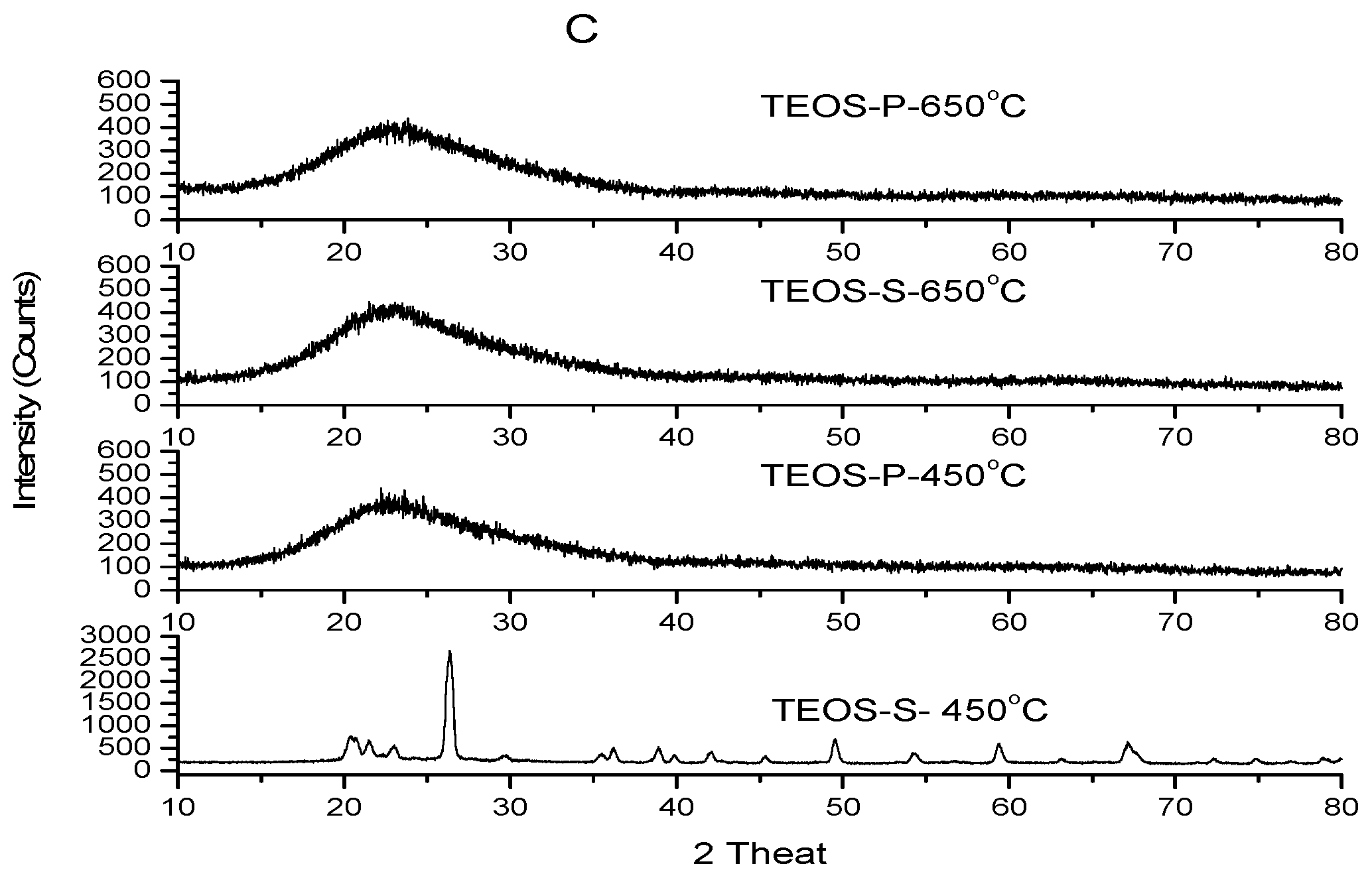
2.1. Synthesis and X-ray Diffraction Patterns (XRD)
2.2. FTIR Spectra of Samples
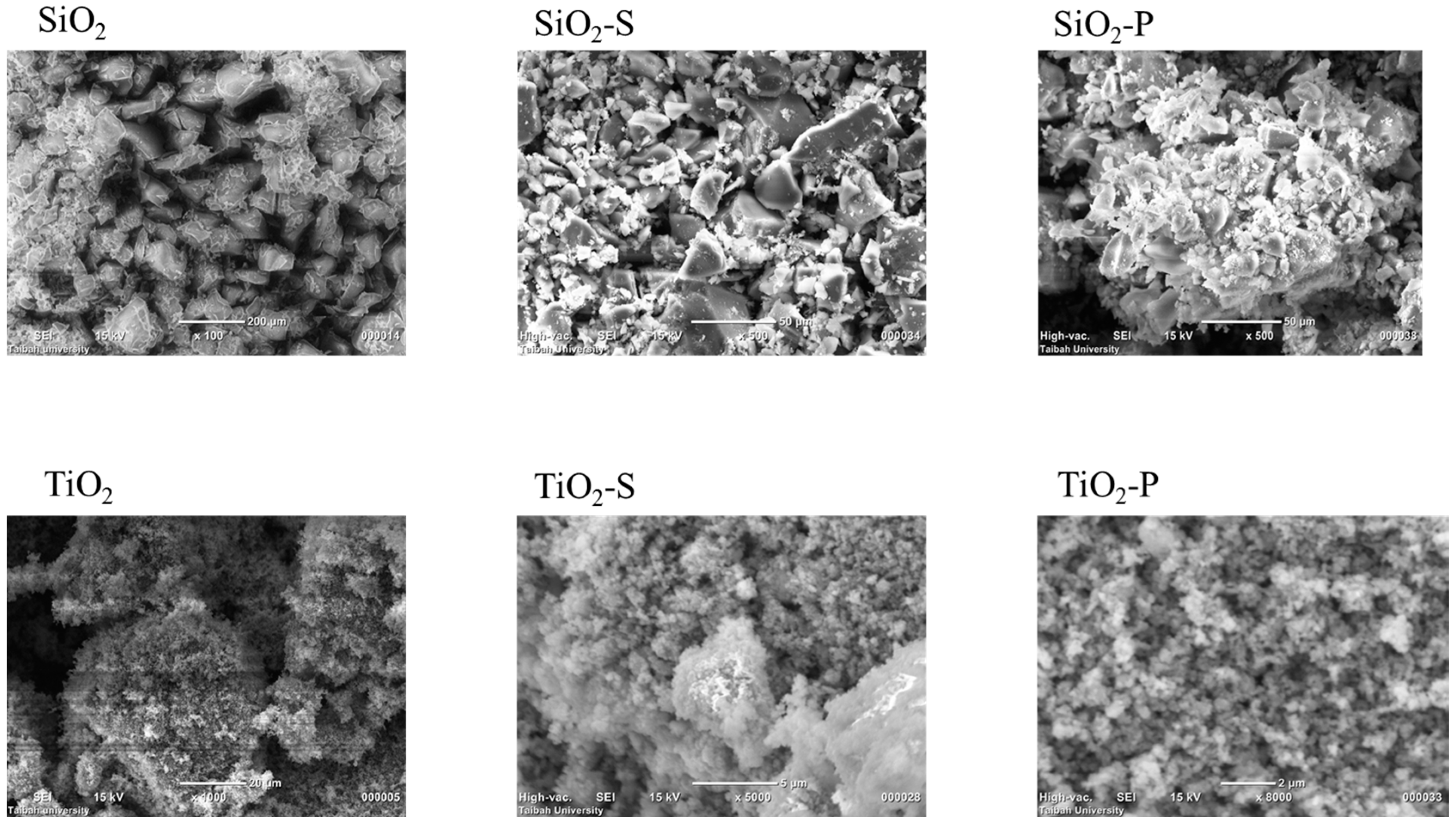
2.3. SEM of Catalysts
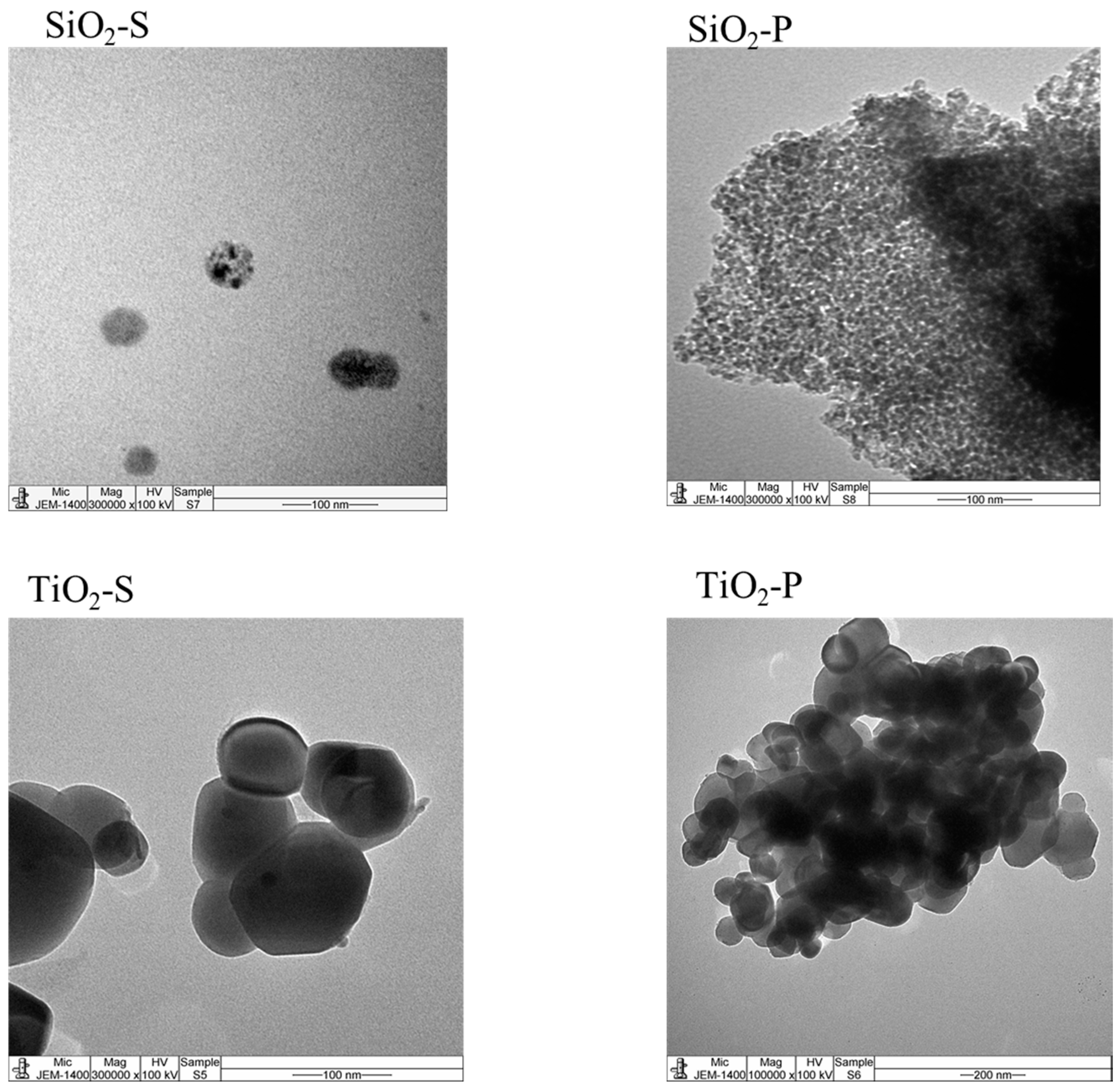
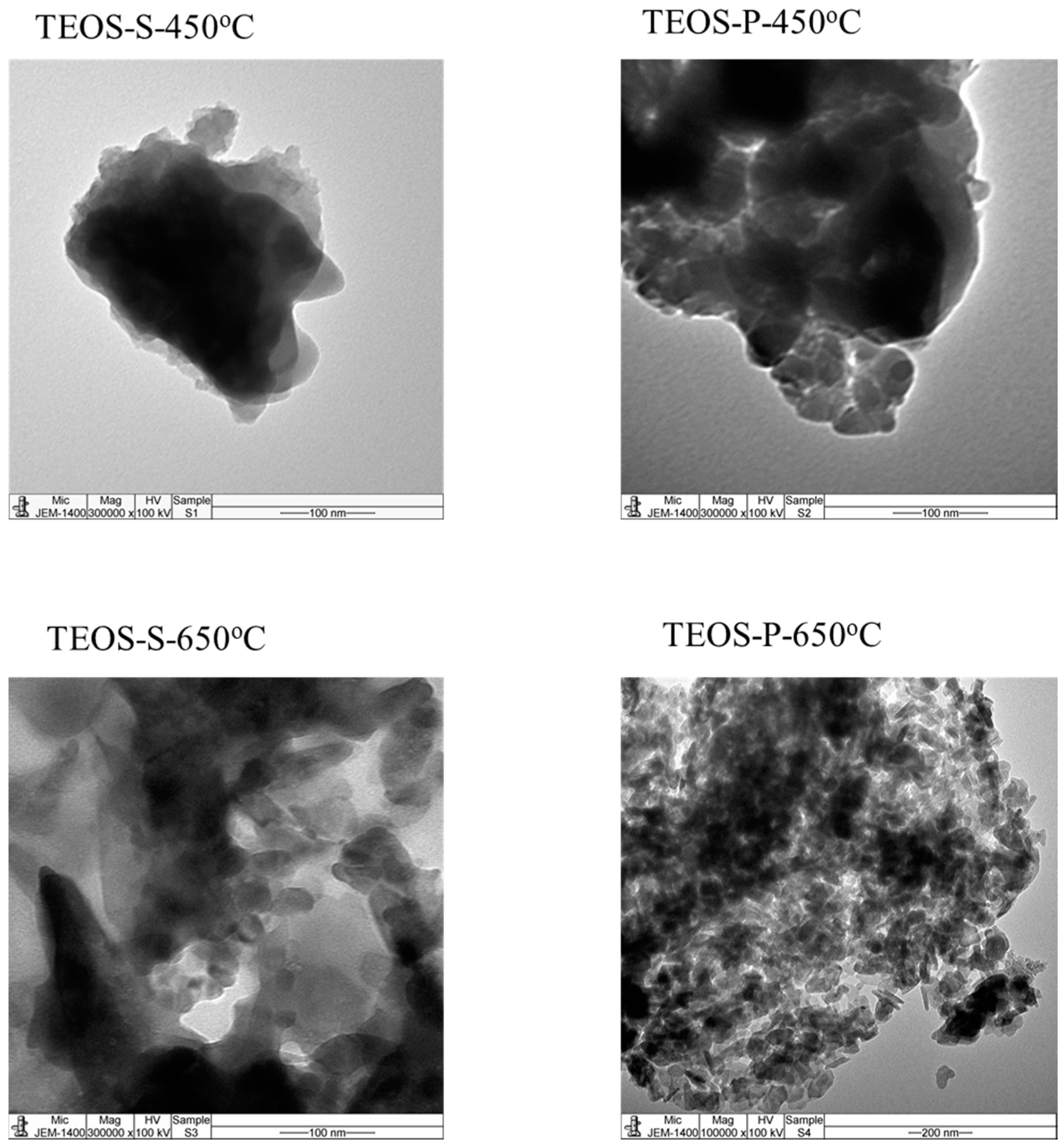
2.4. TEM of Catalysts
2.5. Surface Area Measurements of the Catalysts
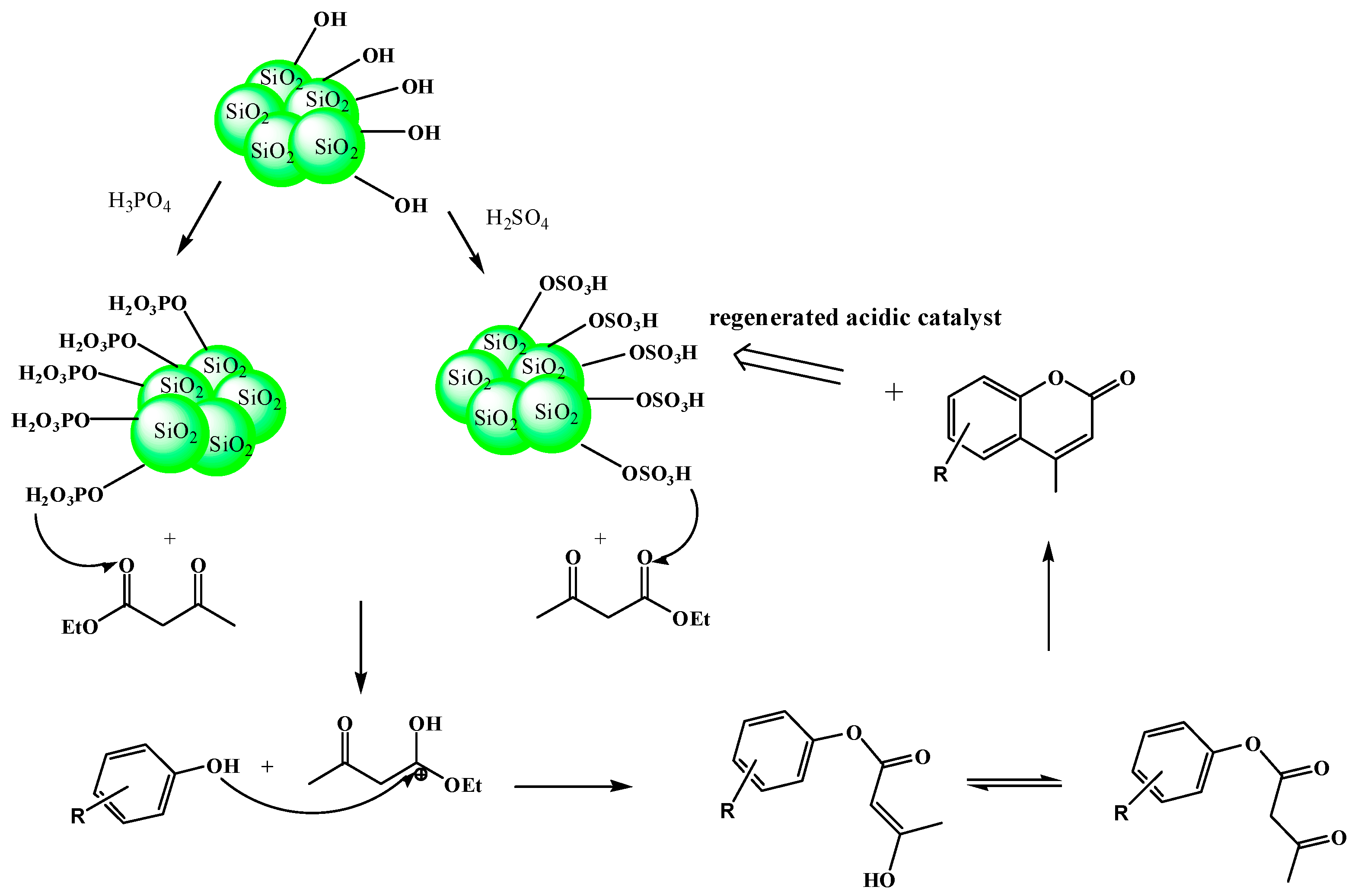
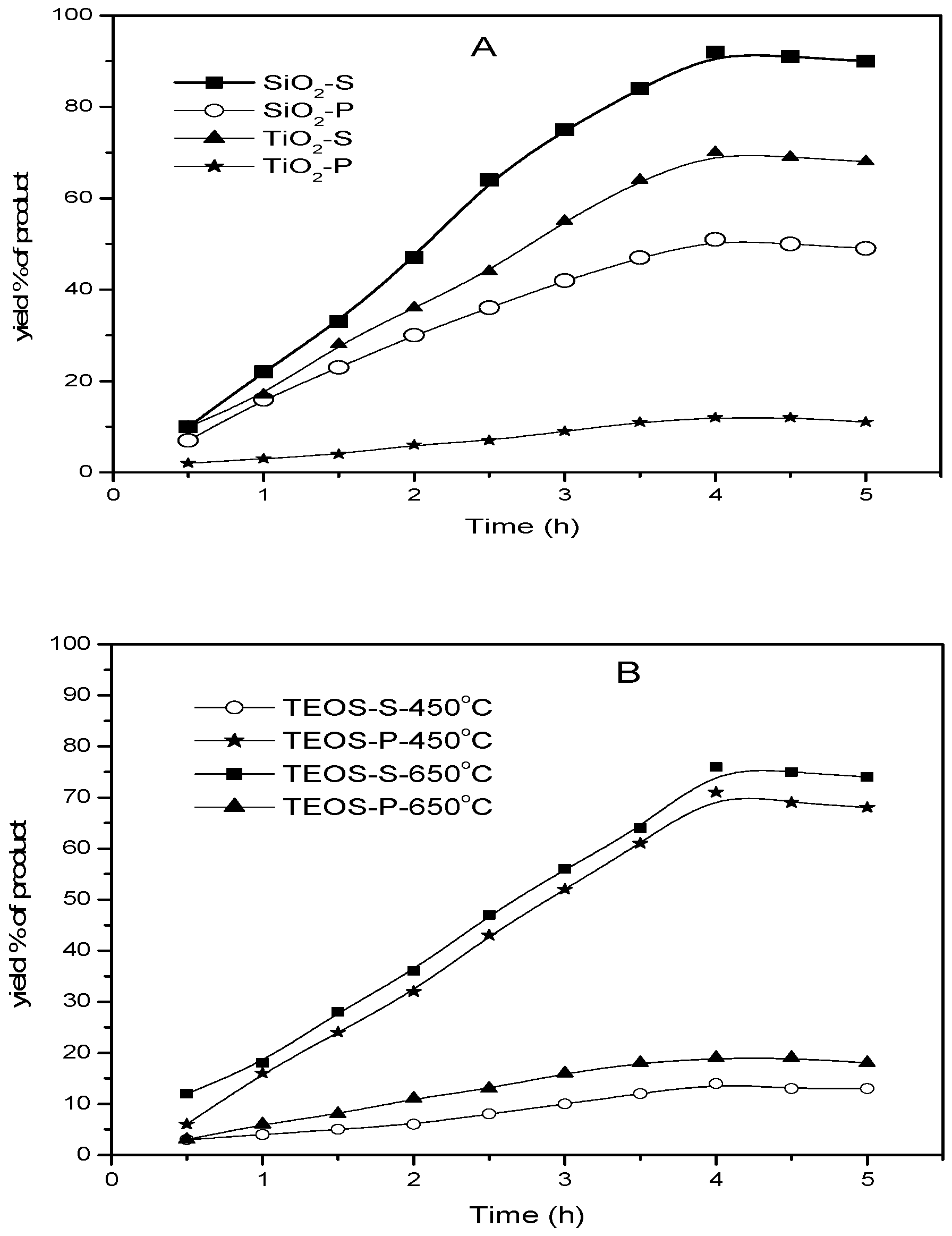
2.6. Catalytic Activity of the Catalysts
3. Experimental
3.1. Materials
3.2. Catalysts Preparation
3.2.1. Sulfated Catalysts
3.2.2. Phosphated Catalysts
3.2.3. Sulfated and/or Phosphated TEOS
3.3. Catalyst Characterization
3.4. Capacity of Catalyst Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sarvari, H.-M.; Sodagar, E.; Doroodmand, M.M. Nano sulfated titania acid catalyst in direct synthesis of fatty acid amides. J. Org. Chem. 2011, 76, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Khan, S.B.; Asiri, A.M.; Ahmad, I. Zirconia-based catalyst for the one-pot synthesis of coumarin through Pechmann reaction. Nanoscale Res. Lett. 2016, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, J. A deactivation mechanism of sulfated titania in the esterification of acetic acid and n-butanol. React. Kinet. Mech. Catal. 2014, 11, 215–233. [Google Scholar] [CrossRef]
- Goswami, P. Dually activated Organo- and Nano-cocatalyzed synthesis of coumarin derivatives. Synth. Commun. 2009, 39, 2271–2278. [Google Scholar] [CrossRef]
- Bahekar, S.S.; Shinde, D.B. Samarium(III) catalyzed one-pot construction of coumarins. Tetrahedron Lett. 2004, 45, 7999–8001. [Google Scholar] [CrossRef]
- Kumar, B.V.; Naik, H.S.B.; Girija, D. ZnO nanoparticle as catalyst for efficient green one-pot synthesis of coumarins through Knoevenagel condensation. J. Chem. Sci. 2011, 123, 615–621. [Google Scholar] [CrossRef]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- O’kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications, and Mode of Action; Wiley and Sons: Chichester, UK, 1997. [Google Scholar]
- Gunnewegh, E.A.; Hoefnagel, A.J.; van Bekkum, H. Zeolite catalysed synthesis of coumarin derivatives. J. Mol. Catal. A Chem. 1995, 100, 87–92. [Google Scholar] [CrossRef]
- Bogdal, D. Coumarins: Fast Synthesis by Knoevenagel Condensation under Microwave Irradiation. J. Chem. Res. 1998, 468–469. [Google Scholar] [CrossRef]
- Zahradnik, M. The Production and Application of Fluorescent Brightening Agents; John Wiley and Sons: New York, NY, USA, 1992. [Google Scholar]
- Singer, L.A.; Long, N.P. Vinyl Radicals. Stereoselectivity in Hydrogen Atom Transfer to Equilibrated Isomeric Vinyl Radicals. J. Am. Chem. Soc. 1966, 88, 5213–5219. [Google Scholar] [CrossRef]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Developmental effects of coumarin and the anticoagulant coumarin derivative warfarin on zebrafish (Danio rerio) embryos. Reprod. Toxicol. 2012, 33, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kolodziej, H. Antibacterial Activity of Extracts and Constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med. 1997, 63, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Maly, D.J.; Leonetti, F.; Backes, B.J.; Dauber, D.S.; Harris, J.L.; Craik, C.S.; Ellman, J.A. Expedient Solid-Phase Synthesis of Fluorogenic Protease Substrates Using the 7-Amino-4-carbamoylmethylcoumarin (ACC) Fluorophore. J. Org. Chem. 2002, 67, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Varu, B.; Motohashi, N.; Tani, S.; Saito, S.; Debnath, S.; Mahapatra, S.; Dastidar, S.G.; Chakrabarty, A.N. Antimicrobial activity of new coumarin derivatives. Arzneimittelforschung 2001, 51, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Garazd, M.M.; Muzychka, O.V.; Vovk, A.I.; Nagorichna, I.V.; Ogorodniichuk, A.S. Modified coumarins. 27. Synthesis and antioxidant activity of 3-substituted 5, 7-dihydroxy-4-methylcoumarins. Chem. Nat. Compd. 2007, 43, 19–23. [Google Scholar] [CrossRef]
- Yun, B.S.; Lee, I.K.; Ryoo, I.J.; Yoo, I.D.J. Coumarins with Monoamine Oxidase Inhibitory Activity and Antioxidative Coumarino-lignans from Hibiscus syriacus. Nat. Prod. 2001, 64, 1238–1240. [Google Scholar] [CrossRef]
- Tyagi, Y.K.; Kumar, A.; Raj, H.G.; Vohra, P.; Gupta, G.; Kumari, R.; Kumar, P.; Gupta, R.K. Synthesis of novel amino and acetyl amino-4-methylcoumarins and evaluation of their antioxidant activity. Eur. J. Med. Chem. 2005, 40, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.F.; Ishikawa, A.; Ono, Y.; Arrhenius, T.; Nadzan, A. Novel chromene derivatives as TNF-α inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 3647–3650. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J.H. Design and Therapeutic Application of Matrix Metalloproteinase Inhibitors. Chem. Rev. 1999, 99, 2735–2776. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saini, A.; Sandhu, J.S. LiBr-Mediated, solvent free von Pechmann reaction: Facile and efficient method for the synthesis of 2H-chromen-2-ones. Arkivoc 2007, 15, 18–23. [Google Scholar] [CrossRef]
- Kumar, V.; Tomar, S.; Patel, R.; Yousaf, A.; Parmar, V.S.; Malhotra, S.V. FeCl3-Catalyzed Pechmann Synthesis of Coumarins in Ionic Liquids. Synth. Commun. 2008, 38, 2646–2654. [Google Scholar] [CrossRef]
- Reddy, Y.T.; Sonar, V.N.; Crooks, P.A.; Dasari, P.K.; Reddy, P.N.; Rajitha, B. Ceric Ammonium Nitrate (CAN): An Efficient Catalyst for the Coumarin Synthesis via Pechmann Condensation using Conventional Heating and Microwave Irradiation. Synth. Commun. 2008, 38, 2082–2088. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Sutradhar, N.; Pahari, S.; Bajaj, H.C.; Panda, A.B. Mesoporous zirconium phosphate: An efficient catalyst for the synthesis of coumarin derivatives through Pechmann condensation reaction. Appl. Catal. A 2011, 394, 93–100. [Google Scholar] [CrossRef]
- Perkin, W.H. III-On Propionic Coumarin and Some of its Derivatives. J. Chem. Soc. 1875, 28, 10–15. [Google Scholar] [CrossRef]
- Maheswara, M.; Siddaiah, V.; Lakishmi, G.; Yerra, V.D.; Rao, K.; Rao, C.V. A solvent-free synthesis of coumarins via Pechmann condensation using heterogeneous catalyst. J. Mol. Catal. A Chem. 2006, 255, 49–52. [Google Scholar] [CrossRef]
- Amoozadeh, A.; Ahmadzadeh, M.; Kolvari, E. Easy Access to Coumarin Derivatives Using Alumina Sulfuric Acid as an Efficient and Reusable Catalyst under Solvent-Free Conditions. J. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Yavari, I.; Hekmat-Shoar, R.; Zonouzi, A. A new and efficient route to 4-carboxymethyl coumarins mediated by vinyl triphenyl phosphonium salt. Tetrahedron Lett. 1998, 39, 2391–2392. [Google Scholar] [CrossRef]
- Narasimhan, N.S.; Mali, F.S.; Barve, M.V. Synthetic Application of Lithiation Reactions; Part XIII. Synthesis of 3-Phenylcoumarins and Their Benzo Derivatives. Synthesis 1979, 39, 906–909. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Kumar, P. A novel synthesis of coumarins employing triphenyl(α-carboxymethylene) phosphorane imidazolide as a C-2 synthon. Tetrahedron Lett. 2009, 50, 236–238. [Google Scholar] [CrossRef]
- Cairns, N.; Harwood, L.M.; David, P.; Astles, D.P. Tandem thermal Claisen–Cope rearrangements of coumarate derivatives. Total syntheses of the naturally occurring coumarins: Suberosin, demethylsuberosin, ostruthin, balsamiferone and gravelliferone. J. Chem. Soc. Perkin Trans. 1994, 1, 3101–3107. [Google Scholar] [CrossRef]
- Karimi, B.; Zareyee, D. Design of a Highly Efficient and Water-Tolerant Sulfonic Acid Nanoreactor Based on Tunable Ordered Porous Silica for the von Pechmann Reaction. Org. Lett. 2008, 10, 3989–3992. [Google Scholar] [CrossRef] [PubMed]
- Verdía, P.; Santamarta, F.; Tojo, E. Knoevenagel Reaction in [MMIm] [MSO4]: Synthesis of Coumarins. Molecules 2011, 16, 4379–4388. [Google Scholar] [CrossRef] [PubMed]
- Khoshkholgh, M.J.; Lotfi, M.; Balalaie, S.; Rominger, F. Efficient synthesis of pyrano[2,3-c] coumarins via intramolecular domino Knoevenagel Hetero-Diels–Alder reactions. Tetrahedron 2009, 65, 4228–4234. [Google Scholar] [CrossRef]
- Essien, E.R.; Olaniyi, O.A.; Adams, L.A.; Shaibu, R.O. Sol-Gel-Derived Porous Silica: Economic Synthesis and Characterization. J. Miner. Mater. Charact. Eng. 2012, 11, 976–981. [Google Scholar] [CrossRef]
- Kalita, P.; Kumar, R. Solvent-free coumarin synthesis via Pechmann reaction using solid catalysts. Microporous Mesoporous Mater. 2012, 149, 1–9. [Google Scholar] [CrossRef]
- Ghosh, A. An Efficient and Simple Procedure for the Synthesis of 4-Substituted Coumarins by von-Pechmann Reaction using Mixture of FeCl3 and Tetrabutylammonium bromide as Catalyst. Int. J. Sci. Res. 2016, 5, 974–976. [Google Scholar] [CrossRef]
- Sethna, S.M.; Shah, N.M.; Shah, R.C. Aluminium chloride, a new reagent for the condensation of β-ketonic esters with phenols. Part I. The condensations of methyl β-resorcylate, β-resorcylic acid, and resacetophenone with ethyl acetoacetate. J. Chem. Soc. 1938, 228–232. [Google Scholar] [CrossRef]
- Robertson, A.; Sandrock, W.A.; Hendry, C.B. CCCXXX—Hydroxy-carbonyl compounds. Part V. The preparation of coumarins and 1:4-pyrones from phenol, p-cresol, quinol, and α-naphthol. J. Chem. Soc. 1931, 2426–2432. [Google Scholar] [CrossRef]
- Woods, L.L.; John Sapp, J. A New One-Step Synthesis of Substituted Coumarins. J. Org. Chem. 1962, 27, 3703–3705. [Google Scholar] [CrossRef]
- Vekariya, R.H.; Patel, H.D. Recent Advances in the Synthesis of Coumarin Derivatives via Knoevenagel Condensation: A Review. Synth. Commun. 2014, 44, 2756–2788. [Google Scholar] [CrossRef]
- Zareyee, D.; Serehneh, M. Recyclable CMK-5 supported sulfonic acid as an environmentally benign catalyst for solvent-free one-pot construction of coumarin through Pechmann condensation. J. Mol. Catal. A Chem. 2014, 391, 88–91. [Google Scholar] [CrossRef]
- Samadizadeh, M.; Nouri, S.; Moghadam, F.K. Magnetic Nanoparticles Functionalized Ethan Sulfonic Acid (MNESA): As an Efficient Catalyst in the Synthesis of Coumarin Derivatives Using Pechman Condensation Under Mild Condition. Res. Chem. Intermed. 2016, 42, 6089–6103. [Google Scholar] [CrossRef]
- Esfahani, F.K.; Zareyee, D.; Yousefi, R. Sulfonated Core-Shell Magnetic Nanoparticle (Fe3O4 @SiO2 @PrSO3H) As A Highly Active and Durable Protonic Acid Catalyst; Synthesis of Coumarin Derivatives Through Pechman Reaction. ChemCatChem 2014, 6, 3333–3337. [Google Scholar] [CrossRef]
- Khder, A.S.; Ahmed, S.A.; Khairou, K.S.; Altass, H.M. Competent, Selective and High Yield of 7-hydroxy-4-methyl Coumarin over Sulfonated Mesoporous Silica as Solid Acid Catalysts. J. Porous Mater. 2017. [Google Scholar] [CrossRef]
- Joshi, R.; Chudasama, U. Synthesis of Coumarins via Pechmann Condensation using inorganic ion exchangers as solid acid catalysts. J. Sci. Ind. Res. 2008, 67, 1092–1097. [Google Scholar]
- Chen, X.R.; Ju, Y.H.; Mou, C.Y. Direct Synthesis of Mesoporous Sulfated Silica-Zirconia Catalysts with High Catalytic Activity for Biodiesel via Esterification. J. Phys. Chem. C 2007, 111, 18731–18737. [Google Scholar] [CrossRef]
- Bouasla, S.; Amaro-Gahete, J.; Esquivel, D.; Lopez, M.I.; Jimenez-Sanchidrian, C.; Teguiche, M.; Romero-Salguero, F.J. Coumarin Derivatives Solvent-Free Synthesis under Microwave Irradiation over Heterogenous Solid Catalysts. Molecules 2017, 22, 2072. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, B.; Mishra, M.K.; Jasra, R.V. Microwave-assisted solvent free synthesis of hydroxy derivatives of 4-methyl coumarin using nano-crystalline sulfated-zirconia catalyst. J. Mol. Catal. A Chem. 2008, 286, 41–46. [Google Scholar] [CrossRef]
- Opanasenko, M.; Shamzhy, M.; Cejka, J. Solid Acid Catalysts for Coumarin Synthesis by the Pechman Reaction: MOFs versus Zeolites. ChemCatChem 2013, 5, 1024–1031. [Google Scholar] [CrossRef]
- Reddy, B.M.; Patil, M.K. Organic Syntheses and Transformations Catalyzed by Sulfated Zirconia. Chem. Rev. 2009, 109, 2185–2208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Huang, M.; Ma, H.-L.; Zhang, Z.-Q.; Gao, J.-M.; Zhu, Y.-L.; Han, X.-J.; Guo, X.-Y. Preparation of a Carbon-Based Solid Acid Catalyst by Sulfonating Activated Carbon in a Chemical Reduction Process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.S.; Rudradas, A.P.; Babu, M.H. The indium(III) chloride-catalyzed von Pechmann reaction: A simple and effective procedure for the synthesis of 4-substituted coumarins. Tetrahedron Lett. 2002, 43, 9195–9197. [Google Scholar] [CrossRef]
- Manhas, M.S.; Ganguly, S.N.; Mukherjee, S.; Jian, A.K.; Bose, A.K. Microwave initiated reactions: Pechmann coumarin synthesis, Biginelli reaction, and acylation. Tetrahedron Lett. 2006, 47, 2423–2425. [Google Scholar] [CrossRef]
- Rajabi, F.; Feiz, A.; Luque, R. An efficient synthesis of coumarin derivatives using a SBA-15 supported Cobalt(II) nanocatalyst. Catal. Lett. 2015, 145, 1621–1625. [Google Scholar] [CrossRef]
- Khaligh, N.G. Synthesis of coumarins via Pechmann reaction catalyzed by 3-methyl-1-sulfonic acid imidazolium hydrogen sulfate as an efficient, halogen-free and reusable acidic ionic liquid. Catal. Sci. Technol. 2012, 2, 1633–1636. [Google Scholar] [CrossRef]
- Atghia, S.V.; Beigbaghlou, S.S. Use of a Highly Efficient and Recyclable Solid-Phase Catalyst Based on Nanocrystalline Titania for the Pechmann Condensation. C. R. Chim. 2014, 1155–1159. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 3rd ed.; Addison-Wesley: Reading, MA, USA, 1967. [Google Scholar]
- Oyamada, J.; Chengguo, J.C.; Fujiwara, Y.; Kitamura, T. Direct Synthesis of Coumarins by Pd(II)-Catalyzed Reaction of Alkoxyphenols and Alkynoates. Chem. Lett. 2002, 31, 380–381. [Google Scholar] [CrossRef]
- Naik, M.A.; Mishra, B.G.; Dubey, A. Combustion synthesized WO3–ZrO2 nanocomposites as catalyst for the solvent-free synthesis of coumarins. Colloids Surf. A 2008, 317, 234–238. [Google Scholar] [CrossRef]
- Shirini, F.; Asieh Yahyazadeh, A.; Mohammadi, K. A solvent-free synthesis of coumarins using 1,3-disulfonic acid imidazolium hydrogen sulfate as a reusable and effective ionic liquid catalyst. Res. Chem. Intermed. 2015, 41, 6207–6218. [Google Scholar] [CrossRef]
- Reddy, B.M.; Thirupathi, B.; Patil, M.K. One-Pot Synthesis of Substituted Coumarins Catalyzed by Silica Gel Supported Sulfuric Acid Under Solvent-Free Conditions. Open Catal. J. 2009, 2, 33–39. [Google Scholar] [CrossRef]




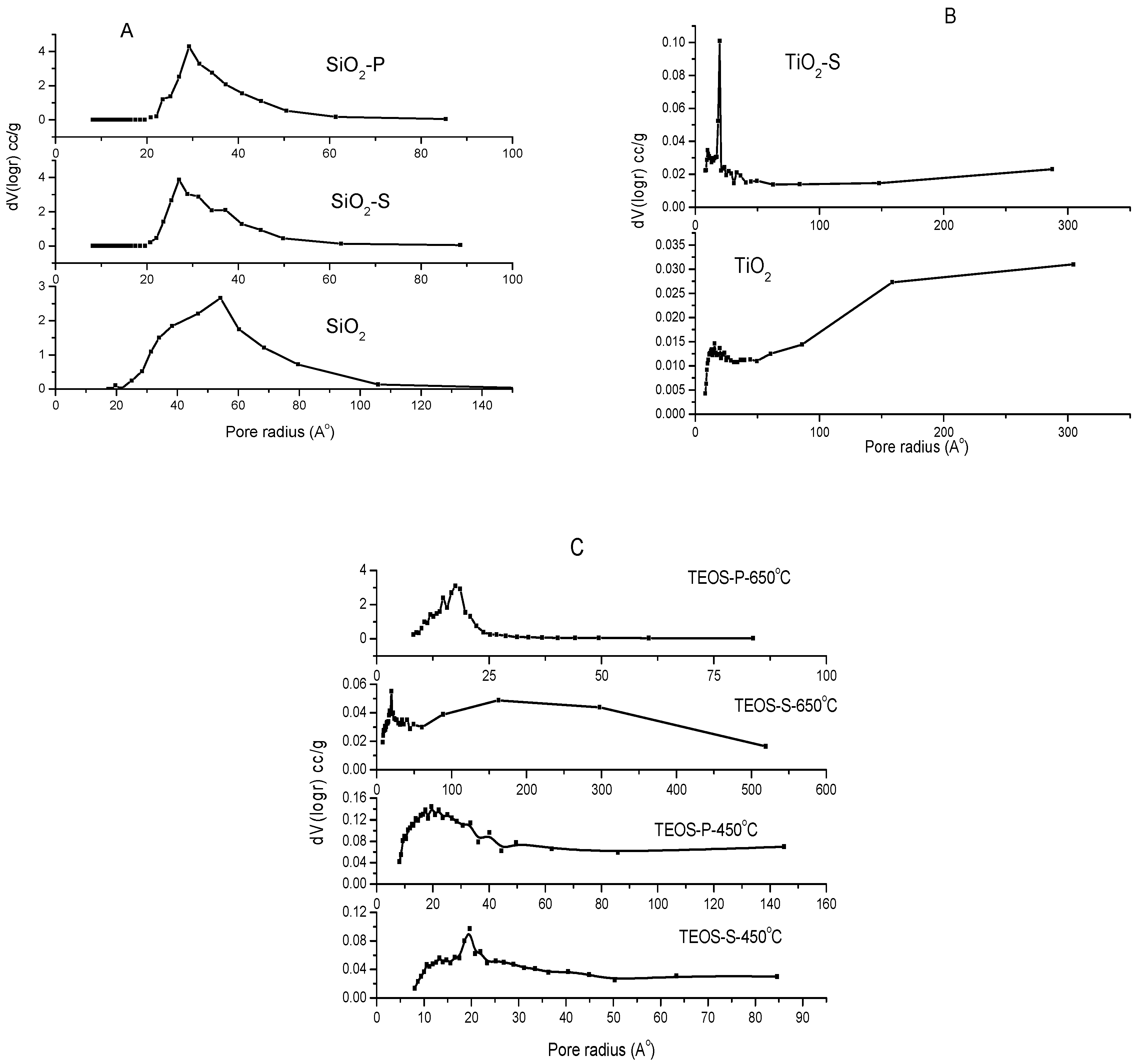

| Sample | SBET m2/g | St m2/g | St m2/g | Vp BET cm3/g | Vp BJH cm3/g | rBET Å | rBJH Å |
|---|---|---|---|---|---|---|---|
| SiO2 | 308.5 | 308.5 | 352.7 | 0.801 | 0.819 | 52.0 | 54.1 |
| SiO2-S | 244.6 | 344.6 | 468.8 | 0.636 | 0.741 | 52.0 | 37.0 |
| SiO2-P | 285.8 | 285.8 | 476 | 0.696 | 0.787 | 48.7 | 29.2 |
| TiO2 | 10.4 | 10.4 | 12.2 | 0.031 | 0.031 | 59.4 | 4.3 |
| TiO2-S | 30.6 | 19.3 | 32.4 | 0.045 | 0.044 | 29.8 | 4.8 |
| TiO2-P | 36.4 | 22.5 | 38.6 | 0.052 | 0.048 | 26.5 | 4.1 |
| TEOS-S-450 °C | 20.8 | 20.8 | 50.7 | 0.102 | 0.116 | 98.3 | 19.6 |
| TEOS-P-450 °C | 50.3 | 50.3 | 103.5 | 0.118 | 0.144 | 46.8 | 11.1 |
| TEOS-S-650 °C | 20 | 20 | 35.7 | 0.061 | 0.068 | 133.7 | 18.6 |
| TEOS-P-650 °C | 767.4 | 441.3 | 890.8 | 0.664 | 0.696 | 34.6 | 34.9 |
| (A) | |||||
| Catalyst | wt % of Catalyst | Phenol Type | Yield % | M.P °C of Product | Theoritical M.P °C [5,62] |
| - | 0 | Phenol derivatives | NR * | ||
| SiO2-S | 20 | Resorcinol | 100 | 186–187 | 185–187 |
| SiO2-P | 20 | Resorcinol | 72 | ||
| TiO2-S | 20 | Resorcinol | 93 | ||
| TiO2-P | 20 | Resorcinol | 20 | ||
| SiO2-S | 20 | Pyrogallol | 82 | 242–244 | 242–243 |
| SiO2-P | 20 | Pyrogallol | 51 | ||
| TiO2-S | 20 | Pyrogallol | 85 | ||
| TiO2-P | 20 | Pyrogallol | 59 | ||
| SiO2-S | 20 | β-Naphthol | 71 | 181–182 | 180–181 |
| SiO2-P | 20 | β-Naphthol | 63 | ||
| TiO2-S | 20 | β-Naphthol | 51 | ||
| TiO2-P | 20 | β-Naphthol | 44 | ||
| SiO2-S | 20 | α-Naphthol | 74 | 155–156 | 154–156 |
| SiO2-P | 20 | α-Naphthol | 40 | ||
| TiO2-S | 20 | α-Naphthol | 60 | ||
| TiO2-P | 20 | α-Naphthol | 47 | ||
| SiO2-S | 20 | Catechol | 47 | 193–195 | - |
| SiO2-P | 20 | Catechol | 43 | ||
| TiO2-S | 20 | Catechol | 50 | ||
| TiO2-P | 20 | Catechol | 28 | ||
| SiO2-S | 20 | Phenol | 31 | 79–81 | 78–80 |
| SiO2-P | 20 | Phenol | 9 | ||
| TiO2-S | 20 | Phenol | 23 | ||
| TiO2-P | 20 | Phenol | 7 | ||
| (B) | |||||
| - | 0 | Phenol derivatives | NR * | ||
| TEOS-S-450 °C | 20 | Resorcinol | 26 | 186–187 | 185–187 |
| TEOS-P-450 °C | 20 | Resorcinol | 95 | ||
| TEOS-S-650 °C | 20 | Resorcinol | 100 | ||
| TEOS-P-650 °C | 20 | Resorcinol | 54 | ||
| TEOS-S-450 °C | 20 | Pyrogallol | 17 | 242–244 | 242–243 |
| TEOS-P-450 °C | 20 | Pyrogallol | 70 | ||
| TEOS-S-650 °C | 20 | Pyrogallol | 71 | ||
| TEOS-P-650 °C | 20 | Pyrogallol | 47 | ||
| TEOS-S-450 °C | 20 | β-Naphthol | 49 | 181–182 | 180–181 |
| TEOS-P-450 °C | 20 | β-Naphthol | 56 | ||
| TEOS-S-650 °C | 20 | β-Naphthol | 58 | ||
| TEOS-P-650 °C | 20 | β-Naphthol | 60 | ||
| TEOS-S-450 °C | 20 | α-Naphthol | 34 | 155–156 | 154–156 |
| TEOS-P-450 °C | 20 | α-Naphthol | 47 | ||
| TEOS-S-650 °C | 20 | α-Naphthol | 60 | ||
| TEOS-P-650 °C | 20 | α-Naphthol | 50 | ||
| TEOS-S-450 °C | 20 | Catechol | 22 | 193–195 | - |
| TEOS-P-450 °C | 20 | Catechol | 48 | ||
| TEOS-S-650 °C | 20 | Catechol | 38 | ||
| TEOS-P-650 °C | 20 | Catechol | 36 | ||
| TEOS-S-450 °C | 20 | Phenol | 6 | 79–81 | 78–80 |
| TEOS-P-450 °C | 20 | Phenol | 7 | ||
| TEOS-S-650 °C | 20 | Phenol | 12 | ||
| TEOS-P-650 °C | 20 | Phenol | 10 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan, N.R.E.; Hagar, M.; Afifi, T.H.; Al-wadaani, F.; Okasha, R.M. Catalytic Activity of Sulfated and Phosphated Catalysts towards the Synthesis of Substituted Coumarin. Catalysts 2018, 8, 36. https://doi.org/10.3390/catal8010036
Radwan NRE, Hagar M, Afifi TH, Al-wadaani F, Okasha RM. Catalytic Activity of Sulfated and Phosphated Catalysts towards the Synthesis of Substituted Coumarin. Catalysts. 2018; 8(1):36. https://doi.org/10.3390/catal8010036
Chicago/Turabian StyleRadwan, Nagi R. E., Mohamed Hagar, Tarek H. Afifi, Fahd Al-wadaani, and Rawda M. Okasha. 2018. "Catalytic Activity of Sulfated and Phosphated Catalysts towards the Synthesis of Substituted Coumarin" Catalysts 8, no. 1: 36. https://doi.org/10.3390/catal8010036
APA StyleRadwan, N. R. E., Hagar, M., Afifi, T. H., Al-wadaani, F., & Okasha, R. M. (2018). Catalytic Activity of Sulfated and Phosphated Catalysts towards the Synthesis of Substituted Coumarin. Catalysts, 8(1), 36. https://doi.org/10.3390/catal8010036






