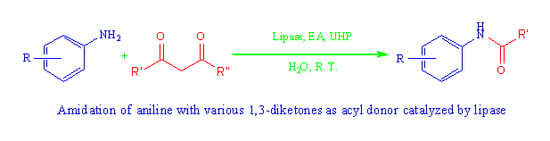
Lipase-Mediated Amidation of Anilines with 1,3-Diketones via C–C Bond Cleavage
Abstract
:1. Introduction
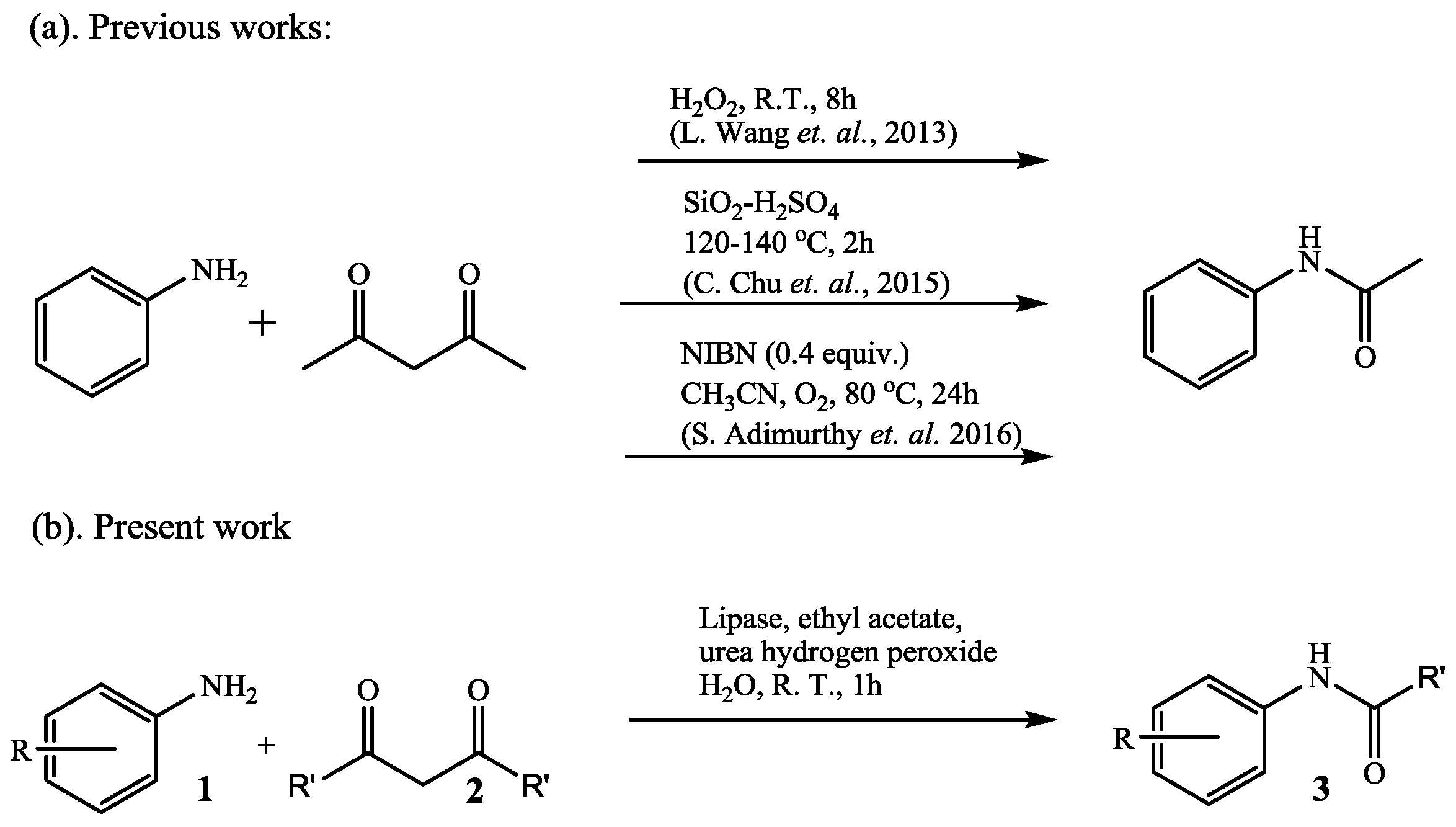
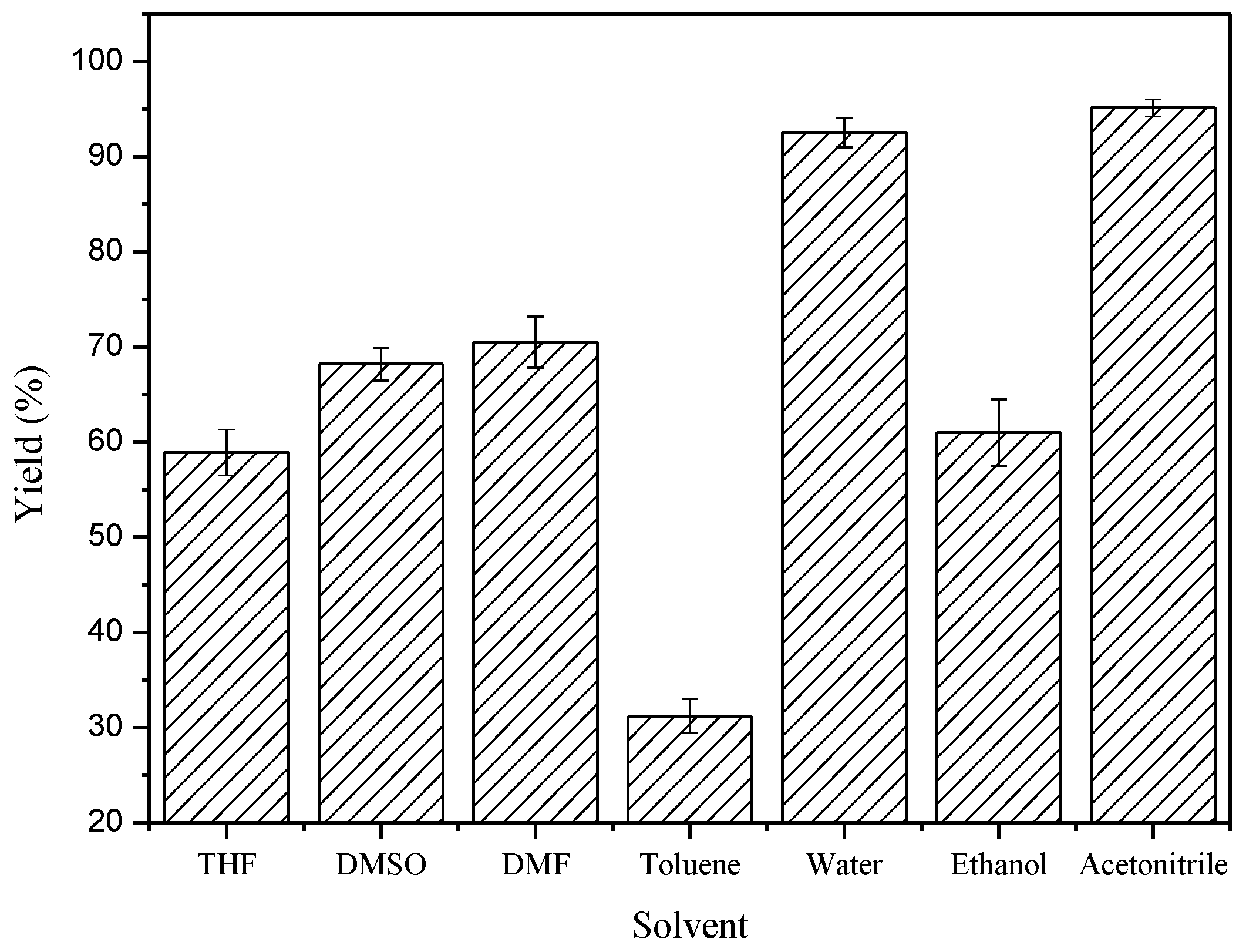
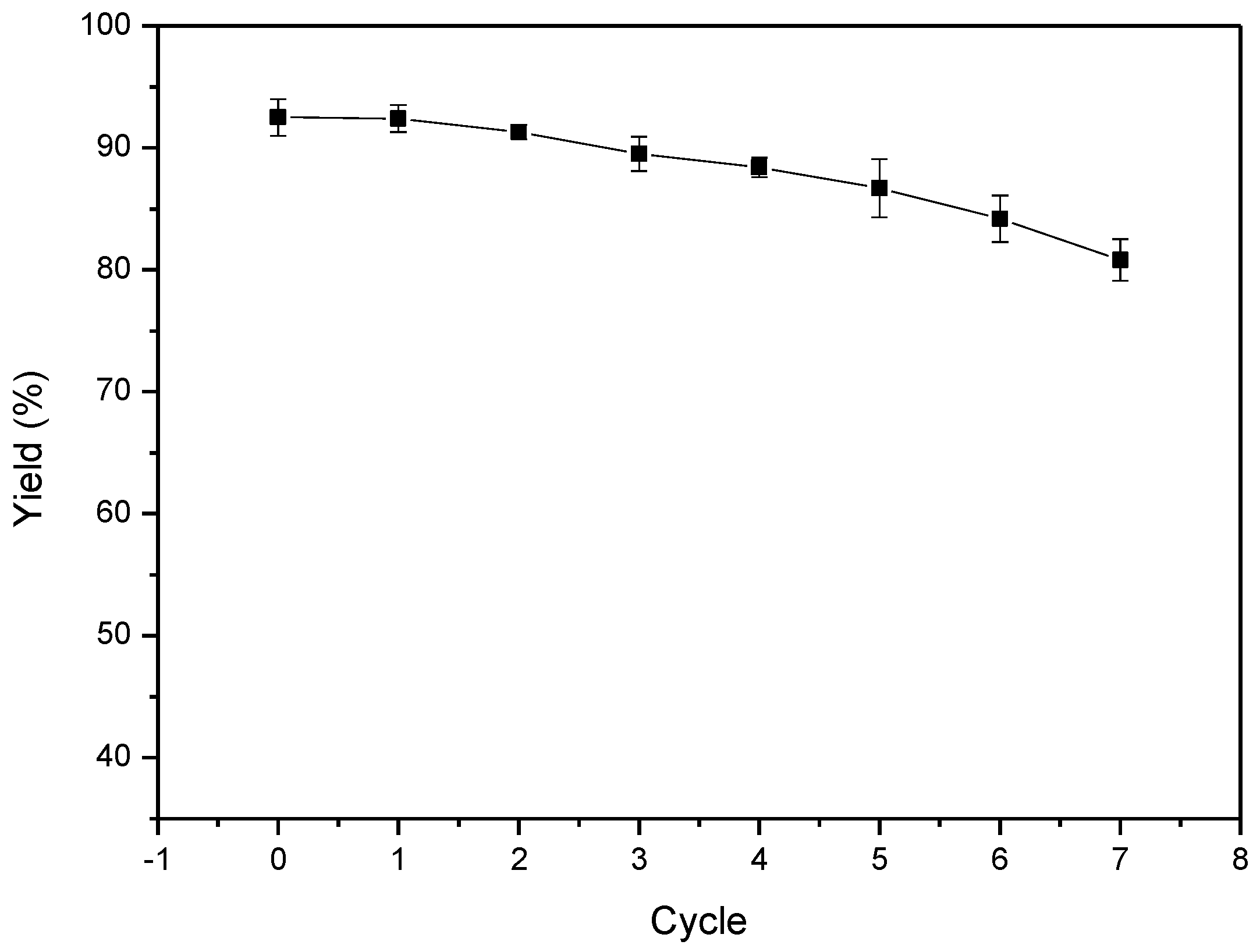
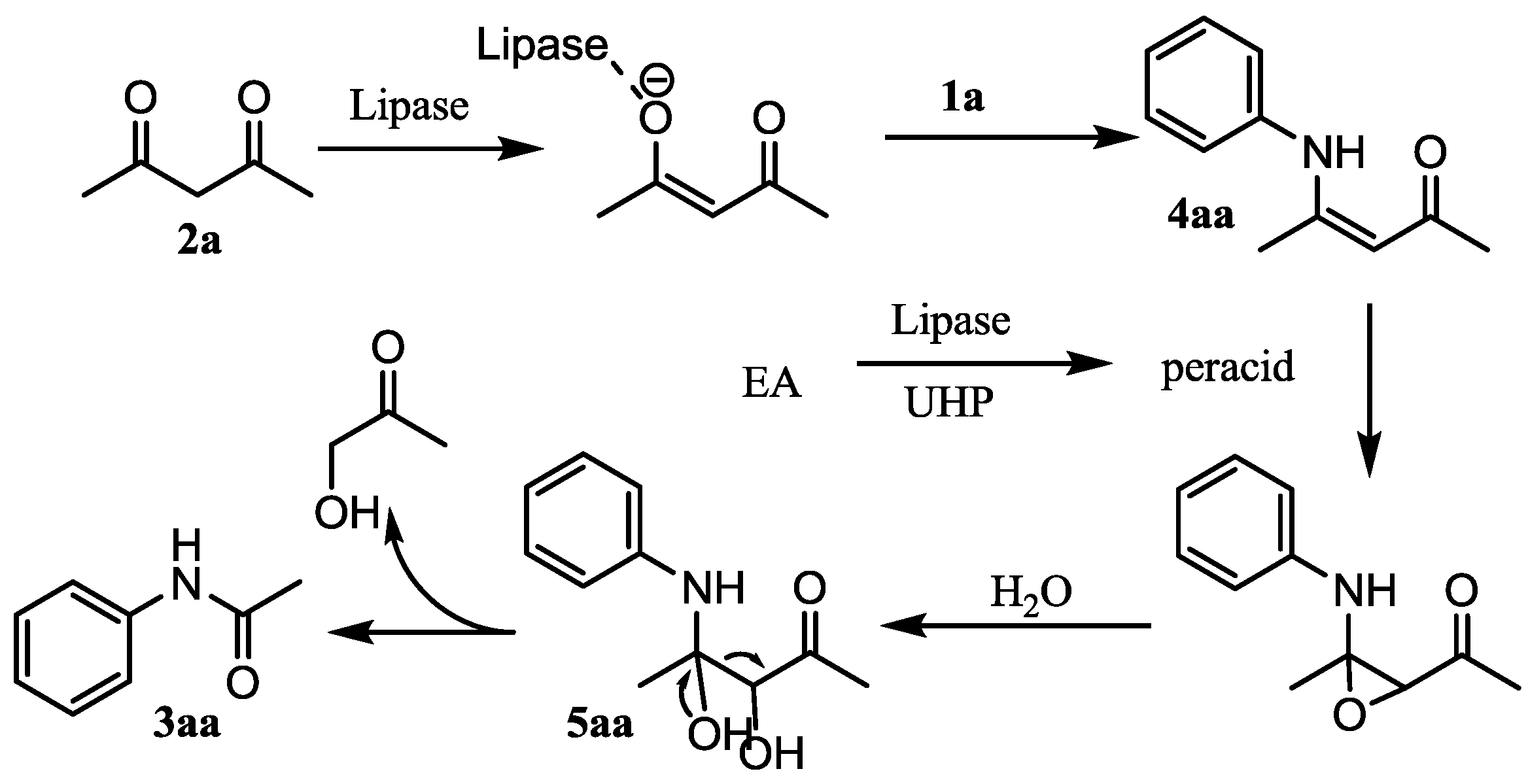
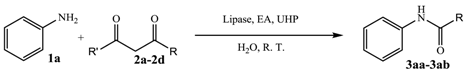
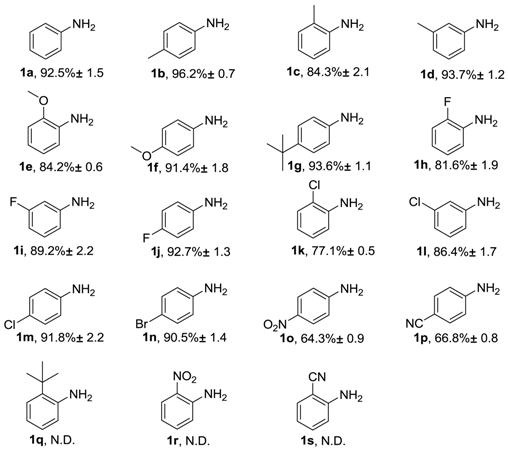
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. General Procedure of the Lipase-Mediated Oxidative Formation of Amides
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bode, J.W. Emerging methods in amide-and peptide-bond formation. Curr. Opin. Drug Discov. Dev. 2006, 9, 765–975. [Google Scholar] [CrossRef]
- Humphrey, J.M.; Chamberlin, A.R. Chemical Synthesis of Natural Product Peptides: Coupling Methods for the Incorporation of Noncoded Amino Acids into Peptides. Chem. Rev. 1997, 97, 2243–2266. [Google Scholar] [CrossRef] [PubMed]
- Valeur, E.; Bradley, M. Amide bond formation: Beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, C.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Crochet, P.; Cadierno, V. Metal-catalyzed amide bond forming reactions in an environmentally friendly aqueous medium: nitrile hydrations and beyond. Green Chem. 2013, 15, 46–66. [Google Scholar] [CrossRef]
- Allen, C.L.; Williams, J.M.J. Metal-catalysed approaches to amide bond formation. Chem. Soc. Rev. 2011, 40, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Kang, S.; Hong, H. N-Heterocyclic carbene-based well-defined ruthenium hydride complexes for direct amide synthesis from alcohols and amines under base-free conditions. Tetrahedron 2015, 71, 4565–4569. [Google Scholar] [CrossRef]
- Dhake, K.P.; Qureshi, Z.S.; Singhal, R.S.; Bhanage, B.M. Candida antarctica lipase B-catalyzed synthesis of acetamides using [BMIm (PF 6)] as a reaction medium. Tetrahedron Lett. 2009, 50, 2811–2814. [Google Scholar] [CrossRef]
- Gotor, V. Non-conventional hydrolase chemistry: Amide and carbamate bond formation catalyzed by lipases. Bioorg. Med. Chem. 1999, 7, 2189–2197. [Google Scholar] [CrossRef]
- Kuo, C.H.; Lin, J.A.; Chien, C.M.; Tsai, C.H.; Liu, Y.C.; Shieh, C.J. Formation of amide bond catalyzed by lipase in aqueous phase for peptide synthesis. J. Mol. Catal. B-Enzym. 2016, 129, 15–20. [Google Scholar] [CrossRef]
- Nechab, M.; Blidi, L.E.; Vanthuyne, N.; Gastaldi, S.; Bertrand, M.P.; Gil, G. N-Acyl glycinates as acyl donors in serine protease-catalyzed kinetic resolution of amines. Improvement of selectivity and reaction rate. Org. Biomol. Chem. 2008, 6, 3917–3920. [Google Scholar] [CrossRef] [PubMed]
- Ebert, C.; Gardossi, L.; Linda, P. Control of enzyme hydration in penicillin amidase catalysed synthesis of amide bond. Tetrahedron Lett. 1996, 37, 9377–9380. [Google Scholar] [CrossRef]
- Giordano, R.C.; Ribeiro, M.P.A.; Giordano, R.L.C. Kinetics of β-lactam antibiotics synthesis by penicillin G acylase (PGA) from the viewpoint of the industrial enzymatic reactor optimization. Biotechnol. Adv. 2006, 24, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Kavala, V.; Wang, C.C.; Barange, D.K.; Kuo, C.W.; Lei, P.M.; Yao, C.F. Synthesis of Isocoumarin Derivatives via the Copper-Catalyzed Tandem Sequential Cyclization of 2- Halo-N-phenyl Benzamides and Acyclic 1,3-Diketones. J. Org. Chem. 2012, 77, 5022–5029. [Google Scholar] [CrossRef] [PubMed]
- Kawata, A.; Takata, K.; Kuninobu, Y.; Takai, K. Indium-Catalyzed Retro-Claisen Condensation. Angew. Chem. Int. Ed. 2007, 46, 7793–7795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Wang, M.; Zhang, Y.C.; Wang, L. A novel and metal-free approach towards α-ketoamides using a TBHP/I2-promoted tandem reaction of amines with β-diketones via C–C bond cleavage. RSC Adv. 2013, 3, 1311–1316. [Google Scholar] [CrossRef]
- Payette, J.N.; Yamamoto, H. Nitrosobenzene-Mediated C–C Bond Cleavage Reactions and Spectral Observation of an Oxazetidin-4-one Ring System. J. Am. Chem. Soc. 2008, 130, 12276–12278. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, C.; Zhang, Z.G.; Wu, P.Y.; Jiang, X.F. Transition-Metal-Free Aerobic Oxidative Cleavage of C–C Bonds in α-Hydroxy Ketones and Mechanistic Insight to the Reaction Pathway. Angew. Chem. 2012, 124, 12738–12742. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.; Li, P.H.; Zhang, X.L.; Wang, L. H2O2-mediated oxidative formation of amides from aromatic amines and 1,3-diketones as acylation agents via C–C bond cleavage at room temperature in water under metal-free conditions. Green Chem. 2013, 15, 3289–3294. [Google Scholar] [CrossRef]
- Rao, S.N.; Mohan, D.C.; Adimurthy, S. AIBN-promoted amidation of anilines with 1,3-diketones via oxidative cleavage of C–C bond under aerobic conditions. Tetrahedron 2016, 72, 4889–4894. [Google Scholar] [CrossRef]
- Guo, R.Q.; Zhu, C.L.; Sheng, Z.; Li, Y.Z.; Yin, W.; Chu, C.H. Silica sulfuric acid mediated acylation of amines with 1,3-diketones via C–C bond cleavage under solvent-free conditions. Tetrahedron Lett. 2015, 56, 6223–6226. [Google Scholar] [CrossRef]
- Björkling, F.; Godtfredsen, S.E.; Kirk, O. Lipase-mediated formation of peroxycarboxylic acids used in catalytic epoxidation of alkenes. J. Chem. Soc., Chem. Commun. 1990, 1301–1303. [Google Scholar]
- Kirk, O.; Christensen, M.W.; Damhus, T.; Godtfredsen, S.E. Enzyme catalyzed degradation and formation of peroxycarboxylic acids. Biocatalysis 1994, 11, 65–77. [Google Scholar] [CrossRef]
- Yang, F.J.; Zhang, X.W.; Li, F.X.; Wang, Z.; Wang, L. A lipase-glucose oxidase system for the efficient oxidation of N-heteroaromatic compounds and tertiary amines. Green Chem. 2016, 18, 3518–3521. [Google Scholar] [CrossRef]
- López-Iglesias, M.; Busto, E.; Gotor, V.; Gotor-Fernández, V. Use of Protease from Bacillus licheniformis as Promiscuous Catalyst for Organic Synthesis: Applications in C–C and C-N Bond Formation Reactions. Adv. Synth. Catal. 2011, 353, 2345–2353. [Google Scholar] [CrossRef]
- Hult, K.; Berglund, P. Enzyme promiscuity: Mechanism and applications. Trends Biotechnol. 2007, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vongvilai, P.; Sakulsombat, M.; Fischer, A.; Ramström, O. Asymmetric Synthesis of Substituted Thiolanes through Domino Thia-Michael–Henry Dynamic Covalent Systemic Resolution using Lipase Catalysis. Adv. Synth. Catal. 2014, 356, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Klaas, M.R.; Warwel, S. Lipase-catalyzed preparation of peroxy acids and their use for epoxidation. J. Mol. Catal. A Chem. 1997, 117, 311–319. [Google Scholar] [CrossRef]
- Kotlewska, A.J.; Rantwijk, F.; Sheldon, R.A.; Arends, I.W.C.E. Epoxidation and Baeyer–Villiger oxidation using hydrogen peroxide and a lipase dissolved in ionic liquids. Green Chem. 2011, 13, 2154–2160. [Google Scholar] [CrossRef]
- Yang, F.J.; Zhang, X.W.; Li, F.X.; Wang, Z.; Wang, L. Chemoenzymatic Synthesis of α-Cyano Epoxides by a Tandem-Knoevenagel–Epoxidation Reaction. Eur. J. Org. Chem. 2016, 7, 1251–1254. [Google Scholar] [CrossRef]
- Abdulmalek, E.; Arumugam, M.; Basri, M.; Rahman, M.B.A. Optimization of Lipase-Mediated Synthesis of 1-Nonene Oxide Using Phenylacetic Acid and Hydrogen Peroxide. Int. J. Mol. Sci. 2012, 13, 13140–13149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Khaw, N.R.B.J.; Li, Z. Efficient epoxidation of alkenes with hydrogen peroxide, lactone, and lipase. Green Chem. 2009, 11, 2047–2051. [Google Scholar] [CrossRef]
- Yang, F.J.; Wang, Z.; Zhang, X.W.; Jiang, L.Y.; Li, Y.Z.; Wang, L. A Green Chemoenzymatic Process for the Synthesis of Azoxybenzenes. Chemcatchem 2015, 7, 3450–3453. [Google Scholar] [CrossRef]
- Hernandez, K.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Hydrogen Peroxide in Biocatalysis. A Dangerous Liaison. Curr. Org. Chem. 2012, 16, 2652–2672. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, B.; Ayala, M.; Vazquez-Duhalt, R. Suicide inactivation of peroxidases and the challenge of engineering more robust enzymes. Chem. Biol. 2002, 9, 555–565. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Lipase B from Candida antarctica immobilized on octadecyl Sepabeads: A very stable biocatalyst in the presence of hydrogen peroxide. Process Biochem. 2011, 46, 873–878. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Ankudey, E.G.; Olivo, H.F.; Peeples, T.L. Lipase-mediated epoxidation utilizing urea–hydrogen peroxide in ethyl acetate. Green Chem. 2006, 8, 923–926. [Google Scholar] [CrossRef]
- Ríos, M.Y.; Salazar, E.; Olivo, H.F. Baeyer-Villiger oxidation of substituted cyclohexanones via lipase-mediated perhydrolysis utilizing urea-hydrogen peroxide in ethyl acetate. Green Chem. 2007, 9, 459–462. [Google Scholar] [CrossRef]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Alcalde, M.; Ferrer, M.; Plou, F.J.; Ballesteros, A. Environmental biocatalysis: From remediation with enzymes to novel green processes. TRENDS Biotechnol. 2006, 24, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Rueda, N.; Santos, J.C.S.; Ortiz, C.; Torres, R.; Barbosa, O.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Chemical Modification in the Design of Immobilized Enzyme Biocatalysts: Drawbacks and Opportunities. Chem. Rec. 2016, 16, 1436–1455. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.; Tacias-Pascacio, V.; Russo, M.; Marzocchella, A.; Virgen-Ortíz, J.; Fernandez-Lafuente, R. Stabilization of Candida antarctica Lipase B (CALB) immobilized on octyl agarose by treatment with polyethyleneimine (PEI). Molecules 2016, 21, 751. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Virgen-Ortíz, J.J.; Gorines, B.C.; Otero, C.; Fernandez-Lafuente, R. Physical crosslinking of lipase from Rhizomucor miehei immobilized on octyl agarose via coating with ionic polymers: Avoiding enzyme release from the support. Process Biochem. 2017, 54, 81–88. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Peirce, S.; Torrestiana-Sanchez, B.; Yates, M.; Rosales-Quintero, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Evaluation of different commercial hydrophobic supports for the immobilization of lipases: Tuning their stability, activity and specificity. RSC Adv. 2016, 6, 100281–100294. [Google Scholar] [CrossRef]
- Izquierdo, D.F.; Barbosa, O.; Burguete, M.I.; Lozano, P.; Luis, S.V.; Fernandez-Lafuente, R.; García-Verdugo, E. Tuning lipase B from Candida antarctica C–C bond promiscuous activity by immobilization on poly-styrene-divinylbenzene beads. RSC Adv. 2014, 4, 6219–6225. [Google Scholar] [CrossRef]
- Ma, J.S.; Zhang, Z.M.; Wang, B.J.; Kong, X.J.; Wang, Y.G.; Cao, S.G.; Feng, Y. Overexpression and characterization of a lipase from Bacillus subtilis. Protein Expr. Purif. 2006, 45, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Yang, C.H.; Wei, X.F.; Xun, E.N.; Wang, R.; Cao, S.G.; Wang, Z.; Wang, L. Optimization of APE1547-catalyzed enantioselective transesterification of (R/S)-2-methyl-1-butanol in an ionic liquid. Biotechnol. Bioproc. Eng. 2011, 16, 337–342. [Google Scholar] [CrossRef]
| Entry | Enzyme | Yield [b] (%) |
|---|---|---|
| 1 | ANL (Lipase from Aspergillus niger) | 64.2 ± 1.9 |
| 2 | CalB (Candida antarctica lipase B) | 87.6 ± 2.2 |
| 3 | APE1547 (Aeropyrum pernix esterase) | 79.3 ± 1.4 |
| 4 | Novozym 435 | 92.5 ± 1.5 |
| 5 | BSL2 (Bacillus subtilis lipase) | ND [d] |
| 6 | CSL (Lipase from Candida sp. 99–125) | ND [d] |
| 7 | denatured Novozym 435 [c] | ND [d] |
| 8 | No UHP | ND [d] |
| 9 | Control | ND [d] |
| Entry | Enzyme Dosage (U) | Oxidant Loading (Equiv.) | Yield (%) |
|---|---|---|---|
| 1 | 50 | 1 | 11.8 ± 1.3 |
| 2 | 100 | 1 | 64.5 ± 2.1 |
| 3 | 150 | 1 | 81.1 ± 1.8 |
| 4 | 180 | 1 | 82.6 ± 2.2 |
| 5 | 200 | 1 | 84.7 ± 1.1 |
| 6 | 150 | 1.2 | 92.5 ± 1.5 |
| 7 | 150 | 1.4 | 93.7 ± 0.7 |
 | |||
| Entry | 1,3-Diketone | Product | Yield (%) |
| 1 | 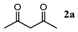 |  | 92.5 ± 1.5 |
| 2 | 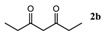 | 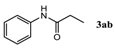 | 89.2 ± 1.4 |
| 3 | 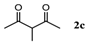 |  | 76.6 ± 0.8 |
| 4 |  | 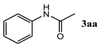 | 87.4 ± 1.7 |
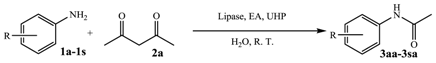 |
 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, F.; Wang, C.; Zheng, L.; Wang, Z.; Zhao, R.; Wang, L. Lipase-Mediated Amidation of Anilines with 1,3-Diketones via C–C Bond Cleavage. Catalysts 2017, 7, 115. https://doi.org/10.3390/catal7040115
Zhang L, Li F, Wang C, Zheng L, Wang Z, Zhao R, Wang L. Lipase-Mediated Amidation of Anilines with 1,3-Diketones via C–C Bond Cleavage. Catalysts. 2017; 7(4):115. https://doi.org/10.3390/catal7040115
Chicago/Turabian StyleZhang, Liu, Fengxi Li, Chunyu Wang, Lu Zheng, Zhi Wang, Rui Zhao, and Lei Wang. 2017. "Lipase-Mediated Amidation of Anilines with 1,3-Diketones via C–C Bond Cleavage" Catalysts 7, no. 4: 115. https://doi.org/10.3390/catal7040115
APA StyleZhang, L., Li, F., Wang, C., Zheng, L., Wang, Z., Zhao, R., & Wang, L. (2017). Lipase-Mediated Amidation of Anilines with 1,3-Diketones via C–C Bond Cleavage. Catalysts, 7(4), 115. https://doi.org/10.3390/catal7040115