Efficient Catalytic Upgrading of Levulinic Acid into Alkyl Levulinates by Resin-Supported Acids and Flow Reactors
Abstract
1. Introduction
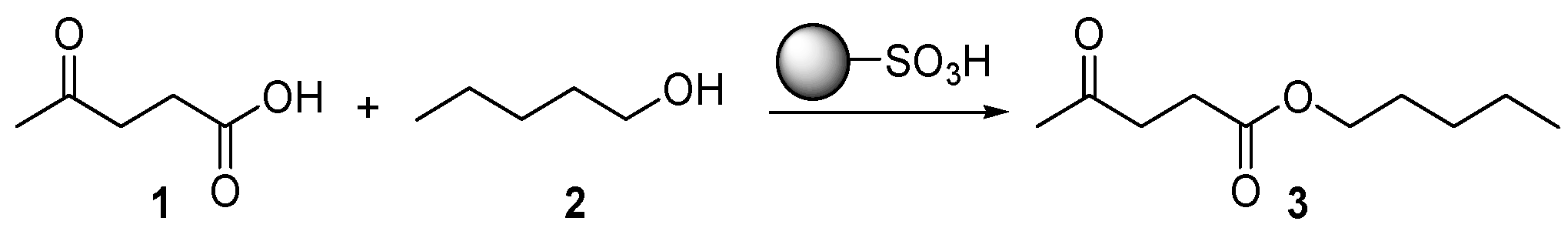
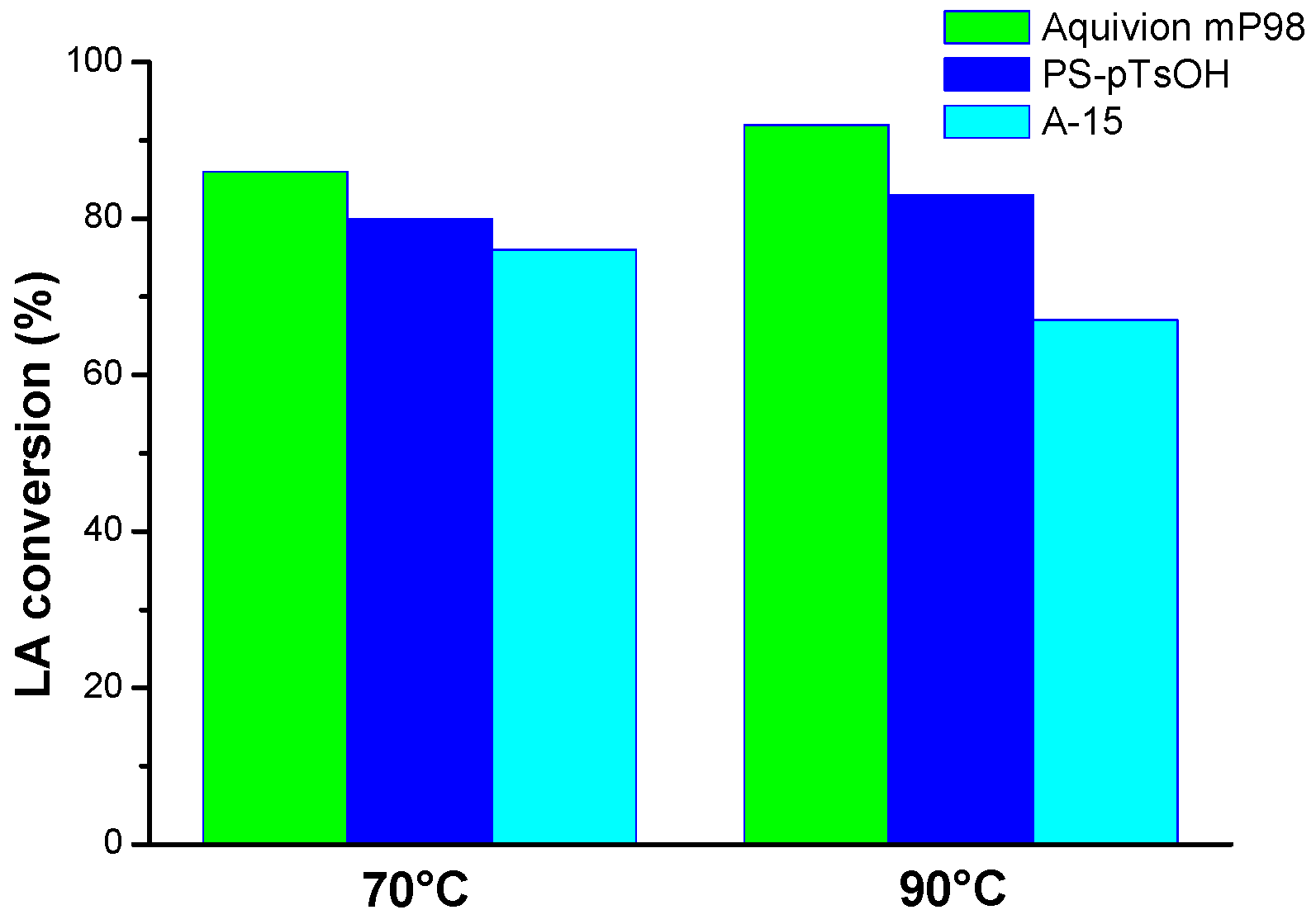
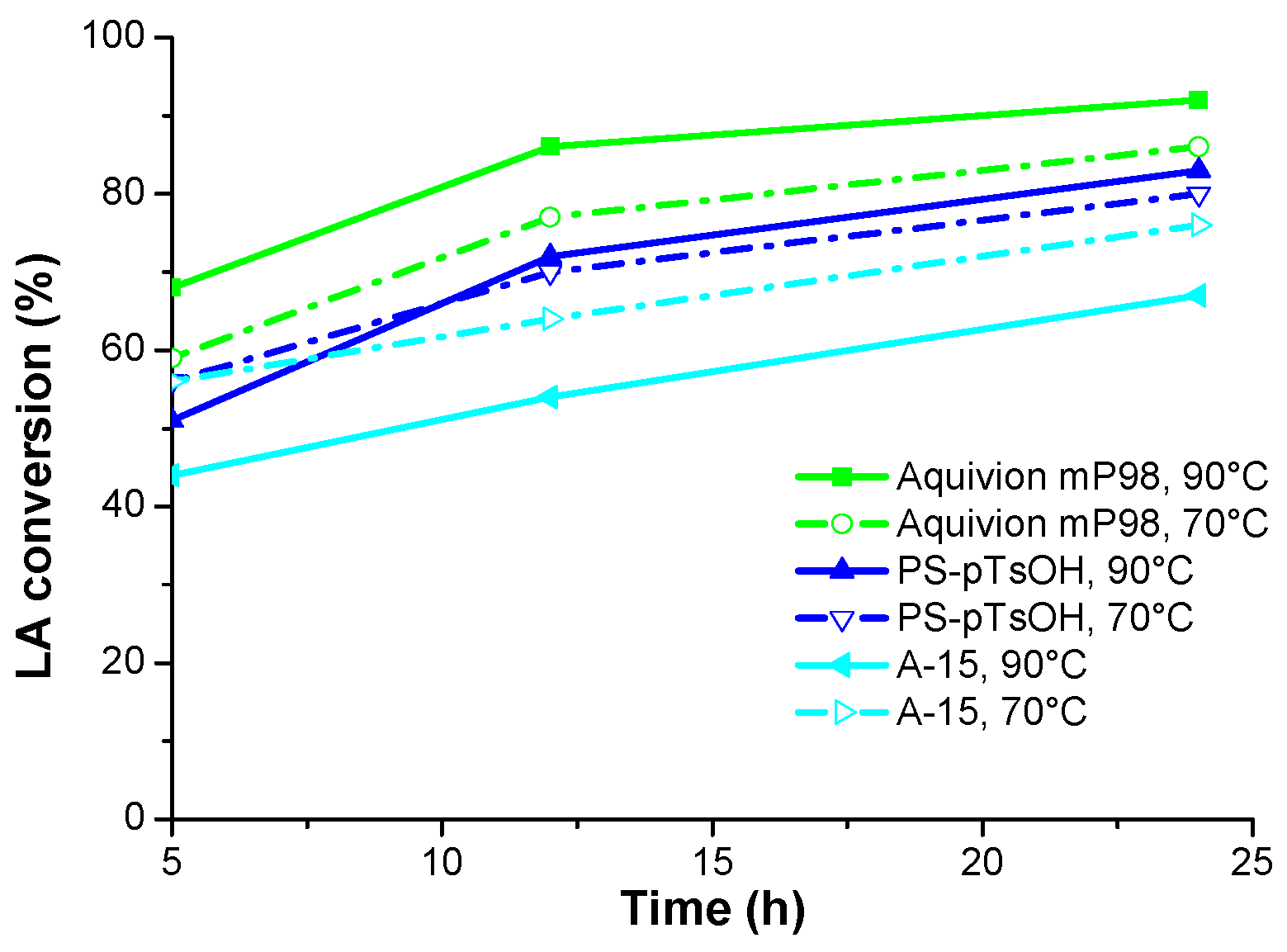
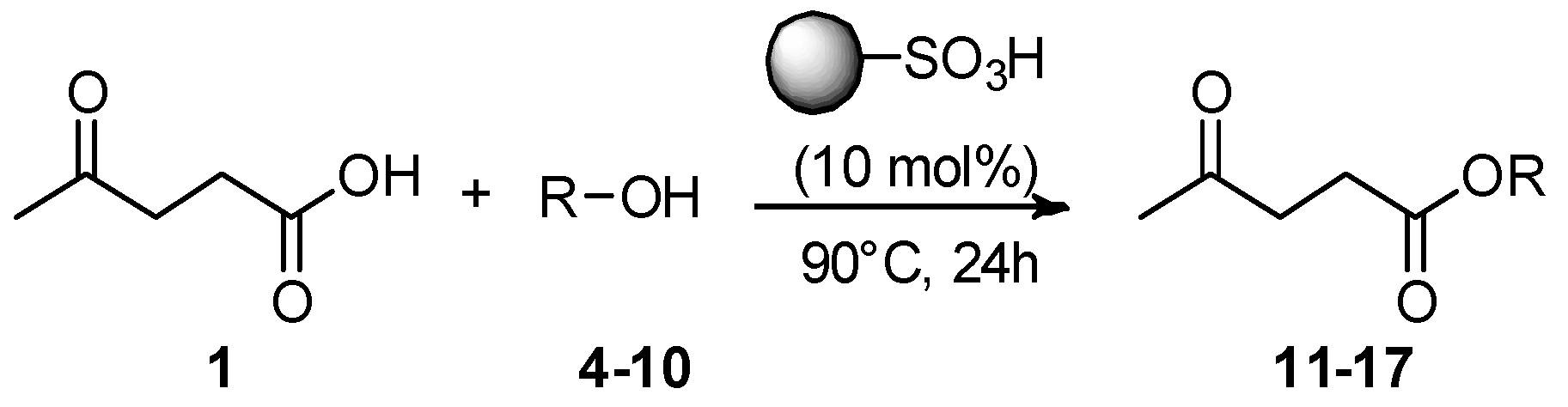
2. Results and Discussion
3. Materials and Methods
3.1. General Remarks
3.2. Representative Experimental Batch Procedure for the Preparation of Pentyllevulinate 3 over Heterogeneous Acid Catalysts
3.3. Esterification Reaction to Pentyllevulinate 3 over Homogeneous p-TsOH Catalyst
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bozell, J.J.; Moens, L.; Wang, D.C.; Fitzpatrick, G.G.; Bilsky, S.W.; Jarnefeld, R.J. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The U.S. Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Werpy, T.A.; White, J.E. (Eds.) Top Value Added Chemicals from Biomass. I. Results of Screening for Potential Candidates from Sugars and Synthesis Gas. U.S. DOE-NREL Report. Available online: https://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on August 2004).
- Climenten, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Palkovits, R. Pentenoic acid pathways for cellulosic biofuels. Angew. Chem. Int. Ed. 2010, 49, 4336–4338. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.F. Alternative Fuel. U.S. Patent 20016309430, 30 October 2001. [Google Scholar]
- Zheng, J.; Zhu, J.; Xu, X.; Wang, W.; Li, J.; Zhao, Y.; Tang, K.; Song, Q.; Kong, D.; Tamg, Y. Continuous hydrogenation of ethyl levulinate to γ-valerolactone and 2-methyl tetrahydrofuran over alumina doped Cu/SiO2 catalyst: The potential of commercialization. Sci. Rep. 2016, 6, 28898. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of gamma-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Jurgen, L.; Petrus, L.; Clarke, L. Valeric biofuels: A platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Braden, D.J.; West, R.M.; Dumesic, J.A. Conversion of cellulose to hydrocarbon fuels by progressive removal of oxygen. Appl. Catal. B Environ. 2010, 100, 184–189. [Google Scholar] [CrossRef]
- Christensen, E.; Yanovitz, J.; Ratcliff, M.; McCormick, R. Renewable Oxygenate Blending Effects on Gasoline Properties. Energy Fuels 2011, 25, 5422–5428. [Google Scholar] [CrossRef]
- Fanick, A.; Cahana, A. Novel Renewable Additive for Diesel Engines; SAE Technical Paper 2014-01-1262; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]
- Groves, A.; Morley, C.; Smith, J.; Stevenson, P.A. Fuel Compositions. U.S. Patent 20050144835 A1, 7 July 2005. [Google Scholar]
- Lake, M.A.; Burton, S.W. Diesel Fuel Compositions Containing Levulinate Ester. U.S. Patent 20100313467 A1, 16 December 2010. [Google Scholar]
- Shrivastav, G.; Khan, T.S.; Agarwal, M.; Haider, M.A. Reformulation of Gasoline to Replace Aromatics by Biomass-Derived Alkyl Levulinates. ACS Sustain. Chem. Eng. 2017, 5, 7118–7127. [Google Scholar] [CrossRef]
- Texaco. Ethyl Levulinate D-975 Diesel Additive Test Program; Texaco/NYSERDA/Biofine Inc.: Glenham, NY, USA, 2000. [Google Scholar]
- Jannsen, A.; Muether, M.; Kolbeck, A.; Lamping, M.; Pischinger, S. The Impact of Different Biofuel Components in Diesel Blends on Engine Efficiency and Emission Performance; SAE Technical Paper 2010-01-2119; SAE International: Warrendale, PA, USA, 2010. [Google Scholar]
- Wang, Z.; Lei, T.; Liu, L.; Zhu, J.; He, X.; Li, Z. Performance Investigations of a Diesel Engine Using Ethyl Levulinate-Diesel Blends. BioResources 2012, 7, 5972–5982. [Google Scholar] [CrossRef]
- Wang, Z.; Chan, X.; Lin, L.; Jan, X.; San, Y.; Shi, X.; He, X.; Zhu, J. Performance and emission characteristics of a diesel engine running on optimized ethyl levulinate–biodiesel–diesel blends. Energy 2016, 95, 29–40. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Munro, M.; Nash, S.; Chuck, C.J. Potential renewable oxygenated biofuels for the aviation and road transport sectors. Fuel 2013, 103, 593–599. [Google Scholar] [CrossRef]
- Dèmolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Luque, R.; Herrero-Davila, L.; Campelo, J.M.; Clark, J.H.; Hidalgo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Biofuels: A technological perspective. Energy Environ. Sci. 2008, 1, 542–564. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Sonar, S.K.; Niphadkar, P.S.; Joshi, P.N.; Deshpande, S.S.; Patil, V.S.; Bokade, V.V. Catalytic upgrading of renewable levulinic acid to ethyl levulinate biodiesel using dodecatungstophosphoric acid supported on desilicated H-ZSM-5 as catalyst. Appl. Catal. A 2013, 460–461, 90–98. [Google Scholar] [CrossRef]
- Pasquale, G.; Vasquez, P.; Romanelli, G.; Baronetti, G. Catalytic upgrading of levulinic acid to ethyl levulinate using reusable silica-included Wells-Dawson heteropolyacid as catalyst. Catal. Commun. 2012, 18, 115–120. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Wen, J. One-step synthesis of mesoporous H4SiW12O40-SiO2 catalysts for the production of methyl and ethyl levulinate biodiesel. Catal. Commun. 2013, 34, 58–63. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Bokade, V.V. Environmentally benign catalytic process for esterification of renewable levulinic acid to various alkyl levulinates biodiesel. Environ. Prog. Sustain. Energy 2015, 34, 795–801. [Google Scholar] [CrossRef]
- Patil, C.R.; Niphadkar, P.S.; Bokade, V.V.; Joshi, P.N. Esterification of levulinic acid to ethyl levulinate over bimodal micro–mesoporous H/BEA zeolite derivatives. Catal. Commun. 2014, 43, 188–191. [Google Scholar] [CrossRef]
- An, S.; Song, D.; Lu, B.; Yang, X.; Guo, Y.H. Morphology Tailoring of Sulfonic Acid Functionalized Organosilica Nanohybrids for the Synthesis of Biomass-Derived Alkyl Levulinates. Chem. Eur. J. 2015, 21, 10786–10798. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Ma, L.; Guo, X.H.; Li, W. Preparation of ethane-bridged organosilica group and keggin type heteropoly acid co-functionalized ZrO2 hybrid catalyst for biodiesel synthesis from eruca sativa gars oil. Catal. Sci. Technol. 2012, 2, 2367–2374. [Google Scholar] [CrossRef]
- Su, F.; Ma, L.; Song, D.; Zhang, X.; Guo, Y. Design of a highly ordered mesoporous H3PW12O40/ZrO2–Si(Ph)Si hybrid catalyst for methyl levulinate synthesis. Green Chem. 2013, 15, 885–890. [Google Scholar] [CrossRef]
- Su, F.; Wu, Q.; Song, D.; Zhang, X.; Wang, M.; Guo, Y. Pore morphology-controlled preparation of ZrO2-based hybrid catalysts functionalized by both organosilica moieties and Keggin-type heteropoly acid for the synthesis of levulinate esters. J. Mater. Chem. A 2013, 1, 13209–13221. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Luque, R.; Macquarrie, D.J. Versatile mesoporous carbonaceous materials for acid catalysis. Chem. Commun. 2007, 6, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Luong, J.H.T. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–34910. [Google Scholar] [CrossRef]
- Nakate, A.V.; Yadav, G.D. Synthesis and Characterization of Sulfonated Carbon-Based Graphene Oxide Monolith by Solvothermal Carbonization for Esterification and Unsymmetrical Ether Formation. ACS Sustain. Chem. Eng. 2016, 4, 1963–1973. [Google Scholar] [CrossRef]
- Oliveira, B.L.; da Silva, V.T. Sulfonated carbon nanotubes as catalysts for the conversion of levulinic acid into ethyl levulinate. Catal. Today 2014, 234, 257–263. [Google Scholar] [CrossRef]
- Li, Z.; Wnetrzak, R.; Kwapinski, W.; Leahy, J.J. Synthesis and Characterization of Sulfated TiO2 Nanorods and ZrO2/TiO2 Nanocomposites for the Esterification of Biobased Organic Acid. ACS Appl. Mater. Interfaces 2012, 4, 4499–4505. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.D.; Yadav, A.R. Synthesis of ethyl levulinate as fuel additives using heterogeneous solid superacidic catalysts: Efficacy and kinetic modeling. Chem. Eng. J. 2014, 243, 556–563. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Karubagi, W.; Nemoto, K.; Fujitani, T. Esterification of levulinic acid with ethanol over sulfated Si-doped ZrO2 solid acid catalyst: Study of the structure–activity relationships. Appl. Catal. A 2014, 476, 186–196. [Google Scholar] [CrossRef]
- Fernandes, D.R.; Rocha, A.S.; Mai, E.F.; Mota, C.J.A.; Teixeira da Silva, V. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal. A 2012, 425, 199–204. [Google Scholar] [CrossRef]
- Maggi, R.; Shijiu, N.R.; Santacroce, V.; Maestri, G.; Bigi, F.; Rothenberg, G. Silica-supported sulfonic acids as recyclable catalyst for esterification of levulinic acid with stoichiometric amounts of alcohols. Beilstein J. Org. Chem. 2016, 12, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Morales, G.; Iglesias, J.; Paniagua, M.; Hernandez, B.; Penedo, S. Efficient conversion of levulinic acid into alkyl levulinates catalyzed by sulfonic mesostructured silicas. Appl. Catal. A 2013, 466, 116–122. [Google Scholar] [CrossRef]
- Barbaro, P.; Liguori, F. Ion Exchange Resins: Catalyst Recovery and Recycle. Chem. Rev. 2009, 109, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Bae, C. Polymer-Supported Acid Catalysis in Organic Synthesis. Curr. Org. Chem. 2011, 8, 208–236. [Google Scholar] [CrossRef]
- Gelbard, G. Organic Synthesis by Catalysis with Ion-Exchange Resins. Ind. Eng. Chem. Res. 2005, 44, 8468–8498. [Google Scholar] [CrossRef]
- Mallesham, B.; Rao, B.G.; Reddy, B.M. Production of biofuel additives by esterification and acetalization of bioglycerol. C. R. Chim. 2016, 19, 1194–1202. [Google Scholar] [CrossRef]
- Tejero, M.A.; Ramìrez, E.; Fitè, C.; Tejero, J.; Cunill, F. Esterification of levulinic acid with butanol over ion exchange resins. Appl. Catal. A 2016, 517, 55–56. [Google Scholar] [CrossRef]
- Vaccaro, L.; Lanari, D.; Marrocchi, A.; Strappaveccia, G. Flow approches towards sustainability. Green Chem. 2014, 16, 3680–3704. [Google Scholar] [CrossRef]
- Marrocchi, A.; Adriaensens, P.; Bartollini, E.; Barkakati, B.; Carleer, R.; Chen, J.; Hensley, D.K.; Petrucci, C.; Tassi, M.; Vaccaro, L. Novel cross-linked polystyrenes with large space network as tailor-made catalyst supports for sustainable media. Eur. Pol. J. 2015, 75, 391–401. [Google Scholar] [CrossRef]
- Castrica, L.; Fringuelli, F.; Gregoli, L.; Pizzo, F.; Vaccaro, L. Amberlite IRA900N3 as a new catalyst for the azidation of alpha,beta-unsaturated ketones under solvent-free conditions. J. Org. Chem. 2006, 71, 9536–9539. [Google Scholar] [CrossRef] [PubMed]
- Fringuelli, F.; Pizzo, F.; Vaccaro, L. First Efficient Regio- and Stereoselective Metal-Catalyzed Azidolysis of 2,3-Epoxycarboxylic Acids in Water. Synlett 2000, 311–314. [Google Scholar] [CrossRef]
- Luis, S.V.; Garcia-Verdugo, E. (Eds.) Chemical Reactions and Processes under Flow Conditions; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Chernysheva, L.; Comino, G.; Brinati, G. Milling Process. Patent WO 2013023983 A1, 21 February 2013. [Google Scholar]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, J.D. Direct Syntheses of Ordered SBA-15 Mesoporous Silica Containing Sulfonic Acid Groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.K.; Lee, J.S. Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresour. Technol. 2010, 101, S62–S65. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Wang, Z.M.; Kim, D.K.; Lee, J.S. Effects of water on the esterification of free fatty acids by acid catalysts. Renew. Energy 2010, 35, 614–618. [Google Scholar] [CrossRef]
- Russbueldt, B.M.E.; Hoelderich, W.F. New sulfonic acid ion-exchange resins for the preesterification of different oils and fats with high content of free fatty acids. Appl. Catal. A Gen. 2009, 362, 47–57. [Google Scholar] [CrossRef]
- Kouzu, M.; Nakagaito, A.; Hidaka, J.-S. Pre-esterification of FFA in plant oil transesterified into biodoesel with the help of solid acid catalysis of sulfonated cation-exchange resin. Appl. Catal. A Gen. 2011, 405, 36–44. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Proc. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Sheldon, R.A. E factors, green chemistry and catalysis: An odyssey. Chem. Commun. 2008, 29, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
| Catalyst Name | Particle Size (mm) | Surface Area (m2/g) | Pore Volume (cm3/g) | Matrix 1 | Swelling Volume 2 (%) | Acid Loading 3 (mmol/g) |
|---|---|---|---|---|---|---|
| A-15 | <0.3 1 | 45 1 | 0.4 1 | Styrene/DVB (macroreticular) | 10 | 4.7 |
| PS-pTsOH | 0.25–0.59 1 | 3.26 | 0.0036 | Styrene/DVB (macroporous) | 50 | 2.9 |
| Aquivion mP98 | ~0.70 | - | - | Tetrafluoroethylene/sulfonylfluoride vinyl ether (non-porous) | 400 | 1.02 |
| Entry | Catalyst (mol %) 1 | LA/1-Pentanol | T (°C) | t (h) | C (%) 2 |
|---|---|---|---|---|---|
| 1 | 4.3 | 1:5 | 70 | 5 | 45 |
| 2 | 8 | 62 | |||
| 3 | 10 | 64 | |||
| 4 | 4.3 | 12 | 46 | ||
| 5 | 8 | 66 | |||
| 6 | 10 | 67 | |||
| 7 | 4.3 | 24 | 53 | ||
| 8 | 8 | 70 | |||
| 9 | 10 | 72 | |||
| 10 | 4.3 | 1:5 | 90 | 5 | 50 |
| 11 | 8 | 68 | |||
| 12 | 10 | 70 | |||
| 13 | 4.3 | 12 | 53 | ||
| 14 | 8 | 69 | |||
| 15 | 10 | 73 | |||
| 16 | 4.3 | 24 | 62 | ||
| 17 | 8 | 73 | |||
| 18 | 10 | 76 (73) 3 | |||
| 19 | 4.3 | 1:10 | 70 | 5 | 47 |
| 20 | 8 | 58 | |||
| 21 | 10 | 59 | |||
| 22 | 4.3 | 12 | 49 | ||
| 23 | 8 | 75 | |||
| 24 | 10 | 77 | |||
| 25 | 4.3 | 24 | 60 | ||
| 26 | 8 | 82 | |||
| 27 | 10 | 86 | |||
| 28 | 4.3 | 1:10 | 90 | 5 | 50 |
| 29 | 8 | 63 | |||
| 30 | 10 | 68 | |||
| 31 | 4.3 | 12 | 58 | ||
| 32 | 8 | 76 | |||
| 33 | 10 | 86 | |||
| 34 | 4.3 | 24 | 66 | ||
| 35 | 8 | 78 | |||
| 36 | 10 | 92 (88) 3,4 |
| Entry | Catalyst (mol %) 1 | LA/1-Pentanol | T (°C) | t (h) | C (%) 2 |
|---|---|---|---|---|---|
| 1 | 4.3 | 1:5 | 70 | 5 | 29 |
| 2 | 8 | 43 | |||
| 3 | 10 | 50 | |||
| 4 | 4.3 | 12 | 32 | ||
| 5 | 8 | 51 | |||
| 6 | 10 | 57 | |||
| 7 | 4.3 | 24 | 53 | ||
| 8 | 8 | 64 | |||
| 9 | 10 | 65 (62) 3 | |||
| 10 | 4.3 | 1:5 | 90 | 5 | 22 |
| 11 | 8 | 38 | |||
| 12 | 10 | 46 | |||
| 13 | 4.3 | 12 | 33 | ||
| 14 | 8 | 39 | |||
| 15 | 10 | 52 | |||
| 16 | 4.3 | 24 | 43 | ||
| 17 | 8 | 46 | |||
| 18 | 10 | 58 | |||
| 19 | 4.3 | 1:10 | 70 | 5 | 50 |
| 20 | 8 | 51 | |||
| 21 | 10 | 56 | |||
| 22 | 4.3 | 12 | 55 | ||
| 23 | 8 | 60 | |||
| 24 | 10 | 64 | |||
| 25 | 4.3 | 24 | 66 | ||
| 26 | 8 | 70 | |||
| 27 | 10 | 76 (73) 3 | |||
| 28 | 4.3 | 1:10 | 90 | 5 | 38 |
| 29 | 8 | 41 | |||
| 30 | 10 | 44 | |||
| 31 | 4.3 | 12 | 50 | ||
| 32 | 8 | 52 | |||
| 33 | 10 | 54 | |||
| 34 | 4.3 | 24 | 58 | ||
| 35 | 8 | 60 | |||
| 36 | 10 | 67 |
| Entry | Catalyst (mol %) 1 | LA/1-Pentanol | T (°C) | t (h) | C (%) 2 |
|---|---|---|---|---|---|
| 1 | 4.3 | 1:5 | 70 | 5 | 32 |
| 2 | 8 | 44 | |||
| 3 | 10 | 58 | |||
| 4 | 4.3 | 12 | 54 | ||
| 5 | 8 | 60 | |||
| 6 | 10 | 62 | |||
| 7 | 4.3 | 24 | 55 | ||
| 8 | 8 | 65 | |||
| 9 | 10 | 68 (64) 3 | |||
| 10 | 4.3 | 1:5 | 90 | 5 | 25 |
| 11 | 8 | 50 | |||
| 12 | 10 | 56 | |||
| 13 | 4.3 | 12 | 50 | ||
| 14 | 8 | 65 | |||
| 15 | 10 | 67 | |||
| 16 | 4.3 | 24 | 50 | ||
| 17 | 8 | 69 | |||
| 18 | 10 | 68 | |||
| 19 | 4.3 | 1:10 | 70 | 5 | 43 |
| 20 | 8 | 50 | |||
| 21 | 10 | 56 | |||
| 22 | 4.3 | 12 | 50 | ||
| 23 | 8 | 65 | |||
| 24 | 10 | 70 | |||
| 25 | 4.3 | 24 | 55 | ||
| 26 | 8 | 73 | |||
| 27 | 10 | 80 | |||
| 28 | 4.3 | 1:10 | 90 | 5 | 50 |
| 29 | 8 | 50 | |||
| 30 | 10 | 51 | |||
| 31 | 4.3 | 12 | 55 | ||
| 32 | 8 | 58 | |||
| 33 | 10 | 72 | |||
| 34 | 4.3 | 24 | 60 | ||
| 35 | 8 | 65 | |||
| 36 | 10 | 83 (78) 3 |
| Entry | Alcohol | Catalyst Type | T (°C) | C (%) 2 |
|---|---|---|---|---|
| 1 | 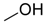 | Aquivion mP98 | 90 | 58 |
| 2 | PS-p-TsOH | 90 | 58 | |
| 3 | A-15 | 70 | 60 | |
| 4 | 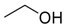 | Aquivion mP98 | 90 | 55 |
| 5 | PS-p-TsOH | 90 | 50 | |
| 6 | A-15 | 70 | 65 | |
| 7 |  | Aquivion mP98 | 90 | 75 |
| 8 | PS-p-TsOH | 90 | 26 | |
| 9 | A-15 | 70 | 70 | |
| 10 | 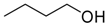 | Aquivion mP98 | 90 | 76 |
| 11 | PS-p-TsOH | 90 | 58 | |
| 12 | A-15 | 70 | 58 | |
| 13 |  | Aquivion mP98 | 90 | 84 |
| 14 | PS-p-TsOH | 90 | 62 | |
| 15 | A-15 | 70 | 80 | |
| 16 | 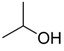 | Aquivion mP98 | 90 | 50 |
| 17 | PS-p-TsOH | 90 | 32 | |
| 18 | A-15 | 70 | 12 | |
| 19 | 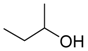 | Aquivion mP98 | 90 | 56 |
| 20 | PS-p-TsOH | 90 | 25 | |
| 21 | A-15 | 70 | 10 |
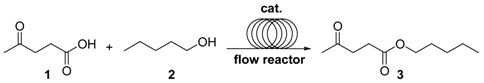
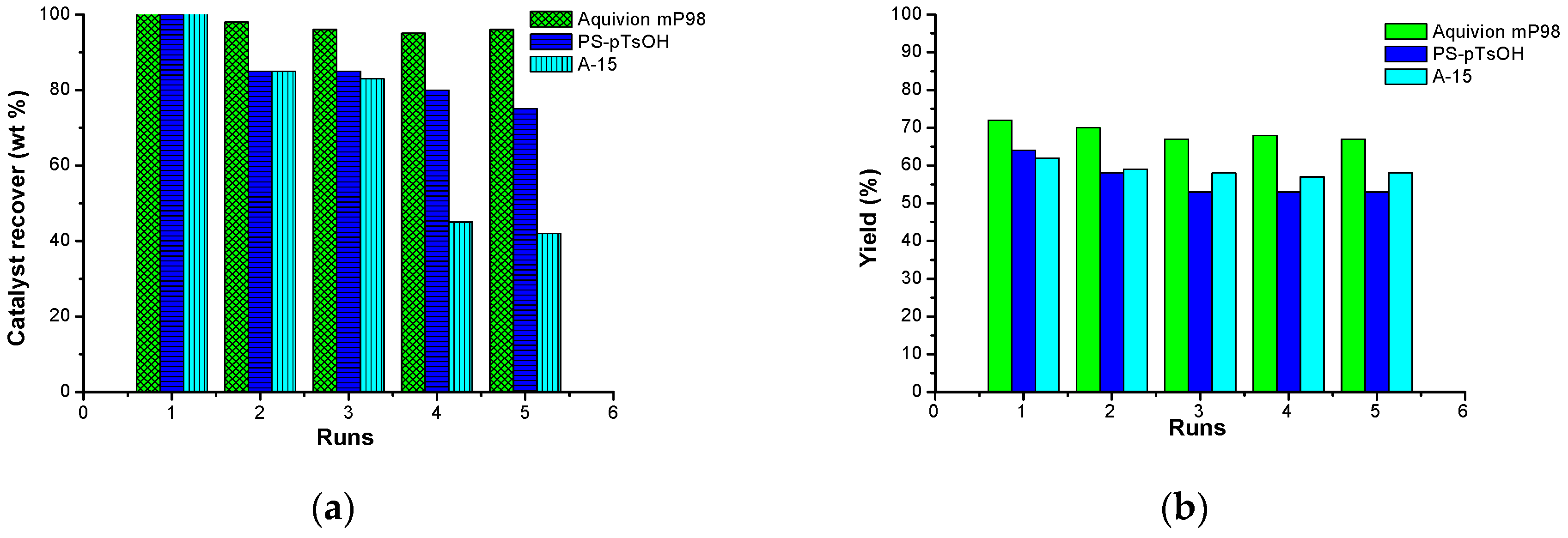
| Entry | Catalyst Type | Cycle | C (%) 2 | gxh−1 | TON 3 | TOF (h−1) 4 |
|---|---|---|---|---|---|---|
| 1 | A-15 | 1 | 93 | 0.93 | 11.5 | 0.88 |
| 2 | 2 | 93 | 0.93 | |||
| 3 | PS-p-TsOH | 1 | 98 | 4.73 | 47.8 | 4.30 |
| 4 | 2 | 98 | 4.73 | |||
| 5 | Aquivion mP98 | 1 | >99 | 4.8 | 37.4 | 4.68 |
| 6 | 2 | >99 | 4.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombettoni, V.; Bianchi, L.; Zupanic, A.; Porciello, A.; Cuomo, M.; Piermatti, O.; Marrocchi, A.; Vaccaro, L. Efficient Catalytic Upgrading of Levulinic Acid into Alkyl Levulinates by Resin-Supported Acids and Flow Reactors. Catalysts 2017, 7, 235. https://doi.org/10.3390/catal7080235
Trombettoni V, Bianchi L, Zupanic A, Porciello A, Cuomo M, Piermatti O, Marrocchi A, Vaccaro L. Efficient Catalytic Upgrading of Levulinic Acid into Alkyl Levulinates by Resin-Supported Acids and Flow Reactors. Catalysts. 2017; 7(8):235. https://doi.org/10.3390/catal7080235
Chicago/Turabian StyleTrombettoni, Valeria, Luca Bianchi, Ana Zupanic, Alessandro Porciello, Maurizio Cuomo, Oriana Piermatti, Assunta Marrocchi, and Luigi Vaccaro. 2017. "Efficient Catalytic Upgrading of Levulinic Acid into Alkyl Levulinates by Resin-Supported Acids and Flow Reactors" Catalysts 7, no. 8: 235. https://doi.org/10.3390/catal7080235
APA StyleTrombettoni, V., Bianchi, L., Zupanic, A., Porciello, A., Cuomo, M., Piermatti, O., Marrocchi, A., & Vaccaro, L. (2017). Efficient Catalytic Upgrading of Levulinic Acid into Alkyl Levulinates by Resin-Supported Acids and Flow Reactors. Catalysts, 7(8), 235. https://doi.org/10.3390/catal7080235









