Co-Detection of Dopamine and Glucose with High Temporal Resolution
Abstract
1. Introduction
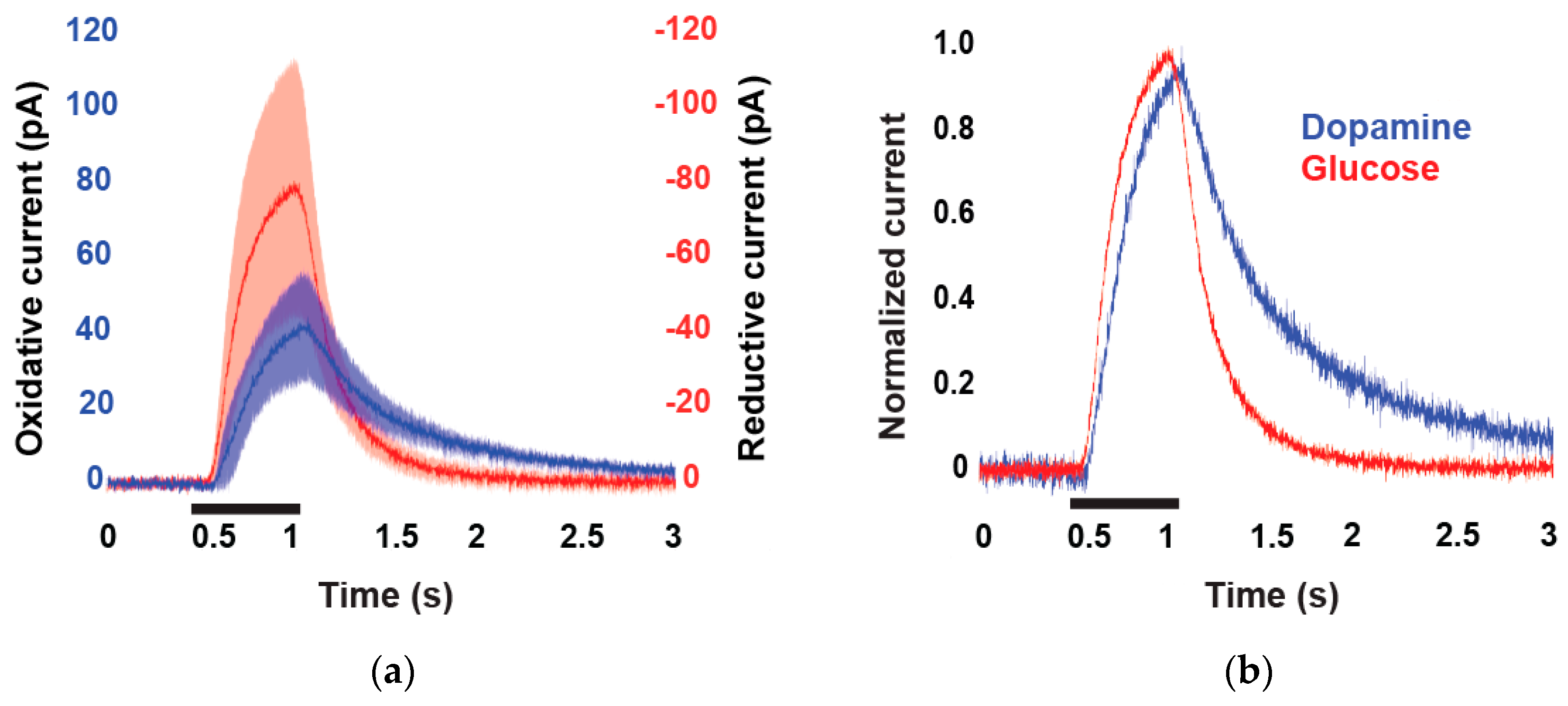
2. Results and Discussion
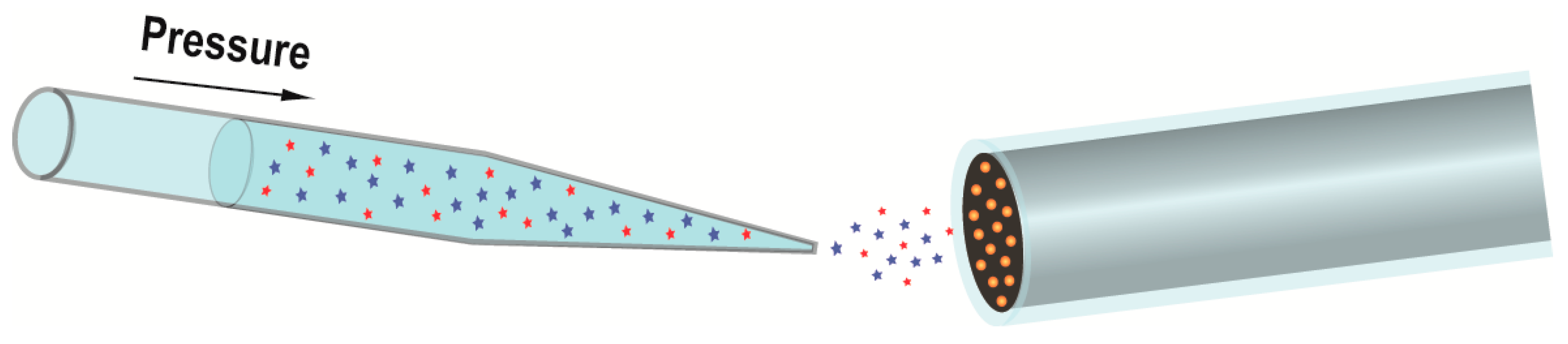
2.1. Preparation of the Biosensor
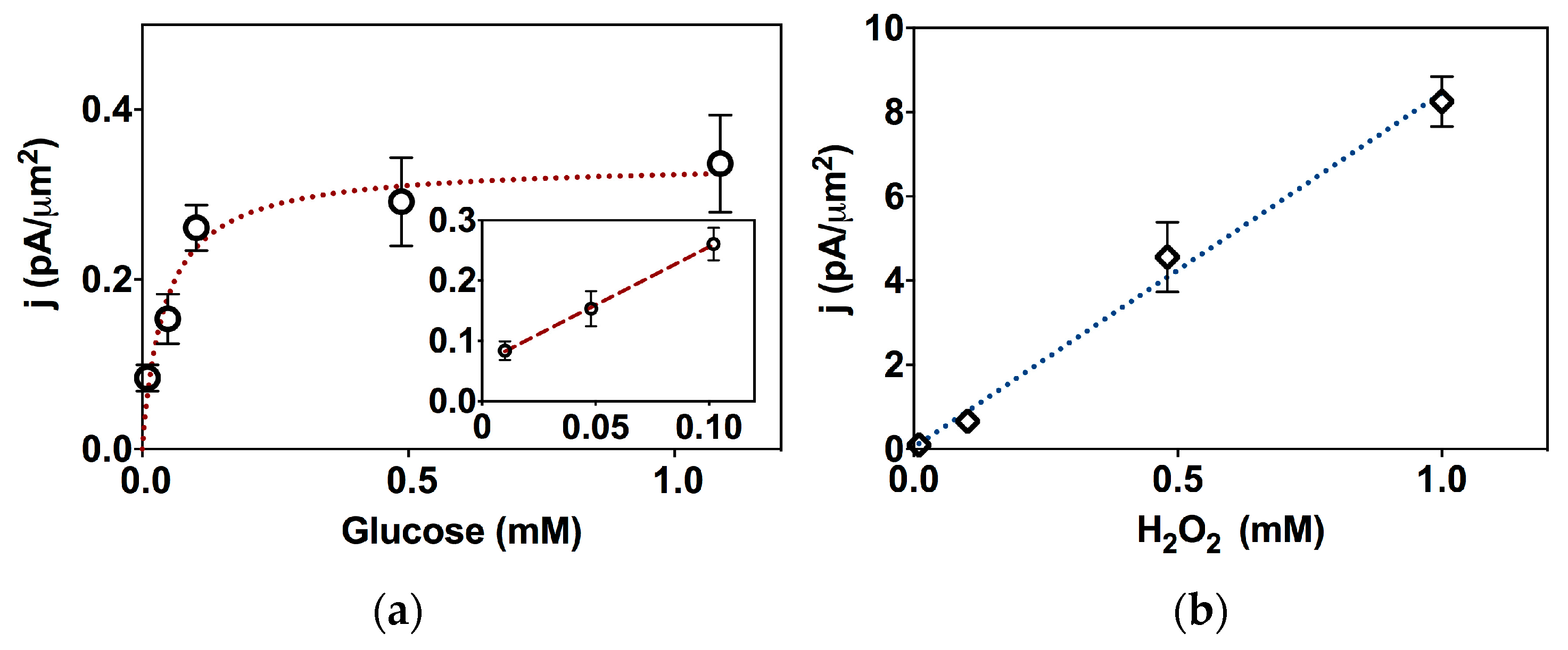
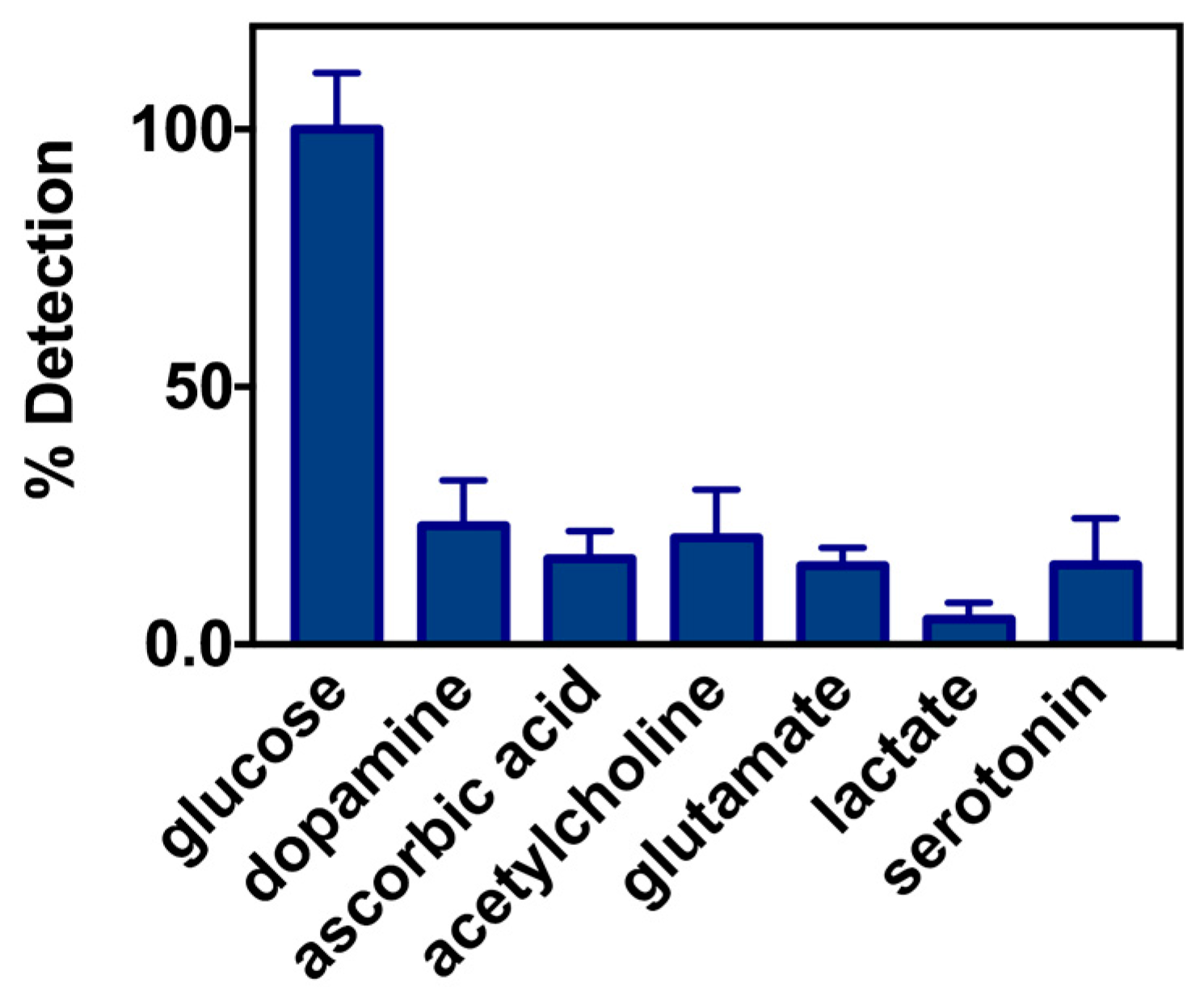
2.2. Biosensor Characterization
3. Materials and Methods
3.1. Chemical Reagents
3.2. Electrochemical Set-Up
3.3. Preparation of a 33 µm CFME
3.4. Functionalization with AuNP
3.5. Immobilization of Enzymes
3.6. Characterization of the Glucose Sensor
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clark, L.C.; Lyons, C. Electrode Systems for Continous Monitoring in cardiovascular surgery cardivascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Misra, S.K.; Schwartz-Duval, A.S.; Daza, E.; Ostadhossein, F.; Bowman, M.; Jain, A.; Taylor, G.; McDonagh, D.; Labriola, L.T.; et al. Real-Time Monitoring of Post-Surgical and Post-Traumatic Eye Injuries Using Multilayered Electrical Biosensor Chip. ACS Appl. Mater. Interfaces 2017, 9, 8609–8622. [Google Scholar] [CrossRef] [PubMed]
- Monosik, R.; Stredansky, M.; Tkac, J.; Sturdik, E. Application of Enzyme Biosensors in Analysis of Food and Beverages. Food Anal. Methods 2012, 5, 40–53. [Google Scholar] [CrossRef]
- Prodromidis, M.I.; Karayannis, M.I. Enzyme based amperometric biosensors for food analysis. Electroanalysis 2002, 14, 241–261. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Bhardwaj, A. Biosensor Technology for Pesticides—A review. Appl. Biochem. Biotechnol. 2015, 175, 3093–3119. [Google Scholar] [CrossRef] [PubMed]
- Dale, N.; Hatz, S.; Tian, F.; Llaudet, E. Listening to the brain: Microelectrode biosensors for neurochemicals. Trends Biotechnol. 2005, 23, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.; de Vries, M.G.; Ngabi, W.; Oomen, P.E.; Cremers, T.I.F.H.; Westerink, B.H.C. In vivo continuous and simultaneous monitoring of brain energy substrates with a multiplex amperometric enzyme-based biosensor device. Biosens. Bioelectron. 2015, 67, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Kiyatkin, E.A.; Wakabayashi, K.T. Parsing glucose entry into the brain: Novel findings obtained with enzyme-based glucose biosensors. ACS Chem. Neurosci. 2014, 6, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.F.; Ledo, A.; Laranjinha, J.; Gerhardt, G.A.; Barbosa, R.M. Microelectrode array biosensor for high-resolution measurements of extracellular glucose in the brain. Sens. Actuators B Chem. 2016, 237, 298–307. [Google Scholar] [CrossRef]
- Santos, R.M.; Laranjinha, J.; Barbosa, R.M.; Sirota, A. Simultaneous measurement of cholinergic tone and neuronal network dynamics in vivo in the rat brain using a novel choline oxidase based electrochemical biosensor. Biosens. Bioelectron. 2015, 69, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, W.; Wang, M. Sensing of Salivary Glucose Using Nano-Structured Biosensors. Biosensors 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Hayat Nawaz, M.A.; Badea, M.; Marty, J.L.; Hayat, A. Nano-engineered biomimetic optical sensors for glucose monitoring in diabetes. Sensors 2016, 16, 1931. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Papa, H.; Gaillard, M.; Gonzalez, L.; Chatterjee, J. Fabrication of Functionalized Carbon Nanotube Buckypaper Electrodes for Application in Glucose Biosensors. Biosensors 2014, 4, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Kitte, S.A.; Gao, W.; Zholudov, Y.T.; Qi, L.; Nsabimana, A.; Liu, Z.; Xu, G. Stainless Steel Electrode for Sensitive Luminol Electrochemiluminescent Detection of H2O2, Glucose, and Glucose Oxidase Activity. Anal. Chem. 2017, 89, 9864–9869. [Google Scholar] [CrossRef] [PubMed]
- Theuer, L.; Lehmann, M.; Junne, S.; Neubauer, P.; Birkholz, M. Micro-Electromechanical Affinity Sensor for the Monitoring of Glucose in Bioprocess Media. Int. J. Mol. Sci. 2017, 18, 1235. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zheng, J.; Wang, B.; Ren, H. Double Biocatalysis Signal Amplification Glucose Biosensor Based on Porous Graphene. Materials 2017, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Zhang, F.; He, X.; Fang, G.; Liu, J.; Wang, S. Design of Cyclic Peptide Based Glucose Receptors and Their Application in Glucose Sensing. Anal. Chem. 2017, 89, 10431–10438. [Google Scholar] [CrossRef] [PubMed]
- Bornhoeft, L.; Biswas, A.; McShane, M. Composite Hydrogels with Engineered Microdomains for Optical Glucose Sensing at Low Oxygen Conditions. Biosensors 2017, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Weltin, A.; Kieninger, J.; Urban, G.A. Microfabricated, amperometric, enzyme-based biosensors for in vivo applications. Anal. Bioanal. Chem. 2016, 408, 4503–4521. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Wu, F.; Hao, J.; Zhang, M.; Yu, P.; Mao, L. In Vivo Analysis with Electrochemical Sensors and Biosensors. Anal. Chem. 2017, 89, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.S.; Wightman, R.M. Electrochemical Analysis of Neurotransmitters. Annu. Rev. Anal. Chem. 2015, 8, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Ferapontova, E.E. Electrochemical Analysis of Dopamine: Perspectives of Specific In Vivo Detection. Electrochim. Acta 2017. [Google Scholar] [CrossRef]
- Fox, M.E.; Wightman, R.M. Contrasting Regulation of Catecholamine Neurotransmission in the Behaving Brain: Pharmacological Insights from an Electrochemical Perspective. Pharmacol. Rev. 2017, 69, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Davie, C.A. A review of Parkinson’s disease. Br. Med. Bull. 2008, 86, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Nutt, D.J.; Lingford-Hughes, A.; Erritzoe, D.; Stokes, P.R. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 2015, 16, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, R.; Qu, J. Effect of different glucose supply conditions on neuronal energy metabolism. Cogn. Neurodyn. 2016, 10, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.K.; Lee, C.A.; Dausch, M.E.; Horman, B.M.; Patisaul, H.B.; McCarty, G.S.; Sombers, L.A. Simultaneous Voltammetric Measurements of Glucose and Dopamine Demonstrate the Coupling of Glucose Availability with Increased Metabolic Demand in the Rat Striatum. ACS Chem. Neurosci. 2017, 8, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Voeroes, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical sensors and biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, T. A Review on Immobilization Techniques of Biosensors. Int. J. Eng. Res. Technol. 2014, 3, 294–298. [Google Scholar]
- Cosnier, S. Biosensors based on electropolymerized films: New trends. Anal. Bioanal. Chem. 2003, 377, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Noll, T.; Noll, G. Strategies for “wiring” redox-active proteins to electrodes and applications in biosensors, biofuel cells, and nanotechnology. Chem. Soc. Rev. 2011, 40, 3564–3576. [Google Scholar] [CrossRef] [PubMed]
- Putzbach, W.; Ronkainen, N.J. Immobilization Techniques in the Fabrication of Nanomaterial-Based Electrochemical Biosensors: A Review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef] [PubMed]
- Vasylieva, N.; Maucler, C.; Meiller, A.; Viscogliosi, H.; Lieutaud, T.; Barbier, D.; Marinesco, S. Immobilization method to preserve enzyme specificity in biosensors: Consequences for brain glutamate detection. Anal. Chem. 2013, 85, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Asuri, P.; Karajanagi, S.S.; Vertegel, A.A.; Dordick, J.S.; Kane, R.S. Enhanced stability of enzymes adsorbed onto nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 1675–1678. [Google Scholar] [CrossRef] [PubMed]
- Cans, A.-S.; Dean, S.L.; Reyes, F.E.; Keating, C.D. Synthesis and characterization of enzyme-Au bioconjugates: HRP and fluorescein-labeled HRP. Nanobiotechnology 2007, 3, 12–22. [Google Scholar] [CrossRef]
- Gagner, J.E.; Lopez, M.D.; Dordick, J.S.; Siegel, R.W. Effect of gold nanoparticle morphology on adsorbed protein structure and function. Biomaterials 2011, 32, 7241–7252. [Google Scholar] [CrossRef] [PubMed]
- Keighron, J.D.; Åkesson, S.; Cans, A.-S. Coimmobilization of acetylcholinesterase and choline oxidase on gold nanoparticles: Stoichiometry, activity, and reaction efficiency. Langmuir 2014, 30, 11348–11355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Luo, X.-L.; Xu, J.J.; Du, Y.; Chen, H.Y. A glucose biosensor based on chitosan–glucose oxidase–gold nanoparticles biocomposite formed by one-step electrodeposition. Anal. Biochem. 2004, 334, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, N.; Niu, Y.; Sun, C. Immobilization of glucose oxidase on gold nanoparticles modified Au electrode for the construction of biosensor. Sens. Actuator B Chem. 2005, 109, 367–374. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, K.; Bai, Y.; Yang, W.; Sun, C. Glucose oxidase/colloidal gold nanoparticles immobilized in Nafion film on glassy carbon electrode: Direct electron transfer and electrocatalysis. Bioelectrochemistry 2006, 69, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Keighron, J.D.; Wigström, J.; Kurczy, M.E.; Bergman, J.; Wang, Y.; Cans, A.S. Amperometric detection of single vesicle acetylcholine release events from an artificial cell. ACS Chem. Neurosci. 2015, 6, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Finot, M.O.; Braybrook, G.D.; McDermott, M.T. Characterization of electrochemically deposited gold nanocrystals on glassy carbon electrodes. J. Electroanal. Chem. 1999, 466, 234–241. [Google Scholar] [CrossRef]
- Ferreyra, N.; Coche-Guérente, L.; Labbé, P. Construction of layer-by-layer self-assemblies of glucose oxidase and cationic polyelectrolyte onto glassy carbon electrodes and electrochemical study of the redox-mediated enzymatic activity. Electrochim. Acta 2004, 49, 477–484. [Google Scholar] [CrossRef]
- Bergman, J.; Wang, Y.; Wigström, J.; Cans, A.S. Counting the number of enzymes immobilized onto a nanoparticle-coated electrode. Anal. Bioanal. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 6th ed.; W. H. Freeman and Company: New York, NY, USA, 2007. [Google Scholar]
- Zaidi, S.A.; Shin, J.H. Recent developments in nanostructure based electrochemical glucose sensors. Talanta 2016, 149, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Harrison, F.E.; May, J.M. Vitamin C Function in the Brain: Vital Role of the Ascorbate Transporter (SVCT2). Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130 (Suppl. 4S), 1007s–1015s. [Google Scholar] [PubMed]
- Bruno, J.P.; Gash, C.; Martin, B.; Zmarowski, A.; Pomerleau, F.; Burmeister, J.; Huettl, P.; Gerhardt, G.A. Second-by-second measurement of acetylcholine release in prefrontal cortex. Eur. J. Neurosci. 2006, 24, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Varner, E.L.; Groskreutz, S.R.; Michael, A.C.; Weber, S.G. In Vivo Monitoring of Dopamine by Microdialysis with 1 min Temporal Resolution Using Online Capillary Liquid Chromatography with Electrochemical Detection. Anal. Chem. 2015, 87, 6088–6094. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.F.; Deitmer, J.W. Glucose and lactate supply to the synapse. Brain Res. Rev. 2010, 63, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.V.; Sulzer, D. Analysis of exocytotic events recorded by amperometry. Nat. Methods 2005, 2, 651–658. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergman, J.; Mellander, L.; Wang, Y.; Cans, A.-S. Co-Detection of Dopamine and Glucose with High Temporal Resolution. Catalysts 2018, 8, 34. https://doi.org/10.3390/catal8010034
Bergman J, Mellander L, Wang Y, Cans A-S. Co-Detection of Dopamine and Glucose with High Temporal Resolution. Catalysts. 2018; 8(1):34. https://doi.org/10.3390/catal8010034
Chicago/Turabian StyleBergman, Jenny, Lisa Mellander, Yuanmo Wang, and Ann-Sofie Cans. 2018. "Co-Detection of Dopamine and Glucose with High Temporal Resolution" Catalysts 8, no. 1: 34. https://doi.org/10.3390/catal8010034
APA StyleBergman, J., Mellander, L., Wang, Y., & Cans, A.-S. (2018). Co-Detection of Dopamine and Glucose with High Temporal Resolution. Catalysts, 8(1), 34. https://doi.org/10.3390/catal8010034




