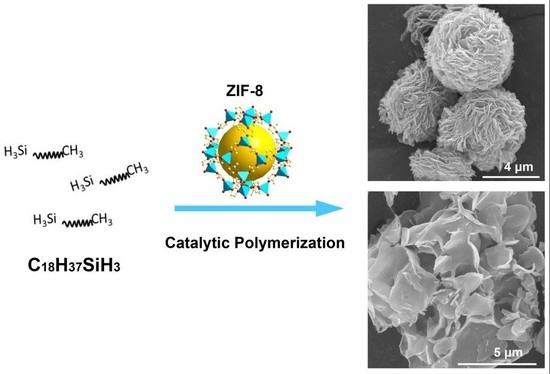
Polymerization of Alkylsilanes on ZIF-8 to Hierarchical Siloxane Microspheres and Microflowers
Abstract
:1. Introduction
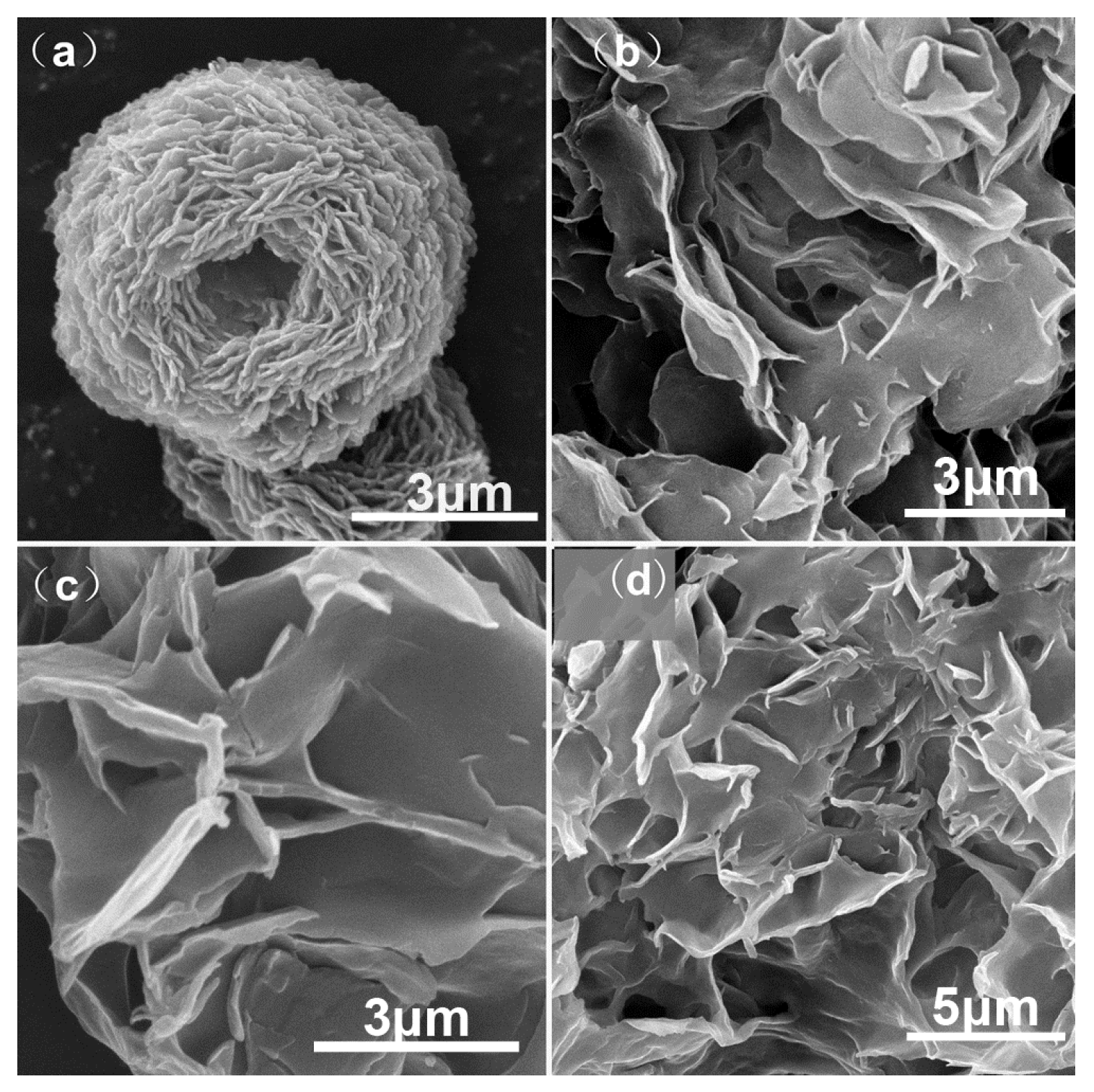
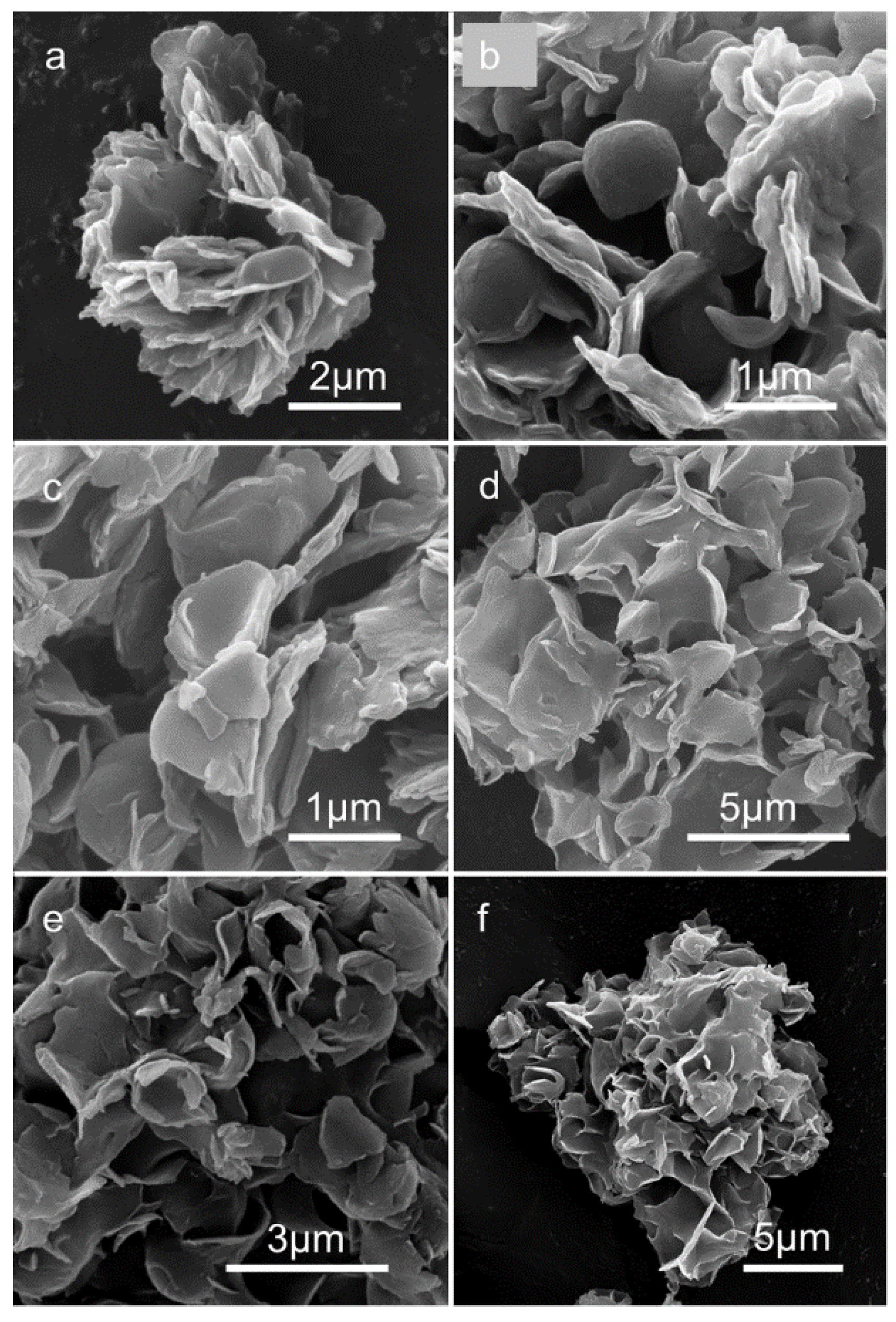
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of ZIF-8
3.2. Polymerization of Octadecylsilane over ZIF-8
3.3. Characterizations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Falcaro, P.; Buso, D.; Hill, A.J.; Doherty, C.M. Patterning techniques for metal organic frameworks. Adv. Mater. 2012, 24, 3153–3168. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Abid, H.R.; Pham, G.H.; Ang, H.M.; Tade, M.O.; Wang, S. Adsorption of CH4 and CO2 on Zr-metal organic frameworks. J. Colloid Interf. Sci. 2012, 366, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Fini, B.M.; Sarjeant, A.A.; Farha, O.K.; Hupp, J.T.; Scheidt, K.A. Urea metal-organic frameworks as effective and size-selective hydrogen-bond catalysts. J. Am. Chem. Soc. 2012, 134, 3334–3337. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-organic frameworks: opportunities for catalysis. Angew. Chem. Int. Ed. Engl. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Ranocchiari, M.; Bokhoven, J.A. Catalysis by metal-organic frameworks: fundamentals and opportunities. Phys. Chem. Chem. Phys. 2011, 13, 6388–6396. [Google Scholar] [CrossRef] [PubMed]
- Maksimchuk, N.V.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Heterogeneous Selective Oxidation of Alkenes to α,β-Unsaturated Ketones over Coordination Polymer MIL-101. Adv. Synth. Catal. 2010, 352, 2943–2948. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Commercial metal-organic frameworks as heterogeneous catalysts. Chem. Commun. 2012, 48, 11275–11288. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, R.; Roeffaers, M.B.; Cremer, D.G.; Vermoortele, F.; Hofkens, J.; Sels, B.F.; Vos, D.E. Metal-organic framework single crystals as photoactive matrices for the generation of metallic microstructures. Adv. Mater. 2011, 23, 1788–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wu, Y.N.; Qiao, J.; Zhang, J.; McDonald, A.; Li, G.; Li, F. The removal of bisphenol A from aqueous solutions by MIL-53(Al) and mesostructured MIL-53(Al). J. Colloid Interf. Sci. 2013, 405, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; García, H.; Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Mller, U.; Luinstra, G.; Yaghi, O.M. Process for Producing Polyalkylene Carbonates. US Patent 6,617,467 B1, 9 September 2003. [Google Scholar]
- Christopher, J.C.; Matthew, G.D.; Matthew, D.J.; Gabriele, K.K.; Matthew, D.L.; Stephen, W. Air-Stable Titanium Alkoxide Based Metal-Organic Framework as an Initiator for Ring-Opening Polymerization of Cyclic Esters. Inorg. Chem. 2006, 45, 6595–6597. [Google Scholar]
- Vitorino, M.J.; Devic, T.; Tromp, M.; Férey, G.; Visseaux, M. Lanthanide Metal-Organic Frameworks as Ziegler-Natta Catalysts for the Selective Polymerization of Isoprene. Macromol. Chem. Phys. 2009, 210, 1923–1932. [Google Scholar]
- Antonietta, G.; Robert, S.A. Fire performance of poly(dimethyl siloxane) composites evaluated by cone calorimetry. Compos. Part A-Appl. Sci. Manuf. 2008, 39, 398–405. [Google Scholar]
- Farhang, A.; Hamid, M.; Ali-Asgar, K. Modification of polysiloxane polymers for biomedical applications: A review. Polym. Int. 2001, 50, 1279–1287. [Google Scholar]
- Yang, D.; Zhang, W.; Jiang, B.; Guo, Y. Silicone rubber ablative composites improved with zirconium carbide or zirconia. Compos. Part A Appl. Sci. Manuf. 2013, 44, 70–77. [Google Scholar] [CrossRef]
- Prasad, B.L.V.; Savka, I.S.; Christopher, M.S.; Vladimir, Z.; Kenneth, J.K. Gold Nanoparticles as Catalysts for Polymerization of Alkylsilanes to Siloxane Nanowires, Filaments, and Tubes. J. Am. Chem. Soc. 2003, 125, 10488–10489. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zi, G.; Yang, F.; Mi, Y.; Yang, X.; Wang, W.; Zou, Q.; Wang, J. The polymerizations of alkylsilane and bis-(γ-triethoxysilylpropyl)-tetrasulfide catalyzed by copper nanoparticles and the effects of transition metal ions on the polymerizations of alkylsilane catalyzed by silver nanoparticles. Mater. Chem. Phys. 2009, 118, 513–518. [Google Scholar] [CrossRef]
- Wei, Q.; Li, B.; Li, C.; Wang, J.; Wang, W.; Yang, X. PVP-capped silver nanoparticles as catalysts for polymerization of alkylsilanes to siloxane composite microspheres. J. Mater. Chem. 2006, 16, 3606. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Hu, W.; Li, B.; Yan, J.; Hu, J. Synthesis of AgBiS2 microspheres by a templating method and their catalytic polymerization of alkylsilanes. Chem. Commun. 2007, 4931–4933. [Google Scholar] [CrossRef]
- Goyal, A.; Kumar, A.; Ajayan, P.M. Metal salt induced synthesis of hybrid metal core-siloxane shell nanoparticles and siloxane nanowires. Chem. Commun. 2010, 46, 964–966. [Google Scholar] [CrossRef] [PubMed]
- De Krafft, K.E.; Wang, C.; Lin, W. Metal-organic framework templated synthesis of Fe2O3/TiO2 nanocomposite for hydrogen production. Adv. Mater. 2012, 24, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Im, J.H.; Kim, T.; Lee, K.; Park, C.R. MOF-derived ZnO and ZnO@C composites with high photocatalytic activity and adsorption capacity. J. Hazard. Mater. 2011, 186, 376–382. [Google Scholar] [CrossRef] [PubMed]
- William, J.R.; Kathryn, L.T.; Lin, W. Surface Modification and Functionalization of Nanoscale Metal-Organic Frameworks for Controlled Release and Luminescence Sensing. J. Am. Chem. Soc. 2007, 129, 9852–9853. [Google Scholar]
- Jiang, Z.; Sun, H.; Qin, Z.; Jiao, X.; Chen, D. Synthesis of novel ZnS nanocages utilizing ZIF-8 polyhedral template. Chem. Commun. 2012, 48, 3620–3622. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Liu, B.; Lan, Y.Q.; Kuratani, K.; Akita, T.; Shioyama, H.; Zong, F.; Xu, Q. From metal-organic framework to nanoporous carbon: toward a very high surface area and hydrogen uptake. J. Am. Chem. Soc. 2011, 133, 11854–11857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.B.; Madhavi, S.; Hng, H.H.; Lou, X.W. Formation of Fe2O3 microboxes with hierarchical shell structures from metal-organic frameworks and their lithium storage properties. J. Am. Chem. Soc. 2012, 134, 17388–17391. [Google Scholar] [CrossRef] [PubMed]
- Uyen, N.T.; Ky, A.L.; Nam, S.P. Expanding Applications of Metal—Organic Frameworks: Zeolite Imidazolate Framework ZIF-8 as an Efficient Heterogeneous Catalyst for the Knoevenagel Reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar]
- Céline, C.; Sandrine, L.; Delphine, B.; Fabien, B.; Vincent, L.; Emmanuel, S.; Anne-Agathe, Q.; Nicolas, B. Catalysis of Transesterification by a Nonfunctionalized Metal-Organic Framework: Acido-Basicity at the External Surface of ZIF-8 Probed by FTIR and ab Initio Calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar]
- Yichang, P.; Yunyang, L.; Gaofeng, Z.; Lan, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar]
- Kai, X.; Shuai, C.; Xiaoyu, P.; Caterina, D. Carbon with hierarchical pores from carbonized metal—Organic frameworks for lithium sulphur batteries. Chem. Commun. 2013, 49, 2192–2194. [Google Scholar]
- Kyo, S.P.; Zheng, N.; Adrien, P.C.; Jae, Y.C. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Na, C.; Zhi, Y.G.; Xiu, P.Y. Zeolitic Imidazolate Framework-8 Nanocrystal Coated Capillary for Molecular Sieving of Branched Alkanes from Linear Alkanes along with High-Resolution Chromatographic Separation of Linear Alkanes. J. Am. Chem. Soc. 2010, 132, 13645–13647. [Google Scholar]
- Jiang, L.; Zhao, Y.; Zhai, J. A lotus-leaf-like superhydrophobic surface: A porous microsphere/nanofiber composite film prepared by electrohydrodynamics. Angew. Chem. Int. Ed. Engl. 2004, 43, 4338–4341. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Xie, C.; Li, Y.; Guo, L.; Nie, M.; Zhang, J.; Yan, Z.; Wang, J.; Wang, W. Polymerization of Alkylsilanes on ZIF-8 to Hierarchical Siloxane Microspheres and Microflowers. Catalysts 2017, 7, 77. https://doi.org/10.3390/catal7030077
Yang L, Xie C, Li Y, Guo L, Nie M, Zhang J, Yan Z, Wang J, Wang W. Polymerization of Alkylsilanes on ZIF-8 to Hierarchical Siloxane Microspheres and Microflowers. Catalysts. 2017; 7(3):77. https://doi.org/10.3390/catal7030077
Chicago/Turabian StyleYang, Lin, Congjia Xie, Yizhou Li, Lei Guo, Minfang Nie, Jinping Zhang, Zhiying Yan, Jiaqiang Wang, and Wei Wang. 2017. "Polymerization of Alkylsilanes on ZIF-8 to Hierarchical Siloxane Microspheres and Microflowers" Catalysts 7, no. 3: 77. https://doi.org/10.3390/catal7030077
APA StyleYang, L., Xie, C., Li, Y., Guo, L., Nie, M., Zhang, J., Yan, Z., Wang, J., & Wang, W. (2017). Polymerization of Alkylsilanes on ZIF-8 to Hierarchical Siloxane Microspheres and Microflowers. Catalysts, 7(3), 77. https://doi.org/10.3390/catal7030077