“Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources
Abstract
:1. Introduction
2. Results
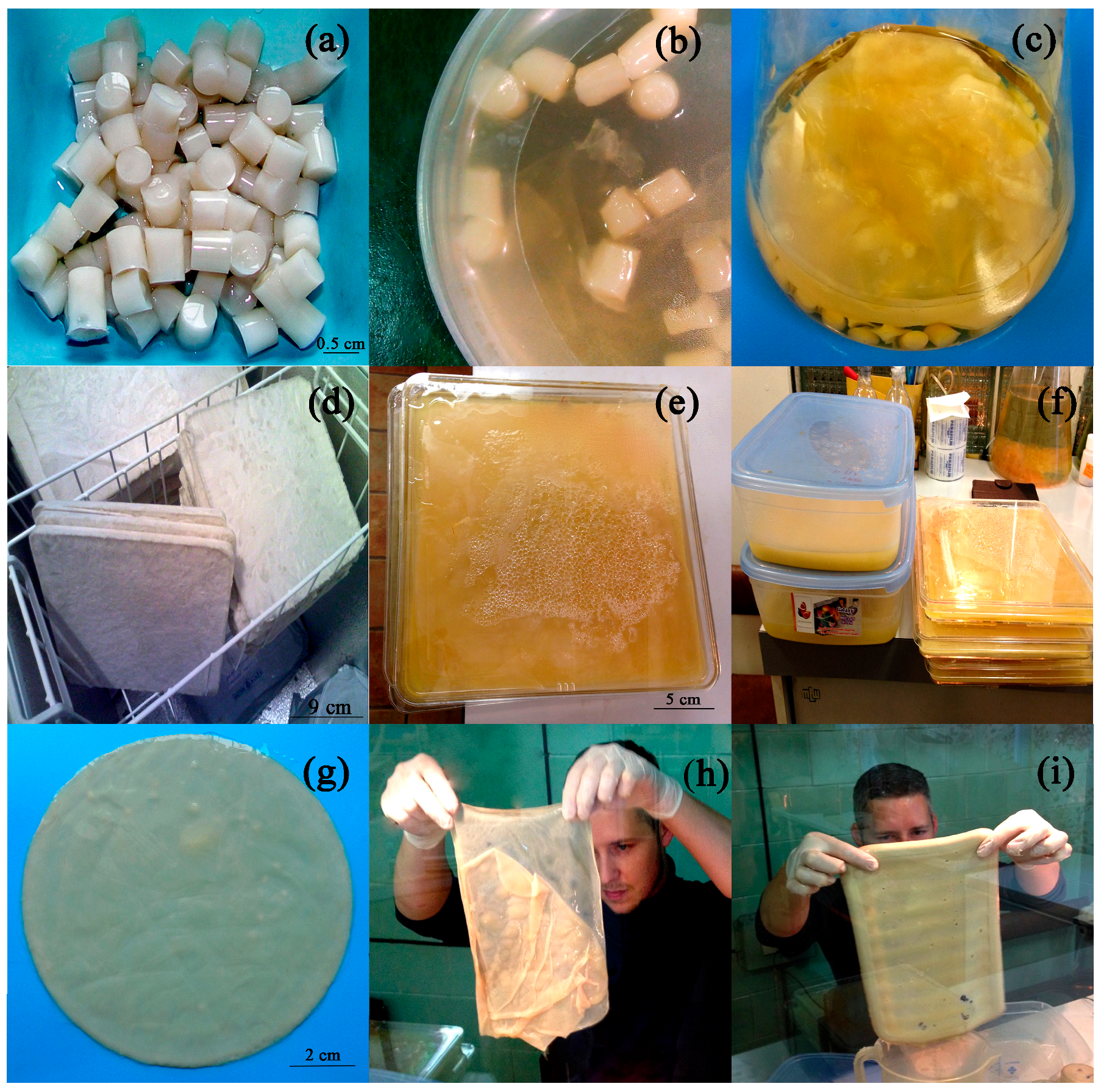
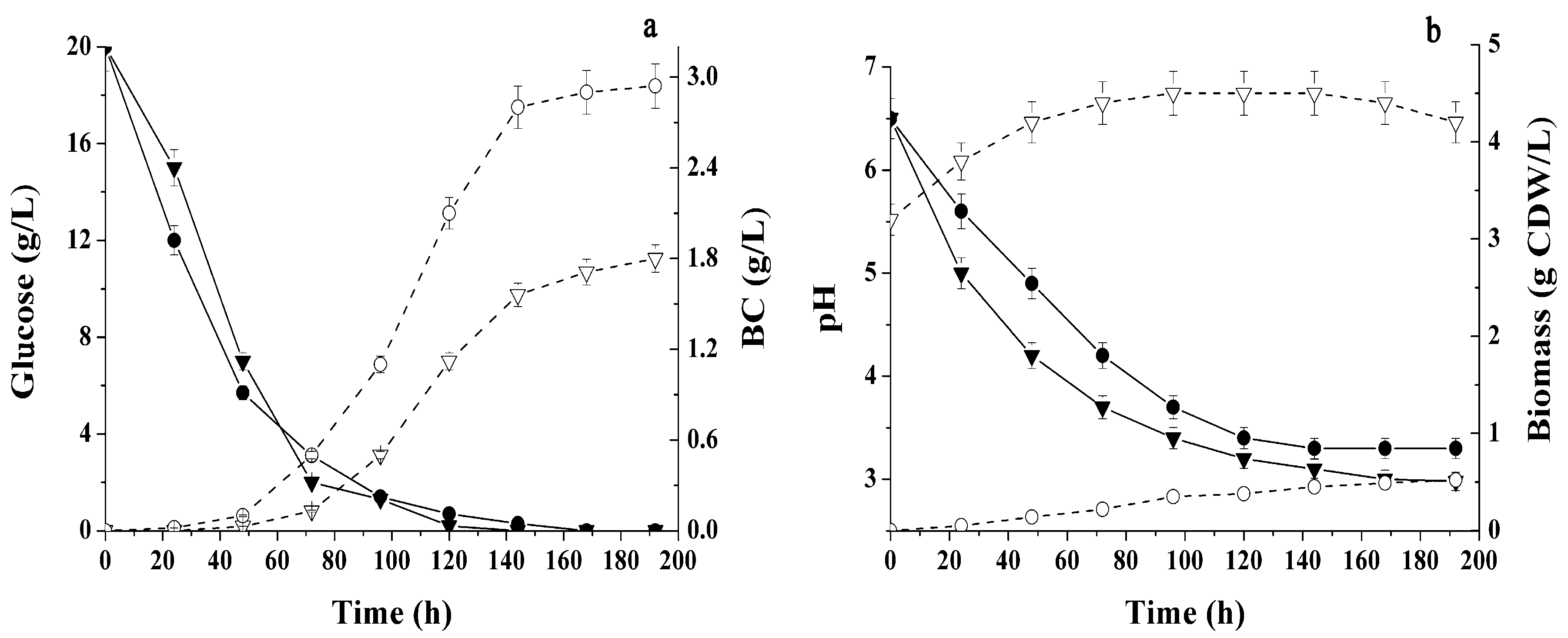
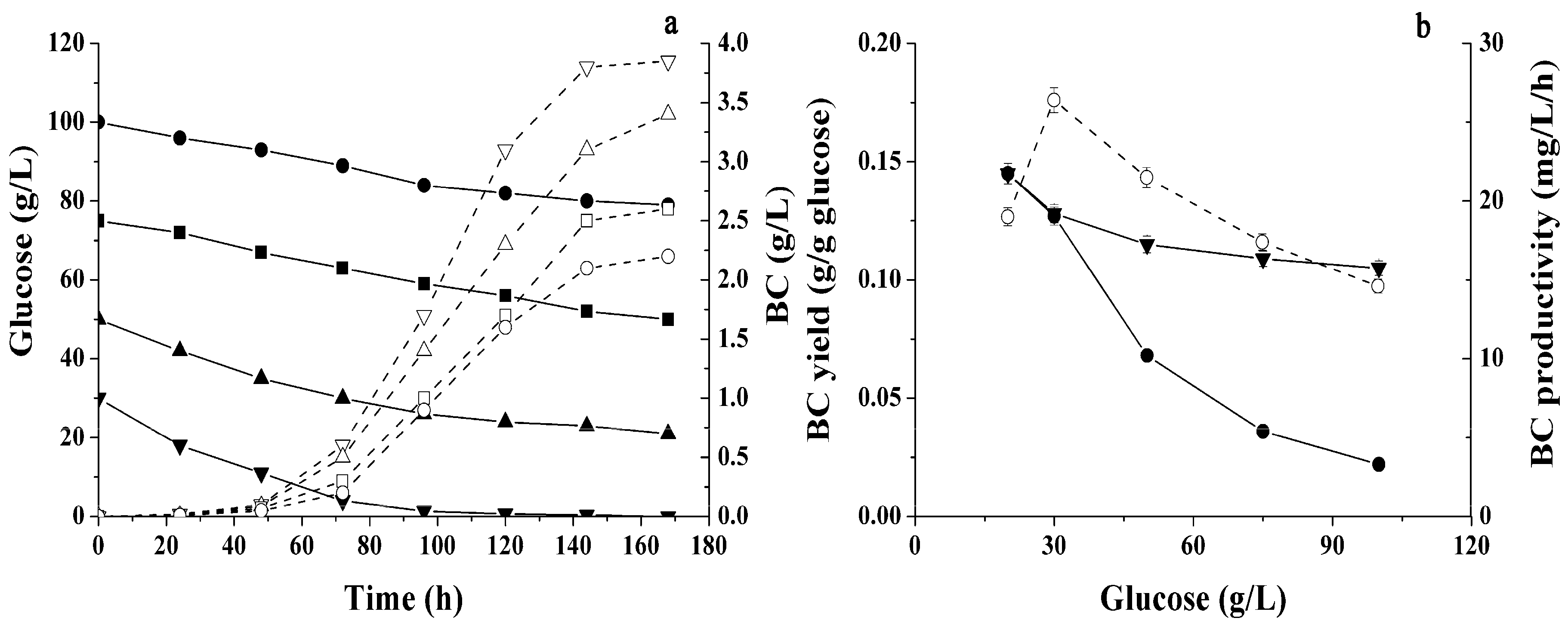
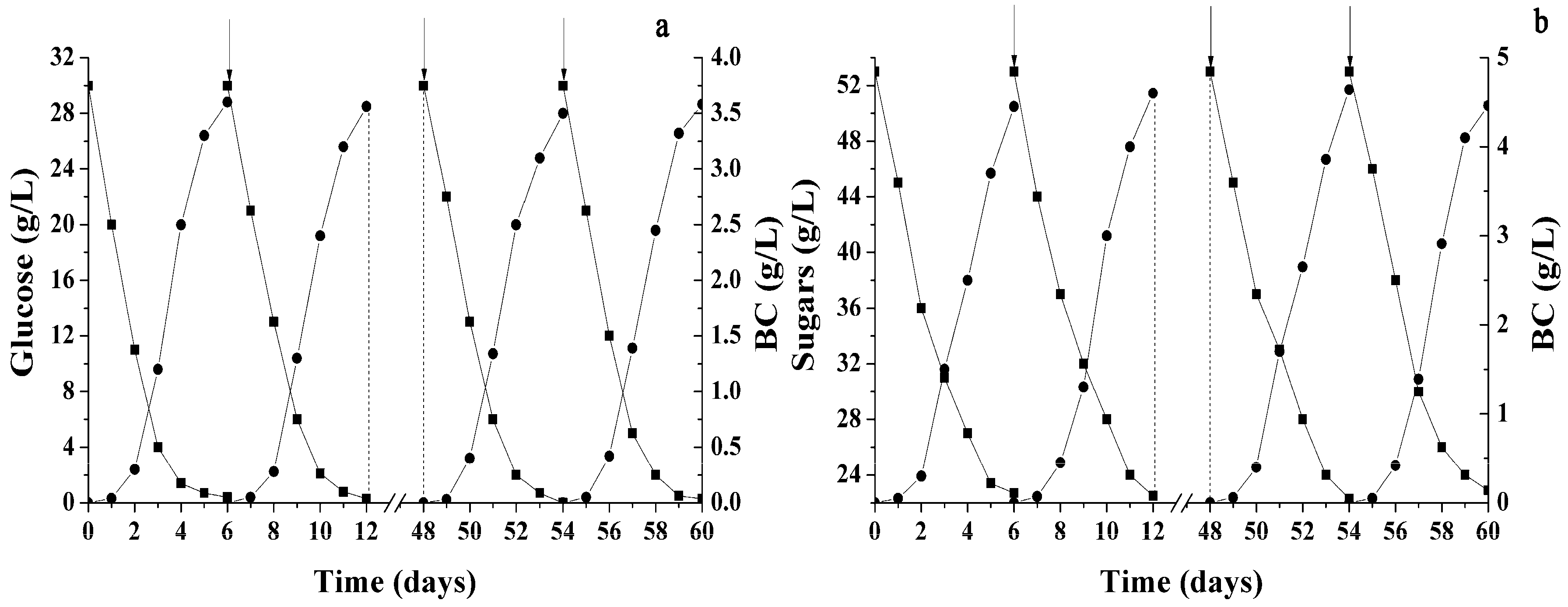
2.1. Conditions Influencing BC Production by Immobilized Cells
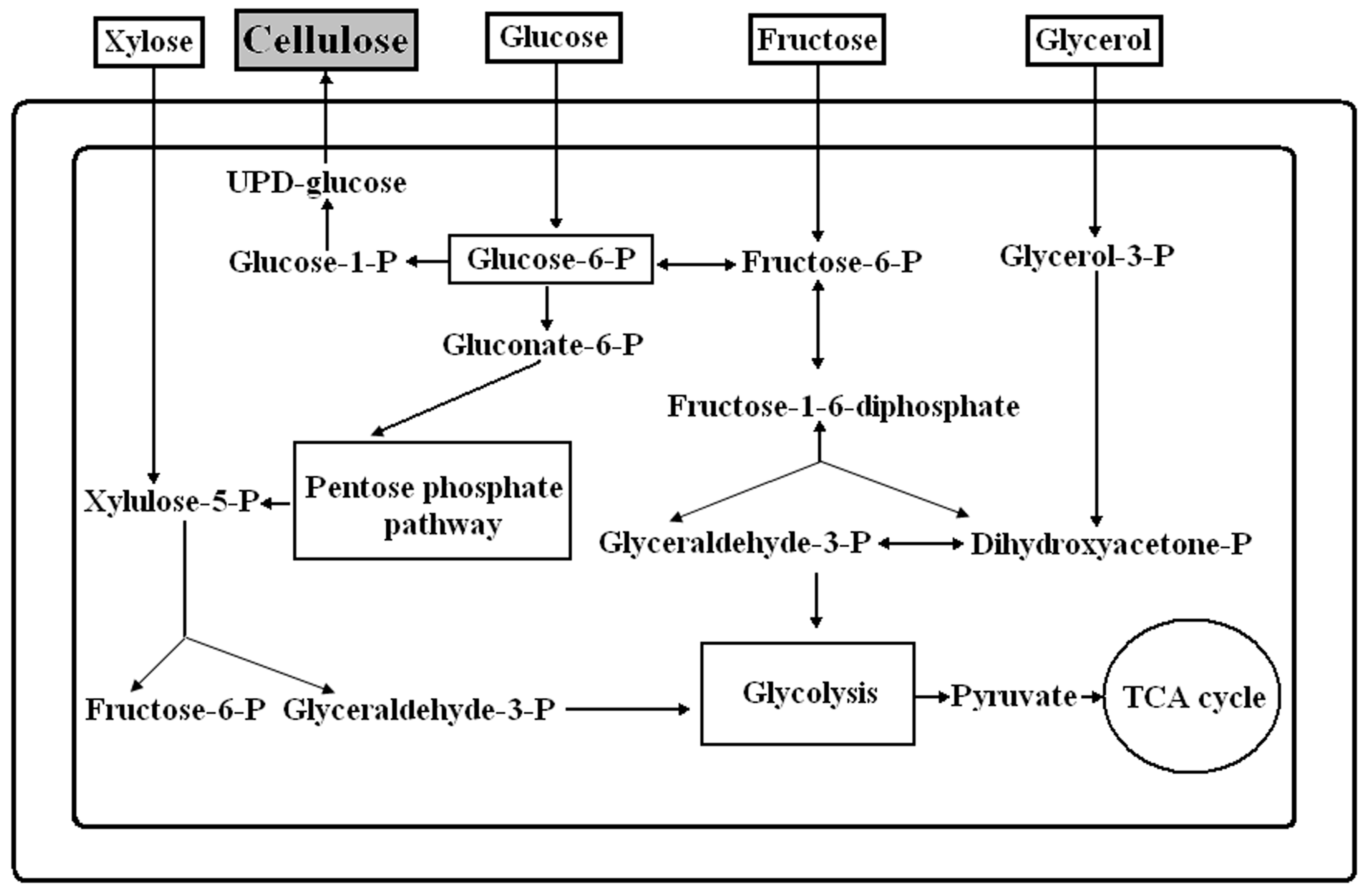
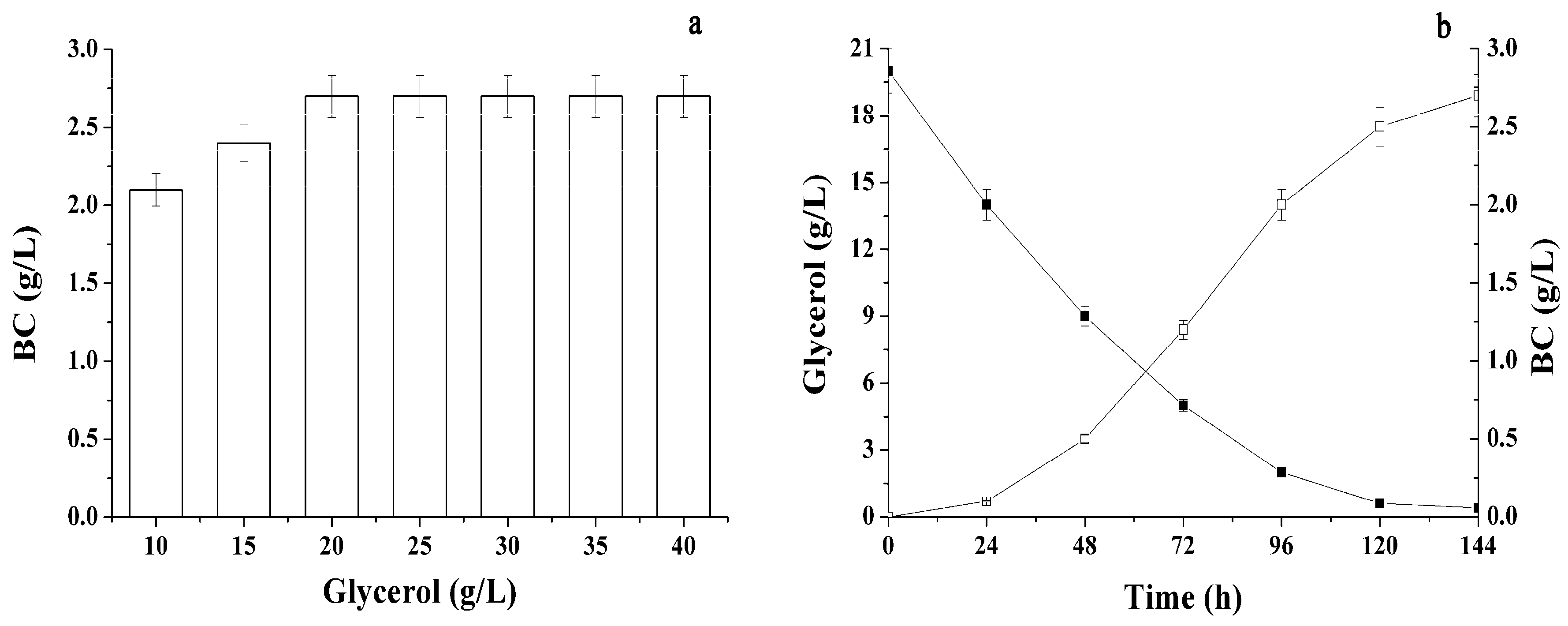
2.2. Different Sugars and Glycerol as Sources for BC Synthesis
2.3. Various Hydrolysates of Renewable Raw Materials as Media for BC Obtaining
3. Discussion
4. Materials and Methods
4.1. Microbial Strains and Culture Media
4.2. Cells’ Immobilization
4.3. Analytical Methods
4.4. BC Production
4.5. Pretreatment and Fermentative Hydrolysis of Biomass
4.6. Characterization of BC Samples
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef] [PubMed]
- Mohite, B.V.; Patil, S.V. A novel biomaterial: Bacterial cellulose and its new era applications. Biotechnol. Appl. Biochem. 2014, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jiang, X. Structure and properties of bacterial cellulose produced using a trickling bed reactor. Appl. Biochem. Biotechnol. 2014, 172, 3844–3861. [Google Scholar] [CrossRef] [PubMed]
- Gayathry, G.; Gopalaswamy, G. Production and characterization of bacterial cellulose fibre from Acetobacter xylinum. Indian J. Fibre Text. Res. 2014, 39, 93–96. [Google Scholar]
- Keshk, S.M.A.S. Bacterial cellulose production and its industrial applications. J. Bioprocess Biotech. 2014, 4, 150. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y. An overview of fermentation production and application of bacterial cellulose. Adv. Mater. Res. 2013, 627, 878–884. [Google Scholar] [CrossRef]
- Kongruang, S. Bacterial cellulose production by Acetobacter xylinum strains from agricultural waste products. Appl. Biochem. Biotechnol. 2008, 148, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.R.; Ishwar, B.B.; Shrikant, A.S.; Rekha, S.S. Microbial cellulose: Fermentative production and applications. Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
- Lin, S.P.; Calvar, I.L.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Pourramezan, G.Z.; Roayaei, A.M.; Qezelbash, Q.R. Optimization of culture conditions for bacterial cellulose production by Acetobacter sp. 4B-2. Biotechnology 2009, 8, 150–154. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial cellulose production from industrial waste and by-product streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef] [PubMed]
- Jalili Tabaii, M.; Emtiazi, G. Comparison of bacterial cellulose production among different strains and fermented media. Appl. Food Biotechnol. 2016, 3, 35–41. [Google Scholar]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.F.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, F.; Yang, X.-X.; Han, S.-F. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour. Technol. 2013, 135, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yang, R.; Liu, X. Production of bacterial cellulose by Acetobacter xylinum through utilizing acetic acid hydrolysate of bagasse as low-cost carbon source. BioResources 2017, 12, 1190–1200. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J. Biol. Eng. 2009, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Hornung, M.; Ludwig, M.; Gerrard, A.M.; Schmauder, H.-P. Optimizing the production of bacterial cellulose in surface culture: Evaluation of substrate mass transfer influences on the bioreaction (Part 1). Eng. Life Sci. 2006, 6, 537–545. [Google Scholar] [CrossRef]
- Meftahi, A.; Khajavi, R.; Rashidi, A.; Rahimi, M.K.; Bahador, A. Effect of purification on nano microbial cellulose pellicle properties. Proced. Mater. Sci. 2015, 11, 206–211. [Google Scholar] [CrossRef]
- Usha Rani, M.; Anu Appaiah, K.A. Production of bacterial cellulose by Gluconacetobacter hansenii UAC09 using coffee cherry husk. J. Food Sci. Technol. 2013, 50, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jia, Q.; Chen, L.; Zhang, L. Effect of organic acids on bacterial cellulose produced by Acetobacter xylinum. RRJMB 2016, 5, 1–6. [Google Scholar]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.W.; McNamara, J.T.; Zimmer, J. Mechanism of activation of bacterial cellulose synthase by cyclic-di-GMP. Nat. Struct. Mol. Biol. 2014, 21, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Waters, C.M. A tangled web: Regulatory connections between quorum sensing and cyclic di-GMP. J. Bacteriol. 2012, 194, 4485–4493. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, L.; Junter, G.-A.; Jouenne, T.; Mignot, L. Exopolysaccharide production by free and immobilized microbial cultures. Enzyme Microb. Technol. 1994, 16, 1048–1054. [Google Scholar] [CrossRef]
- Santos, E.L.I.; Rostro-Alanís, M.; Parra-Saldívar, R.; Alvarez, A.J. A novel method for bioethanol production using immobilized yeast cells in calcium-alginate films and hybrid composite pervaporation membrane. Bioresour. Technol. 2018, 247, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Nikolskaya, A.B.; Lyagin, I.V.; Senko, O.V.; Stepanov, N.A.; Maslova, O.V.; Mamedova, F.; Varfolomeyev, S.D. Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour. Technol. 2012, 114, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Senko, O.V.; Aleskerova, L.E.; Alenina, K.A.; Mazhul, M.M.; Ismailov, A.D. Biosensors based on the luminous bacteria photobaterium phosphoreum immobilized in polyvinyl alcohol cryogel for the monitoring of ecotoxicants. Appl. Biochem. Microbiol. 2014, 50, 477–482. [Google Scholar] [CrossRef]
- Kumar, A. Supermacroporous Cryogels: Biomedical and Biotechnological Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 496. ISBN 978-1482228816. [Google Scholar]
- Borzani, W.; Souza, S.J. Mechanism of the film thickness increasing during the bacterial production of cellulose on non-agitated liquid media. Biotechnol. Lett. 1995, 17, 1271–1272. [Google Scholar] [CrossRef]
- Budhiono, A.; Rosidi, B.; Taher, H.; Iguchi, M. Kinetic aspects of bacterial BC formation in nata-de-coco culture system. Carbohydr. Polym. 1999, 40, 137–143. [Google Scholar] [CrossRef]
- Ha, J.H.; Shehzad, O.; Khan, S.; Lee, S.Y.; Park, J.W.; Khan, T.; Park, J.K. Production of bacterial cellulose by a static cultivation using the waste from beer culture broth. Korean J. Chem. Eng. 2008, 25, 812–815. [Google Scholar] [CrossRef]
- Hsieha, J.-T.; Wang, M.-J.; Lai, J.-T.; Liu, H.-S. A novel static cultivation of bacterial cellulose production by intermittent feeding strategy. J. Taiwan Inst. Chem. Eng. 2016, 63, 46–51. [Google Scholar] [CrossRef]
- Uzyol, H.K.; Saçan, M.T. Bacterial cellulose production by Komagataeibacter hansenii using algae-based glucose. Environ. Sci. Pollut. Res. 2017, 24, 11154–11162. [Google Scholar] [CrossRef] [PubMed]
- Suwanposri, A.; Yukphan, P.; Yamada, Y.; Ochaikul, D. Statistical optimisation of culture conditions for biocellulose production by Komagataeibacter sp. PAP1 using soya bean whey. Maejo Int. J. Sci. Technol. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Rangaswamy, B.E.; Vanitha, K.P.; Hungund, B.S. Microbial cellulose production from bacteria isolated from rotten fruit. Int. J. Polym. Sci. 2015, 2015, 8. [Google Scholar] [CrossRef]
- Jahan, F.; Kumar, V.; Rawat, G.; Saxena, R.K. Production of microbial cellulose by a bacterium isolated from fruit. Appl. Biochem. Biotechnol. 2012, 167, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, X.A.; Xiong, L.; Guo, H.-J.; Luo, J.; Wang, B.; Zhang, H.-R.; Lin, X.-Q.; Chen, X.-D. Utilization of corncob acid hydrolysate for bacterial cellulose production by Gluconacetobacter xylinus. Appl. Biochem. Biotechnol. 2015, 175, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zhu, Y.X.; Yang, G.; Yang, X.X. Wheat straw acid hydrolysate as a potential cost-effective feedstock for production of bacterial cellulose. J. Chem. Technol. Biotechnol. 2011, 86, 675–680. [Google Scholar] [CrossRef]
- Zeng, X.; Small, D.P.; Wan, W. Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter xylinum BPR 2001 from maple syrup. Carbohydr. Polym. 2011, 85, 506–513. [Google Scholar] [CrossRef]
- Tonouchi, N.; Tsuchida, T.; Yoshinaga, F.; Beppu, T.; Horinouchi, S. Characterization of the biosynthetic pathway of cellulose from glucose and fructose in Acetobacter xylinum. Biosci. Biotechnol. Biochem. 1996, 60, 1377–1379. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Stepanov, N.A.; Senko, O.V.; Maslova, O.V. Immobilized Biocatalysts for Bacterial Cellulose Production. U.S. Patent 2636041, 11 November 2017. [Google Scholar]
- Vincenta, M.; Anthony, L.; Pometto, A.L.; van Leeuwen, J.H. Ethanol production via simultaneous saccharification and fermentation of sodium hydroxide treated corn stover using Phanerochaete chrysosporium and Gloeophyllum trabeum. Bioresour. Technol. 2014, 158, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef] [PubMed]
| Shape of Immobilized Biocatalyst | Dry Cell Biomass Concentration in Immobilized Biocatalyst (g/kg) | BC (g/L) | Biomass of Free Cells Accumulated in the Medium (g CDW/L) |
|---|---|---|---|
| Granules | 20 | 2.9 ± 0.1 | 0.50 ± 0.03 |
| 40 | 3.4 ± 0.2 | 0.53 ± 0.04 | |
| 60 | 3.6 ± 0.2 | 0.57 ± 0.05 | |
| Sheets | 20 | 2.8 ± 0.1 | 0.75 ± 0.05 |
| 40 | 3.2 ± 0.2 | 0.88 ± 0.04 | |
| 60 | 3.4 ± 0.2 | 0.98 ± 0.07 |
| Sugar | Concentration (g/L) | BC (g/L) | |
|---|---|---|---|
| Free Cells | Immobilized Cells | ||
| Arabinose | 20 | 0.03 ± 0.01 | 0.07 ± 0.01 |
| Galactose | 20 | 0.08 ± 0.01 | 0.12 ± 0.02 |
| Glucose | 20 | 1.8 ± 0.10 | 3.6 ± 0.20 |
| Xylose | 20 | 0.19 ± 0.02 | 0.24 ± 0.03 |
| Maltose | 20 | 0.04 ± 0.01 | 0.08 ± 0.01 |
| Sucrose | 20 | 1.9 ± 0.20 | 2.8 ± 0.20 |
| Fructose | 20 | 2.1 ± 0.20 | 3.1 ± 0.20 |
| Glucose/Fructose | 10/10 | 1.9 ± 0.10 | 3.2 ± 0.20 |
| Glucose/Sucrose | 10/10 | 1.8 ± 0.20 | 2.4 ± 0.20 |
| Glucose/Xylose | 10/10 | 0.8 ± 0.10 | 1.3 ± 0.10 |
| Glucose/Fructose | 15/5 | 1.8 ± 0.10 | 2.8 ± 0.20 |
| Glucose/Fructose | 5/15 | 2.1 ± 0.10 | 3.5 ± 0.20 |
| Parameters | Cells | Hydrolysate | |||
|---|---|---|---|---|---|
| Aspen | Jerusalem artichoke | Rice Straw | Wheat Straw | ||
| The residual concentration of RS (g/L) | Free | 11.0 ± 0.5 | 17.0 ± 0.4 | 7.8 ± 0.2 | 4.6 ± 0.3 |
| Immobilized | 12.8 ± 0.5 | 22.7 ± 0.4 | 9.8 ± 0.4 | 7.8 ± 0.3 | |
| BC (g/L) | Free | 2.2 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.3 |
| Immobilized | 2.9 ± 0.1 | 4.5 ± 0.2 | 3.5 ± 0.3 | 3.6 ± 0.2 | |
| BC (g/g RS) | Free | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 |
| Immobilized | 0.1 ± 0.01 | 0.15 ± 0.02 | 0.12 ± 0.01 | 0.12 ± 0.01 | |
| BC productivity (g/L/day) | Free | 0.37 ± 0.2 | 0.42 ± 0.3 | 0.40 ± 0.3 | 0.40 ± 0.3 |
| Immobilized | 0.48 ± 0.2 | 0.75 ± 0.3 | 0.60 ± 0.3 | 0.60 ± 0.3 | |
| Hydrolysates | Initial RS (g/L) | Initial Glucose (g/L) | Accumulated BC (g/L) | BC Productivity (g/L/day) |
|---|---|---|---|---|
| Chlorella vulgaris C1 | 45.1 ± 1.6 | 29.0 ± 0.8 | 2.62 ± 0.12 | 0.440 ± 0.020 |
| Laminaria saccharina (brown) | 36.6 ± 1.1 | 5.1 ± 0.1 | 0.07 ± 0.01 | 0.012 ± 0.001 |
| Acanthophora muscoides (red) | 56.0 ± 1.4 | 13.2 ± 0.3 | 0.42 ± 0.01 | 0.070 ± 0.010 |
| Ulva lactuca (green) | 24.1 ± 0.7 | 7.3 ± 0.2 | 0.08 ± 0.02 | 0.013 ± 0.002 |
| Parameters | BC Free Cells | BC Immobilized Cells | ||||
|---|---|---|---|---|---|---|
| Glucose | Glucose | Glycerol | Hydrolysates of | |||
| Jerusalem artichoke | Rice Straw | Biomass of Chlorella. vulgaris | ||||
| Humidity (%) | 97.5 ± 0.3 | 97.2 ± 0.2 | 97.1 ± 0.2 | 97.0 ± 0.2 | 97.3 ± 0.2 | 98.2 ± 0.2 |
| Thickness (microns) | 35 ± 5 | 45 ± 5 | 65 ± 3 | 70 ± 5 | 40 ± 2 | 40 ± 3 |
| Tensile strength (MPa) | 50 ± 10 | 70 ± 10 | 75 ± 15 | 80 ± 15 | 65 ± 10 | 55 ± 10 |
| Young’s modulus (GPa) | 1.2 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.8 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.2 |
| Crystallinity (%) | 81 ± 1 | 82 ± 1 | 78 ± 1 | 79 ± 1 | 79 ± 1 | 77 ± 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanov, N.; Efremenko, E. “Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources. Catalysts 2018, 8, 33. https://doi.org/10.3390/catal8010033
Stepanov N, Efremenko E. “Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources. Catalysts. 2018; 8(1):33. https://doi.org/10.3390/catal8010033
Chicago/Turabian StyleStepanov, Nikolay, and Elena Efremenko. 2018. "“Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources" Catalysts 8, no. 1: 33. https://doi.org/10.3390/catal8010033
APA StyleStepanov, N., & Efremenko, E. (2018). “Deceived” Concentrated Immobilized Cells as Biocatalyst for Intensive Bacterial Cellulose Production from Various Sources. Catalysts, 8(1), 33. https://doi.org/10.3390/catal8010033




