Immobilized Burkholderia cepacia Lipase on pH-Responsive Pullulan Derivatives with Improved Enantioselectivity in Chiral Resolution
Abstract
1. Introduction
2. Results and Discussion
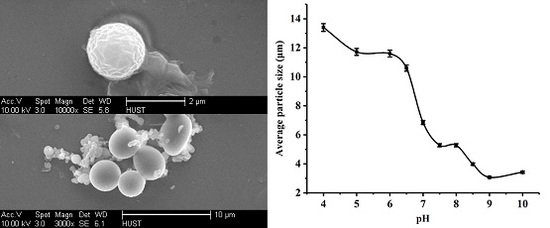
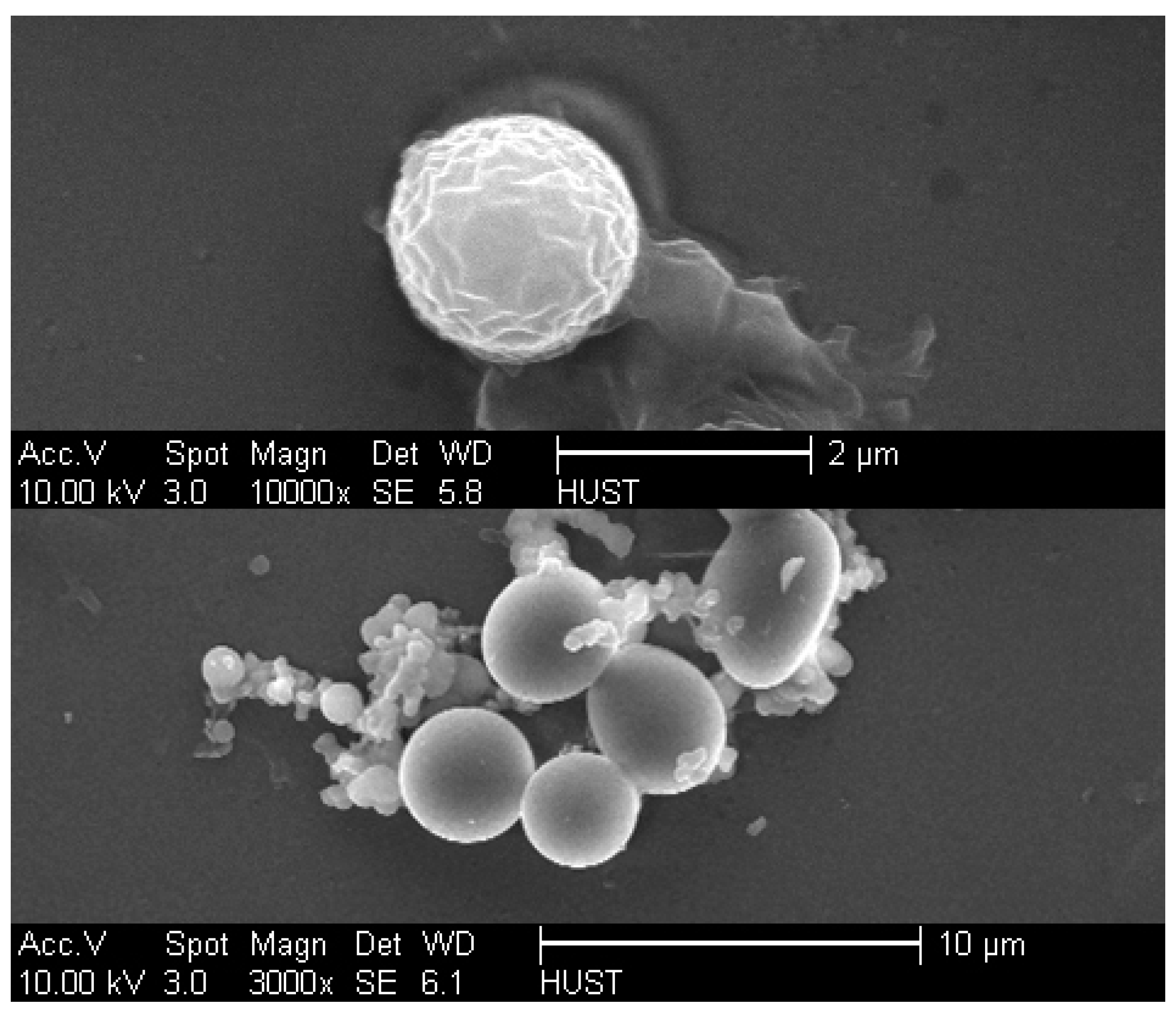
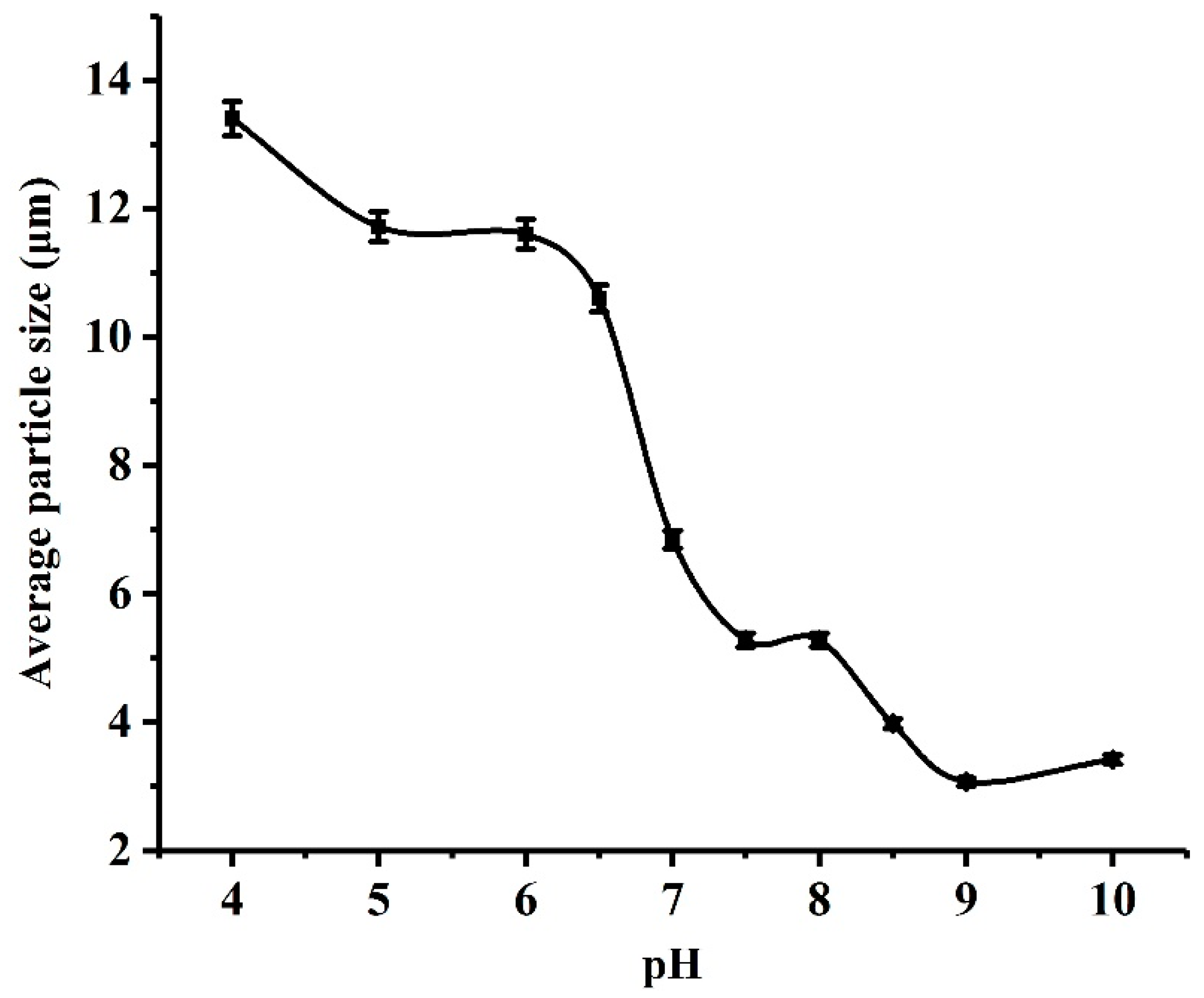
2.1. Particle Characterization and pH Responsibility
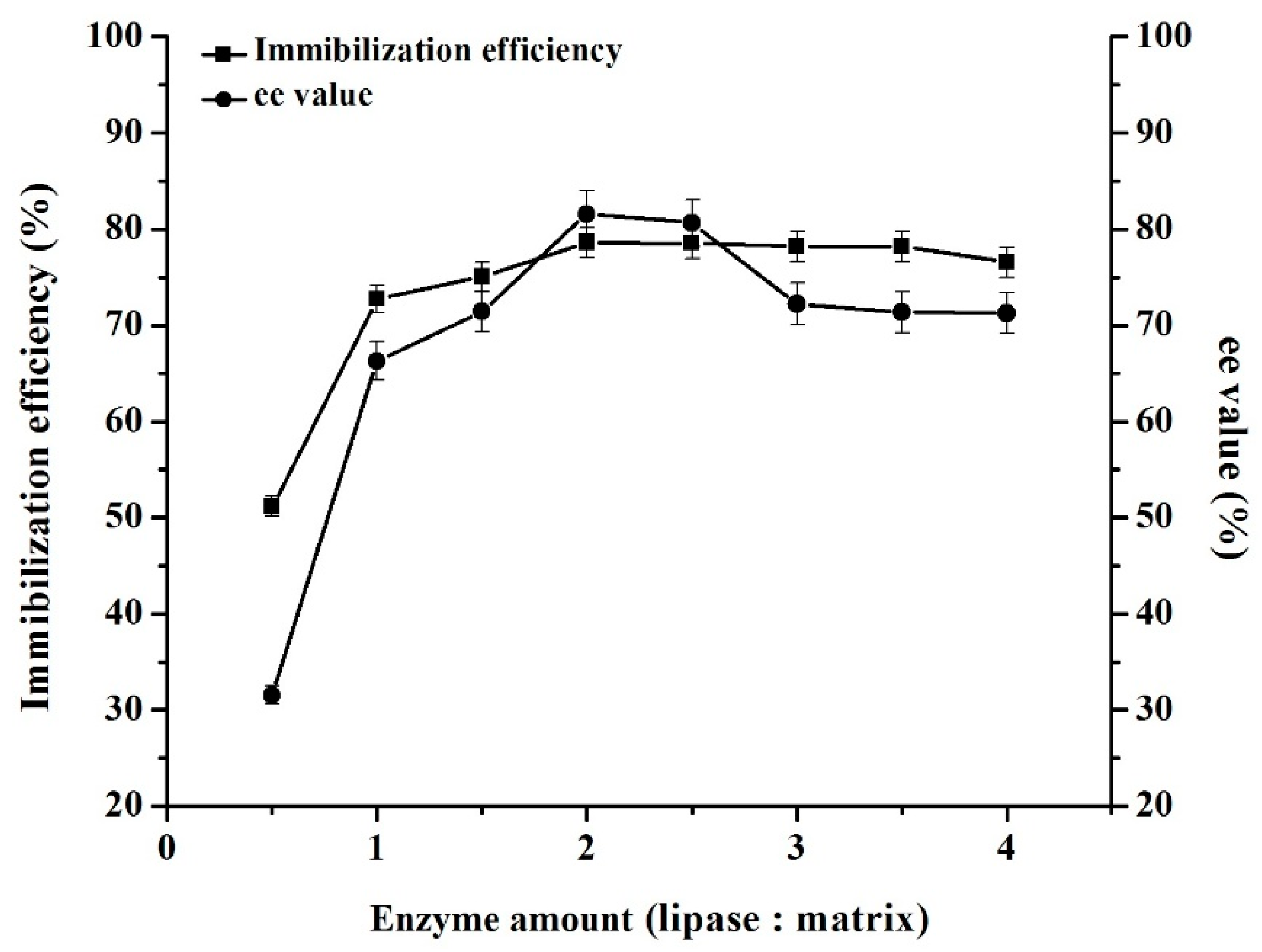
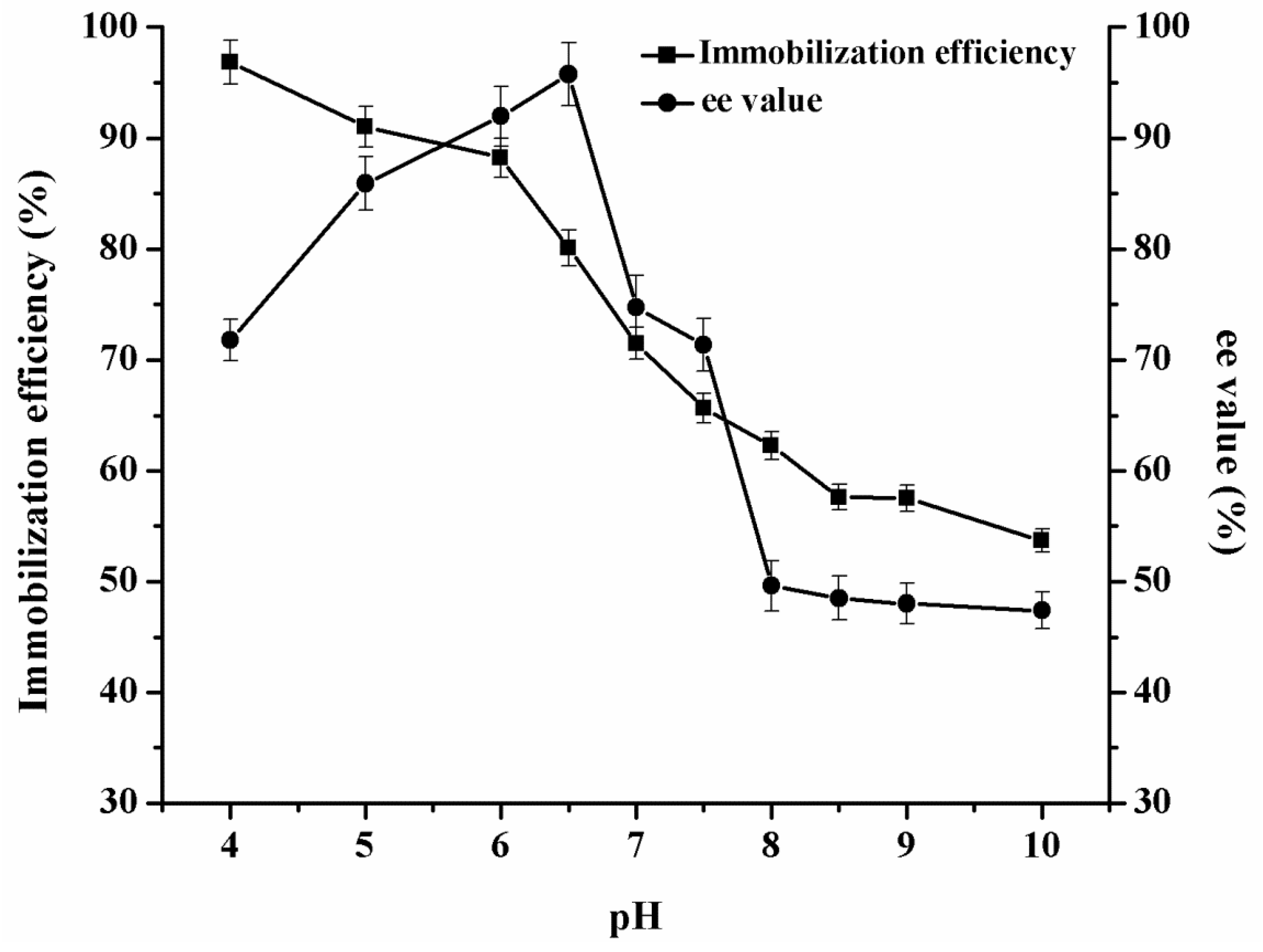
2.2. Lipase Immobilization and Characterization
2.3. Catalytic Performance
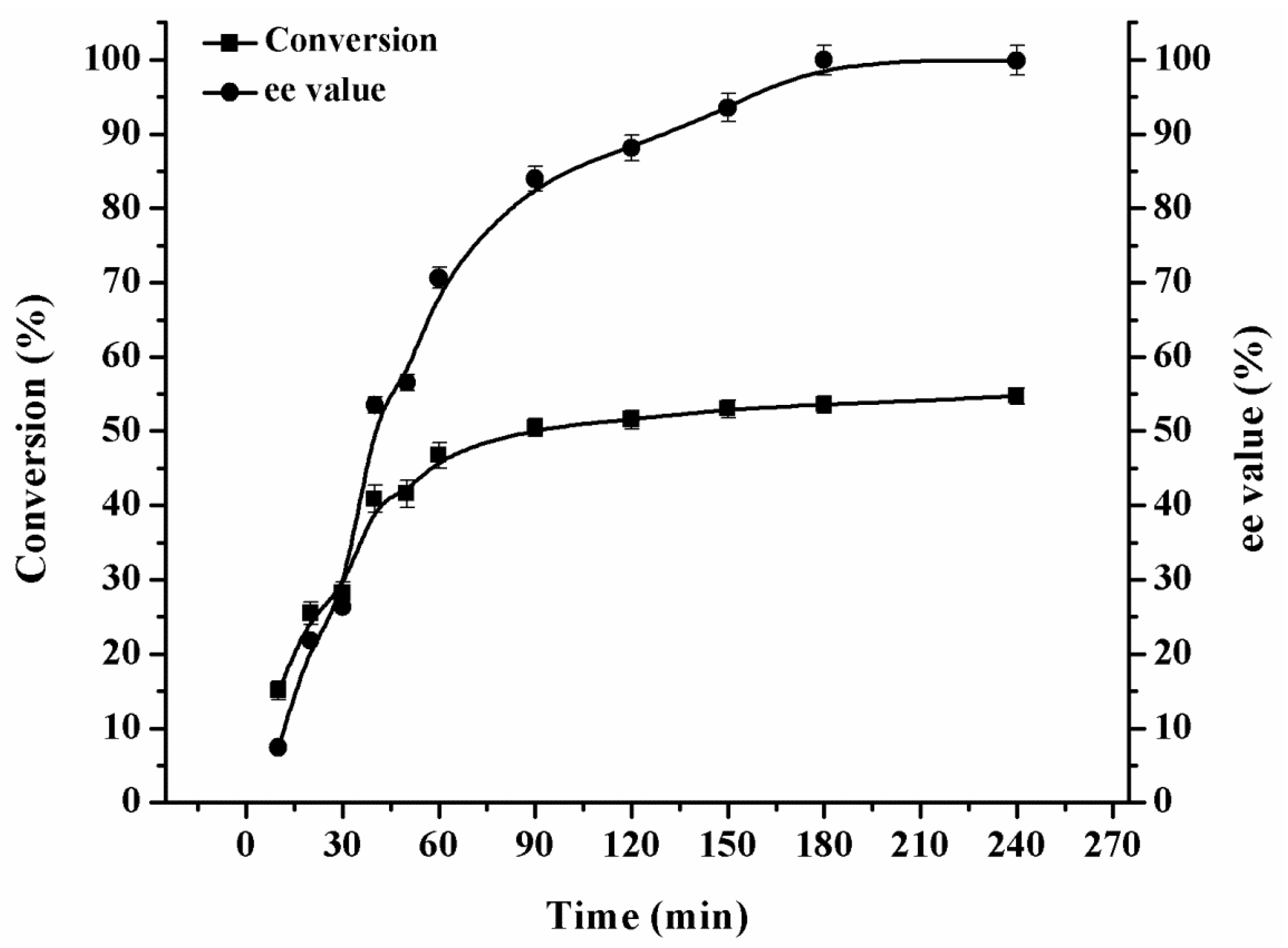
2.3.1. Effect of Reaction Time
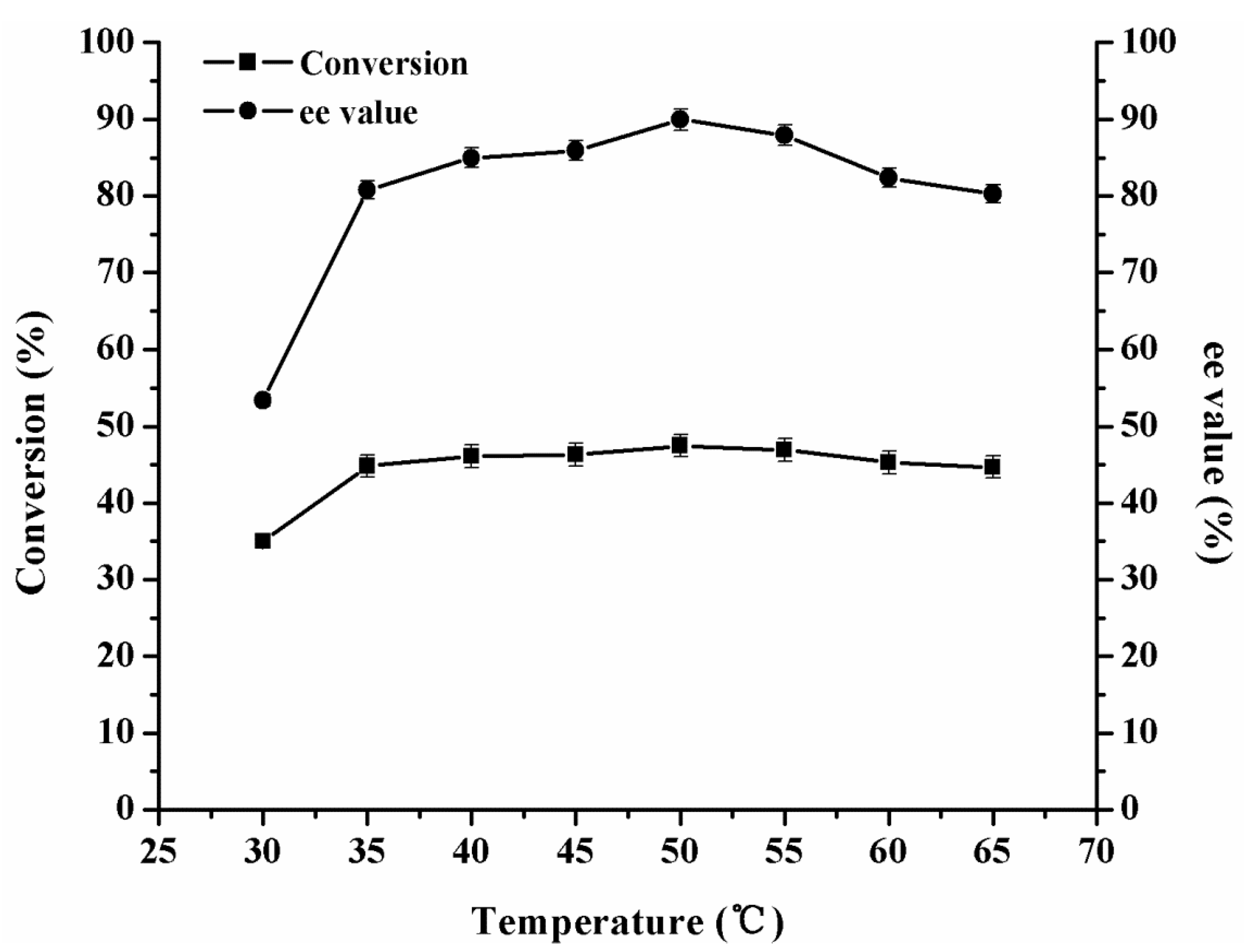
2.3.2. Effect of Temperature
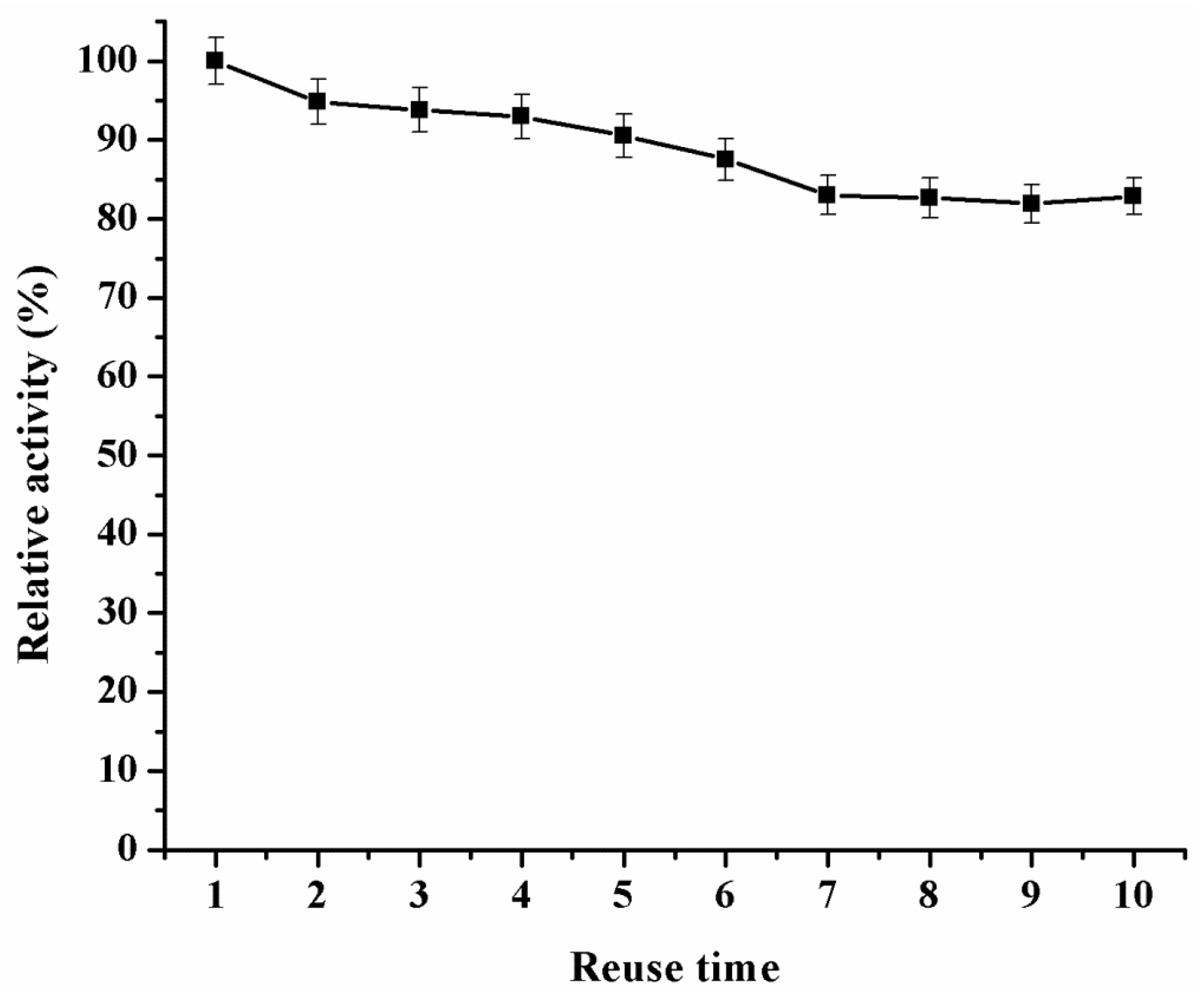
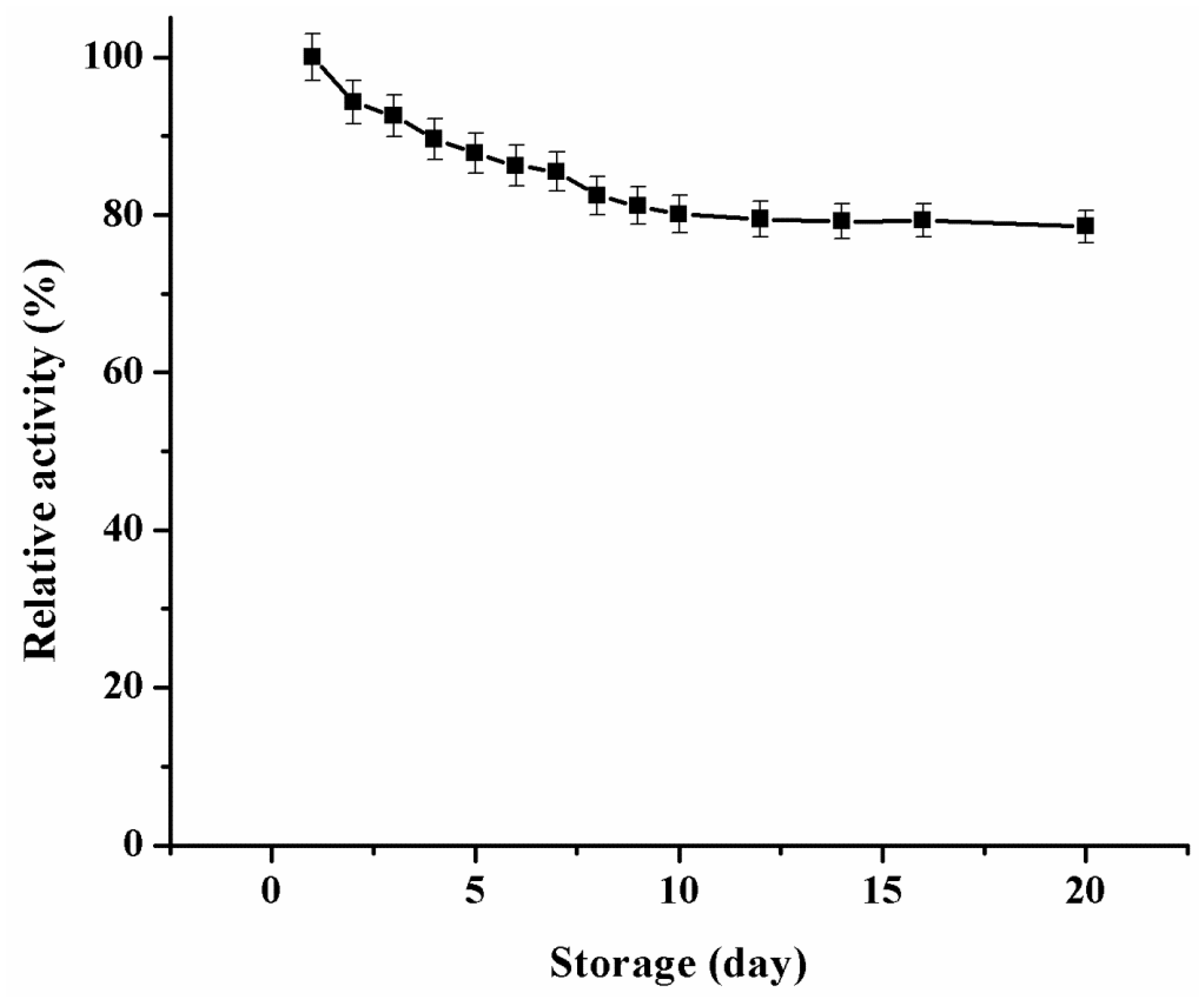
2.3.3. Catalytic Reusability and Storage Stability
2.4. In Comparison with Other Enzymes
3. Experimental
3.1. Materials
3.2. Synthesizing pH-Responsive Particles from Pullulan Polysaccharide
3.3. Particle Size and Specific Surface Area Measurement
3.4. Lipase Immobilization
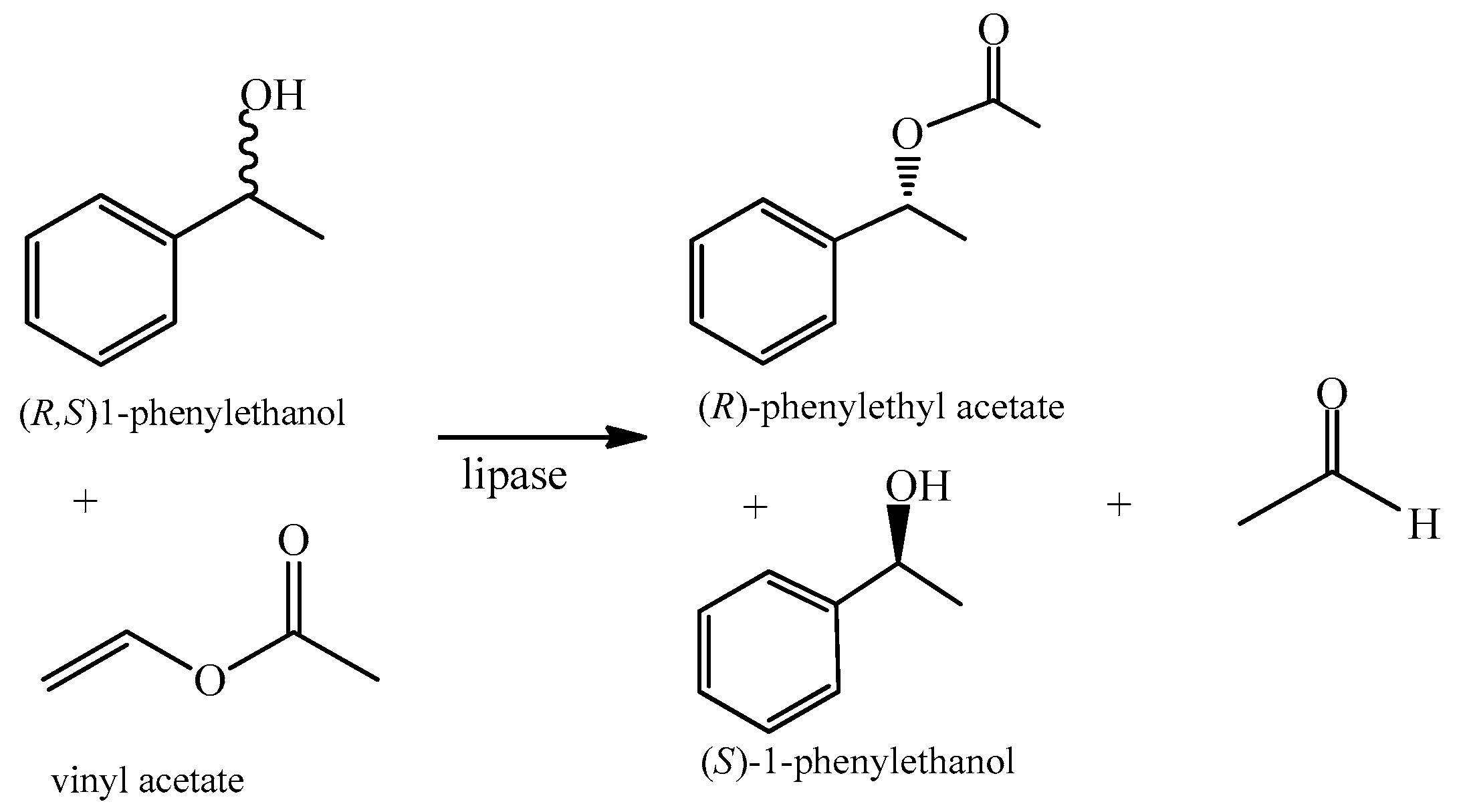
3.5. Catalyzing Resolution of (R, S)-1-Phenylethanol and Efficiency Evaluation
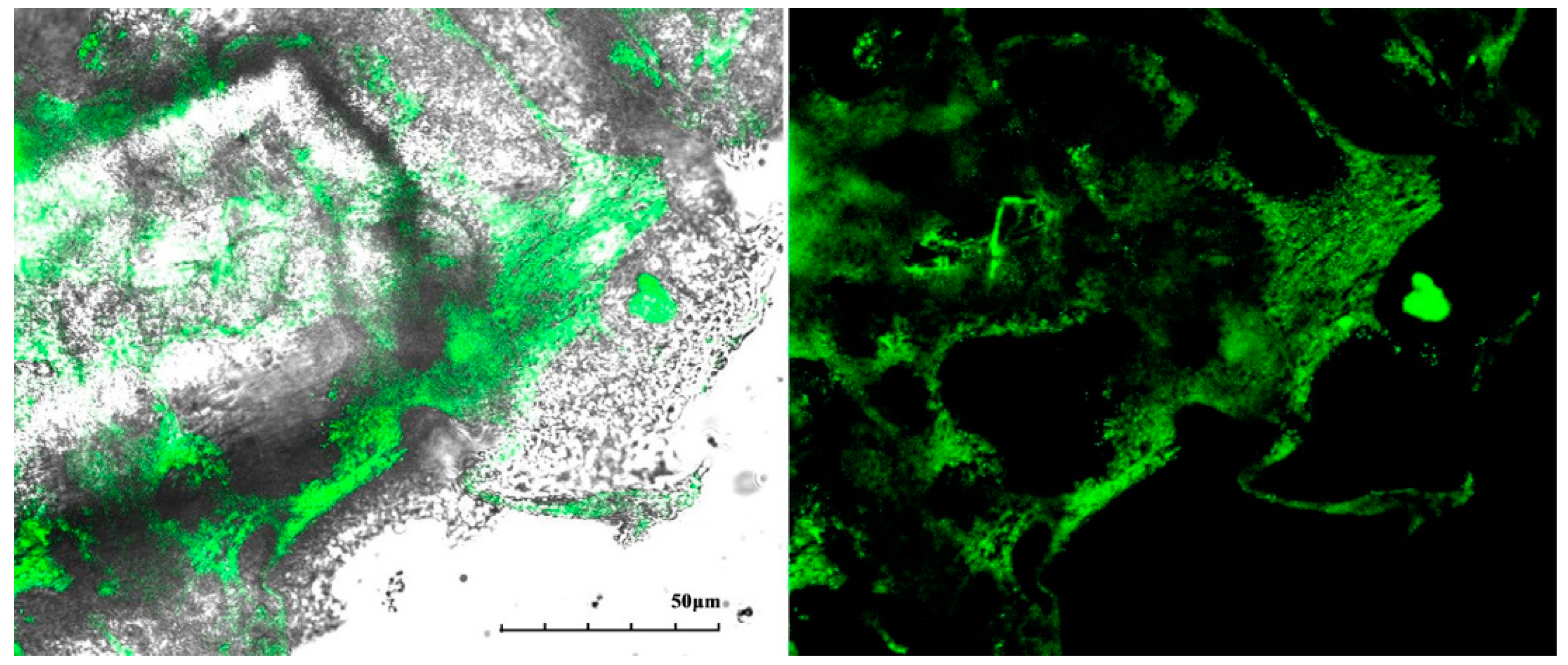
3.6. Characterization via SEM and CLSM for the Probable Mechanism for pH Sensitivity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, A.K.; Mukhopadhyay, M. Overview of Fungal Lipase: A Review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Wang, X.; Li, Q.; Wang, J.; Yan, Y. Improving catalytic performance of Burkholderia cepacia lipase immobilized on macroporous resin NKA. J. Mol. Catal. B Enzym. 2011, 71, 45–50. [Google Scholar] [CrossRef]
- Pan, S.; Xue, L.; Xie, Y.; Yi, Y.; Chong, L.; Yan, Y.; Yun, L. Esterification activity and conformation studies of Burkholderia cepacia lipase in conventional organic solvents, ionic liquids and their co-solvent mixture media. Bioresour. Technol. 2010, 101, 9822–9824. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, D.; Yan, Y. Cloning, expression and characterization of a novel thermal stable and short-chain alcohol tolerant lipase from Burkholderia cepacia strain G63. J. Mol. Catal. B Enzym. 2007, 45, 91–96. [Google Scholar] [CrossRef]
- Drauz, K.; Waldmann, H. Enzyme Catalysis in Organic Synthesis: A Comprehensive Handbook; Wiley-VCH: Hoboken, NJ, USA, 2002; pp. 991–1033. [Google Scholar]
- Ghanem, A.; Aboul-Enein, H.Y. Lipase-Mediated Chiral Resolution of Racemates in Organic Solvents. Cheminform 2004, 15, 3331–3351. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Bao, Y.; Xu, P. Lipase entrapment in protamine-induced bio-zirconia particles: Characterization and application to the resolution of (R, S)-1-phenylethanol. Enzyme Microb. Technol. 2012, 51, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, S.; Xu, L.; Yan, Y. Improving activity and enantioselectivity of lipase via immobilization on macroporous resin for resolution of racemic 1-phenylethanol in non-aqueous medium. BMC Biotechnol. 2013, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.L.; Doan, T.; Won, K.; Ha, S.H.; Koo, Y.M. Immobilization of lipase within carbon nanotube–silica composites for non-aqueous reaction systems. J. Mol. Catal. B Enzym. 2010, 62, 169–172. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An exopolysaccharide and its various applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Fundueanu, G.; Constantin, M.; Ascenzi, P. Preparation and characterization of pH- and temperature-sensitive pullulan microspheres for controlled release of drugs. Biomaterials 2008, 29, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Control. Release 2010, 142, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bian, S.; Huang, Y.; Liang, J.; Fan, Y.; Zhang, X. High drug loading pH-sensitive pullulan-DOX conjugate nanoparticles for hepatic targeting. J. Biomed. Mater. Res. A 2014, 102, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Costantino, H.R.; Griebenow, K.; Langer, R.; Klibanov, A.M. On the pH memory of lyophilized compounds containing protein functional groups. Biotechnol. Bioeng. 1997, 53, 345–348. [Google Scholar] [CrossRef]
- Hirohara, H.; Nabeshima, S.; Fujimoto, M.; Nagase, T. Enzyme Immobilization with Pullulan Gel. U.S. Patent 4247642, 27 January 1981. [Google Scholar]
- Chua, L.S.; Sarmidi, M.R. Immobilised lipase-catalysed resolution of (R, S)-1-phenylethanol in recirculated packed bed reactor. J. Mol. Catal. B Enzym. 2004, 28, 111–119. [Google Scholar] [CrossRef]
- Cui, C.; Xie, R.; Tao, Y.; Zeng, Q.; Chen, B. Improving performance of Yarrowia lipolytica lipase lip2-catalyzed kinetic resolution of (R, S)-1-phenylethanol by solvent engineering. Biocatal. Biotransform. 2015, 33, 38–43. [Google Scholar] [CrossRef]
- Ríos, A.P.D.L.; Rantwijk, F.V.; Sheldon, R.A. Effective resolution of 1-phenyl ethanol by Candida antarctica lipase B catalysed acylation with vinyl acetate in protic ionic liquids (PILs). Green Chem. 2012, 14, 1584–1588. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, Y.H. Novel pH-sensitive polymers containing sulfonamide groups. Macromol. Rapid Commun. 1999, 20, 269–273. [Google Scholar] [CrossRef]
- Paddock, S.W. Confocal laser scanning microscopy. Biotechniques 1999, 27, 992–1004. [Google Scholar] [PubMed]
- Ke, C.; Li, X.; Huang, S.; Xu, L.; Yan, Y. Enhancing enzyme activity and enantioselectivity of Burkholderia cepacia lipase via immobilization on modified multi-walled carbon nanotubes. RSC Adv. 2014, 4, 57810–57818. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Singhal, R.S. Pullulan-complexed α-amylase and glucosidase in alginate beads: Enhanced entrapment and stability. Carbohydr. Polym. 2014, 105, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Fan, Y.; Chen, Y.; Xu, L.; Yan, Y. A new lipase-inorganic hybrid nanoflower with enhanced enzyme activity. RSC Adv. 2016, 6, 19413–19416. [Google Scholar] [CrossRef]
- Ozyilmaz, E.; Sayin, S.; Arslan, M.; Yilmaz, M. Improving catalytic hydrolysis reaction efficiency of sol–gel-encapsulated Candida rugosa lipase with magnetic β-cyclodextrin nanoparticles. Colloids Surf. B 2014, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Siódmiak, T.; Ziegler-Borowska, M.; Marszałł, M.P. Lipase-immobilized magnetic chitosan nanoparticles for kinetic resolution of (R, S)-ibuprofen. J. Mol. Catal. B Enzym. 2013, 94, 7–14. [Google Scholar] [CrossRef]
- Bai, W.; Yang, Y.; Tao, X.; Chen, J.; Tan, T. Immobilization of lipase on aminopropyl-grafted mesoporous silica nanotubes for the resolution of (R, S)-1-phenylethanol. J. Mol. Catal. B Enzym. 2012, 76, 82–88. [Google Scholar] [CrossRef]
- Hara, P.; Mikkola, J.; Murzin, D.Y.; Kanerva, L.T. Supported ionic liquids in Burkholderia cepacia lipase-catalyzed asymmetric acylation. J. Mol. Catal. B Enzym. 2010, 67, 129–134. [Google Scholar] [CrossRef]
- Xue, P.; Yan, X.H.; Wang, Z. Lipase immobilized on HOOC-MCF: A highly enantioselective catalyst for transesterification resolution of (R, S)-1-phenylethanol. Chin. Chem. Lett. 2007, 18, 929–932. [Google Scholar] [CrossRef]
- Na, K.; You, H.B. Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: Characterization, aggregation, and adriamycin release in vitro. Pharm. Res. 2002, 19, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Sato, M.; Kojima, S. Fatty acid methyl ester production using lipase-immobilizing silica particles with different particle sizes and different specific surface areas. Enzyme Microb. Technol. 2006, 39, 889–896. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford method for protein quantitation. In Methods in Molecular Biology; Walker, J.M., Ed.; Humana Press: London, UK, 1994; Volume 32, pp. 9–15. [Google Scholar]
- Li, X.; Xu, L.; Wang, G.; Zhang, H.; Yan, Y. Conformation studies on Burkholderia cenocepacia lipase via resolution of racemic 1-phenylethanol in non-aqueous medium and its process optimization. Process Biochem. 2013, 48, 1905–1913. [Google Scholar] [CrossRef]
- Chen, C.S.; Fujimoto, Y.; Girdaukas, G.; Sih, C.J. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 1982, 104, 7294–7299. [Google Scholar] [CrossRef]
- Feng, S.; Li, G.; Fan, Y.; Yan, Y. Enhanced performance of lipase via microcapsulation and its application in biodiesel preparation. Sci. Rep. 2016, 6, 29670. [Google Scholar] [CrossRef]
| Lipase from | Immobilization Carrier | Conversion (%) | ees (%) | Time (h) | References |
|---|---|---|---|---|---|
| C. rugosa | Magnetic β-cyclodextrin nanoparticles | 49.5 | 98.0 | 24 | 28 |
| C. rugosa | Magnetic chitosan nanoparticles | 50.8 | 79.1 | 140 | 29 |
| Candida sp. 99–125 | Aminopropyl-grafted mesoporous silica nanotubes | NM * | 80.0 | 90 | 30 |
| B. cepacia | Hybrid with calcium phosphate | 47.8 | 91.1 | 24 | 27 |
| B. cepacia | Active carbon cloth | 46.0 | 32.0 | 24 | 31 |
| B. cepacia | pH-responsive particles | 50.0 | 99.2 | 2 | This work |
| B. cepacia | Free lipase | 50.0 | 99.0 | 30 | 9 |
| P. cepacia | Free lipase | 7.1 | 7.7 | 48 | 32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Cui, G.; Ke, C.; Fan, Y.; Yan, Y. Immobilized Burkholderia cepacia Lipase on pH-Responsive Pullulan Derivatives with Improved Enantioselectivity in Chiral Resolution. Catalysts 2018, 8, 13. https://doi.org/10.3390/catal8010013
Xu L, Cui G, Ke C, Fan Y, Yan Y. Immobilized Burkholderia cepacia Lipase on pH-Responsive Pullulan Derivatives with Improved Enantioselectivity in Chiral Resolution. Catalysts. 2018; 8(1):13. https://doi.org/10.3390/catal8010013
Chicago/Turabian StyleXu, Li, Guli Cui, Caixia Ke, Yanli Fan, and Yunjun Yan. 2018. "Immobilized Burkholderia cepacia Lipase on pH-Responsive Pullulan Derivatives with Improved Enantioselectivity in Chiral Resolution" Catalysts 8, no. 1: 13. https://doi.org/10.3390/catal8010013
APA StyleXu, L., Cui, G., Ke, C., Fan, Y., & Yan, Y. (2018). Immobilized Burkholderia cepacia Lipase on pH-Responsive Pullulan Derivatives with Improved Enantioselectivity in Chiral Resolution. Catalysts, 8(1), 13. https://doi.org/10.3390/catal8010013