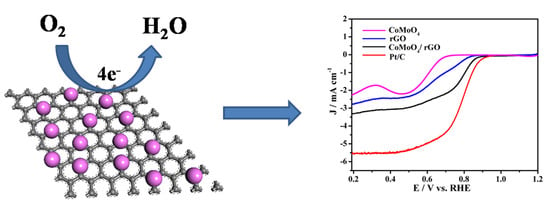
Cross-Linked CoMoO4/rGO Nanosheets as Oxygen Reduction Catalyst
Abstract
1. Introduction
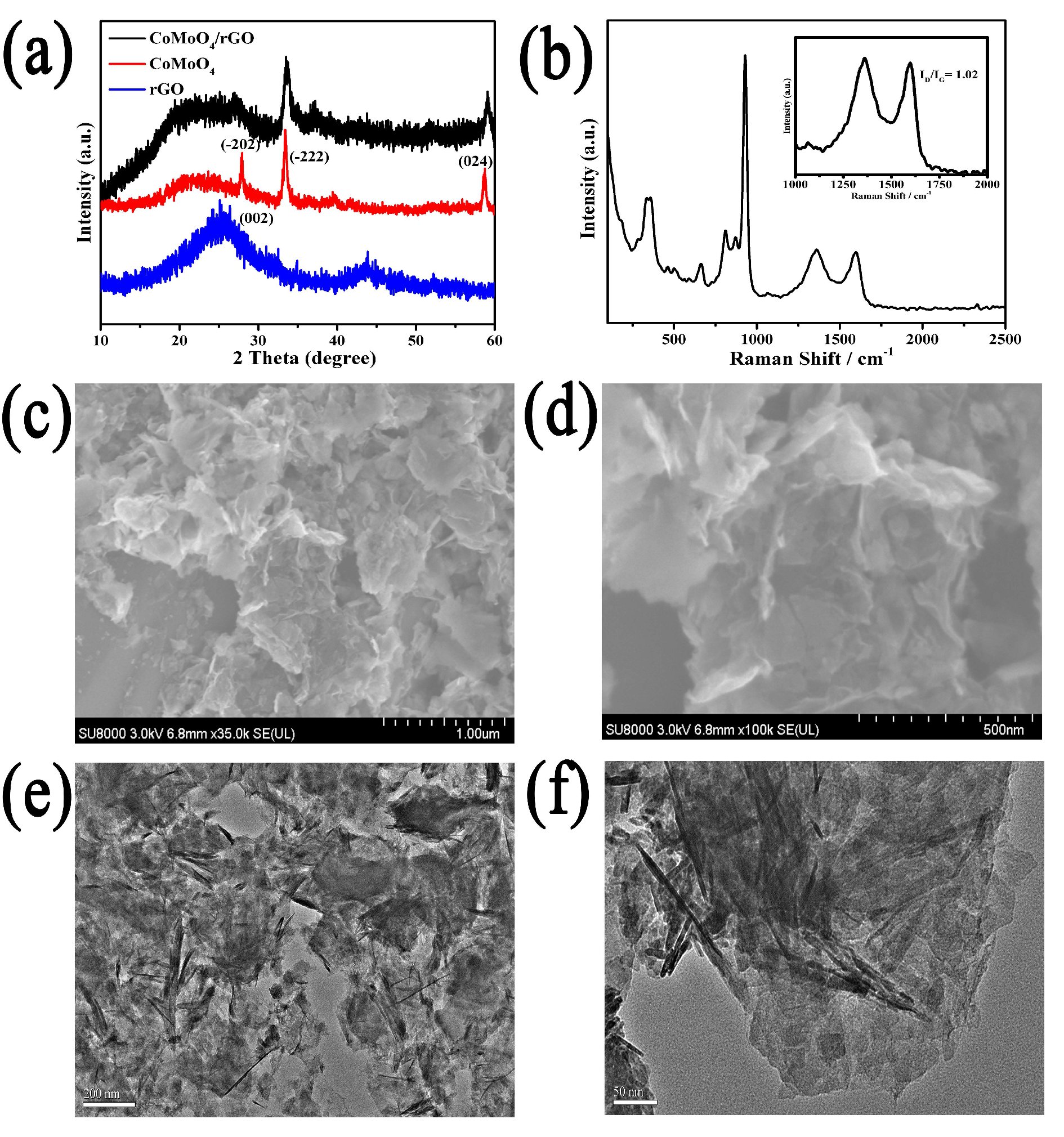
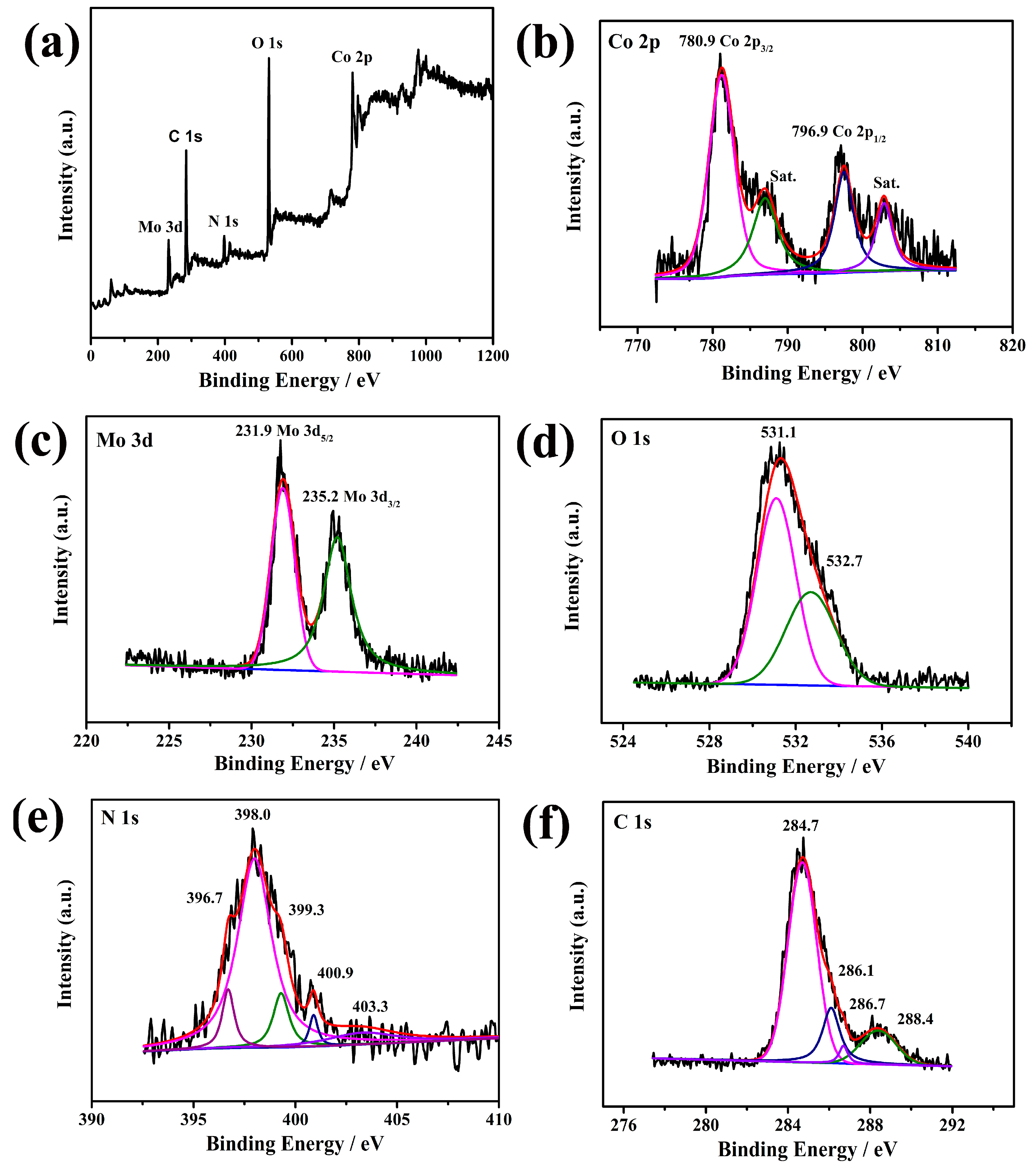
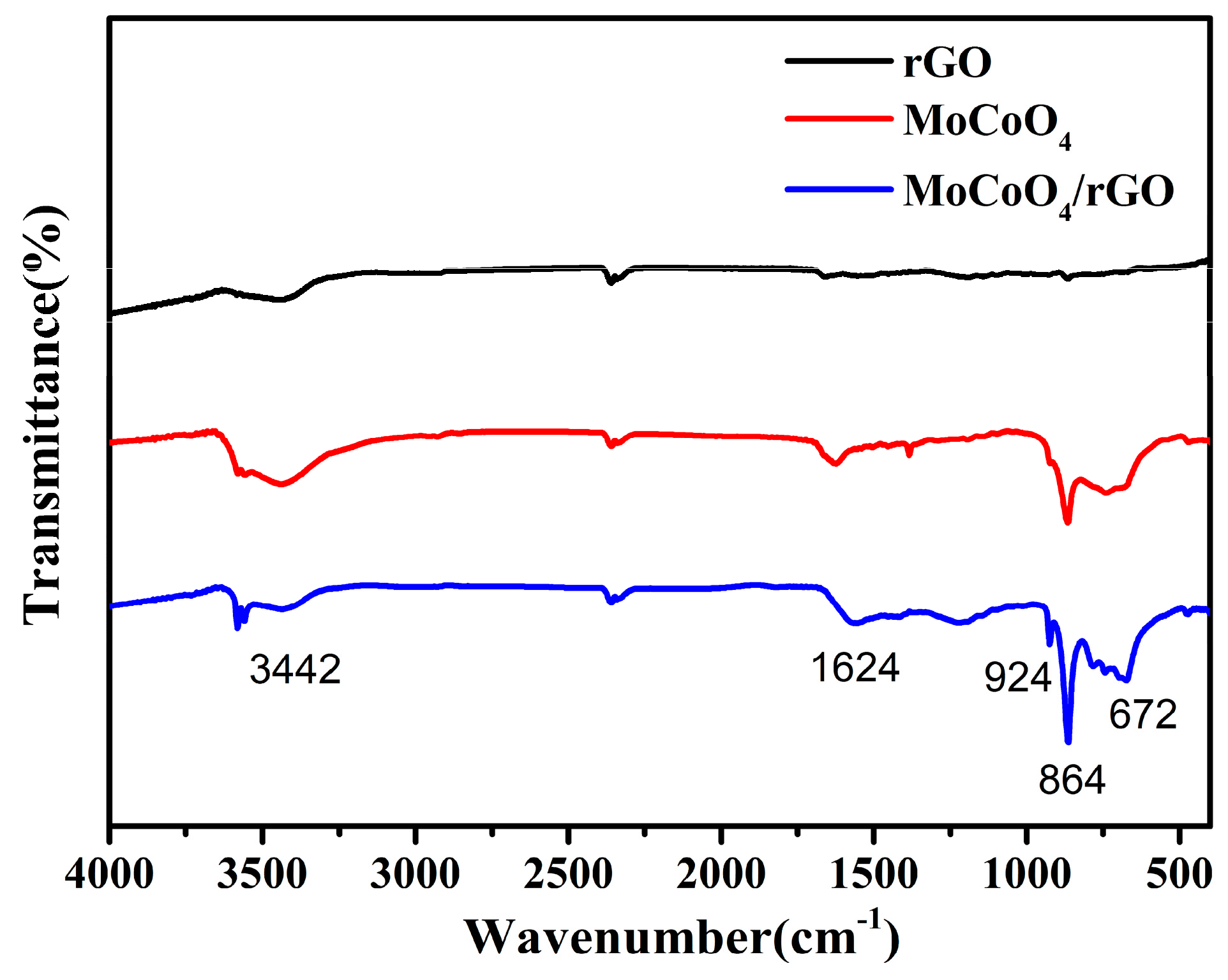
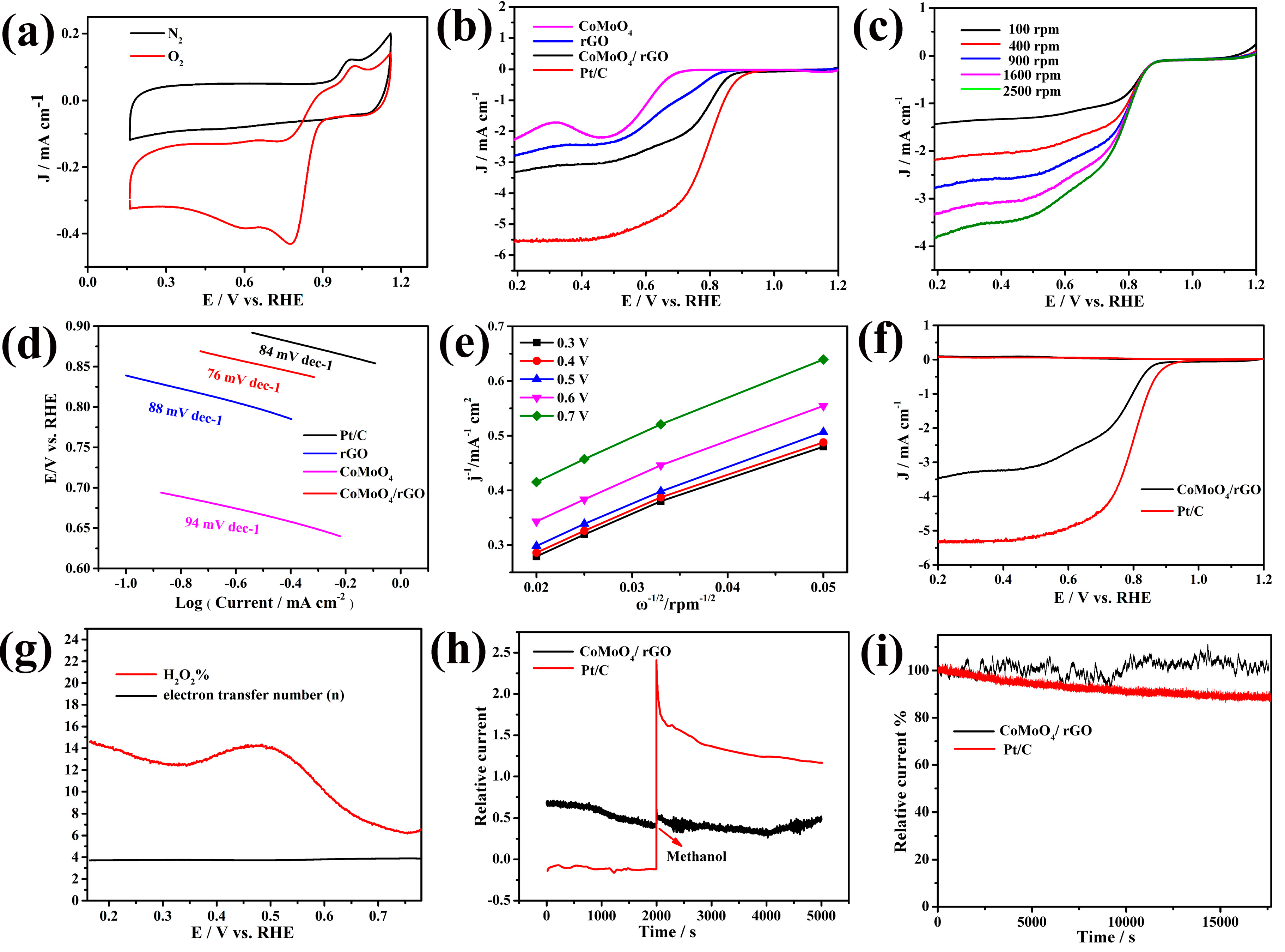
2. Results and Discussion
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis of Graphene Oxide (GO)
3.3. Synthesis of CoMoO4/rGO Catalysts
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Canadell, J.G.; Quéré, C.L.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Conway, T.J.; Gillett, N.P.; Houghton, R.A.; Marland, G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18866–18870. [Google Scholar] [CrossRef] [PubMed]
- Hoel, M.; Kverndokk, S. Depletion of fossil fuels and the impacts of global warming. Resour. Energy Econ. 1996, 18, 115–136. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tryk, D.A.; Wakisaka, M.; Yano, H.; Uchida, H. Overview of recent developments in oxygen reduction electrocatalysis. Electrochim. Acta 2012, 84, 187–201. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Thapa, R.; Kim, H.; Xu, X.; Kim, M.G.; Li, Q.; Park, N.; Liu, M.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, L. Metal molybdate nanorods as non-precious electrocatalysts for the oxygen reduction. Funct. Mater. Lett. 2015, 8, 3. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Markovic, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, D.; Lou, X. Shape-Controlled Synthesis of MnO2 Nanostructures with Enhanced Electrocatalytic Activity for Oxygen Reduction. J. Phys. Chem. C 2009, 114, 1694–1700. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, H.; Li, X.; Xiao, H.; Xiao, W.; Wu, T. Synthesis and characterization of Mn-based composite oxides with enhanced electrocatalytic activity for oxygen reduction. J. Mater. Chem. A 2014, 2, 13345. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, Y. Hierarchical polythiophene-coated MnO2 nanosheets as non-precious electro-catalyst to oxygen reduction. Funct. Mater. Lett. 2010, 3, 89–92. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Vezzù, K.; Delpeuch, A.B.; Negro, E.; Polizzi, S.; Nawn, G.; Bertasi, F.; Pagot, G.; Artyushkova, K.; Atanassov, P.; Noto, V.D. Fe-carbon nitride “Core-shell” electrocatalysts for the oxygen reduction reaction. Electrochim. Acta 2016, 222, 1778–1791. [Google Scholar] [CrossRef]
- Gokhal, R.; Chen, Y.; Serov, A.; Artyushkova, K.; Atanassov, P. Novel dual templating approach for preparation of highly active Fe-N-C electrocatalyst for oxygen reduction. Electrochim. Acta 2017, 224, 49–55. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Kalpana, D.; Renganathan, N.G. Synthesis and characterization of ZnCo2O4 nanomaterial for symmetric supercapacitor applications. Ionics 2009, 15, 107–110. [Google Scholar] [CrossRef]
- Bao, L.; Zang, J.; Li, X. Flexible Zn2SnO4/MnO2 Core/Shell Nanocable-Carbon Microfiber Hybrid Composites for High-Performance Supercapacitor Electrodes. Nano Lett. 2011, 11, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Che, Q.; Li, H.; Zhang, X. Mesoporous NiCo2O4 Nanowire Arrays Grown on Caibon Textiles as Binder- Free Flexible Electrodes for Energy Storage. Adv. Funct. Mater. 2014, 24, 2630–2637. [Google Scholar] [CrossRef]
- Cai, D.; Liu, B.; Wang, D.; Liu, Y.; Wang, L.; Li, H.; Wang, Y.; Wang, C.; Li, Q.; Wang, T. Facile hydrothermal synthesis of hierarchical ultrathin mesoporous NiMoO4 nanosheets for high performance supercapacitors. Electrochim. Acta 2014, 115, 358–363. [Google Scholar] [CrossRef]
- Cai, D.; Liu, B.; Wang, D.; Wang, L.; Liu, Y.; Li, H.; Wang, Y.; Li, Q.; Wang, T. Construction of unique NiCo2O4 nanowire@CoMoO4 nanoplate core/shell arrays on Ni foam for high areal capacitance supercapacitors. J. Mater. Chem. A 2014, 2, 4954–4960. [Google Scholar] [CrossRef]
- Yu, X.; Lu, B.; Xu, Z. Super Long-Life Supercapacitors Based on the Construction of Nanohoneycomb-Like Strongly Coupled CoMoO4–3D Graphene Hybrid Electrodes. Adv. Mater. 2014, 26, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Osgood, T.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Li, M.; Xu, S.; Cherry, C.; Zhu, Y.; Wu, D.; Zhang, C.; Zhang, X.; Huang, R.; Qi, R.; Wang, L.; Chu, P.K. Hierarchical 3-dimensional CoMoO4 nanoflakes on a macroporous electrically conductive network with superior electrochemical performance. J. Mater. Chem. A 2015, 3, 13776–13785. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, J.; Peng, B.; Pan, Y.; Tao, Z.; Chen, J. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem. 2011, 3, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Liang, H.; Hu, J.; Jiang, L.; Wan, L. Controllable Pt Nanoparticle Deposition on Carbon Nanotubes as an Anode Catalyst for Direct Methanol Fuel Cells. J. Phys. Chem. B 2005, 109, 22212–22216. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly Coupled Inorganic/Nanocarbon Hybrid Materials for Advanced Electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent Hybrid of Spinel Manganese-Cobalt Oxide and Graphene as Advanced Oxygen Reduction Electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, H.; Liu, Y.; Qu, J.; Li, J. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction. Nanoscale 2015, 7, 1250–1269. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Shen, A.; Tao, L.; Wang, S. Molecular doping of graphene as metal–free electrocatalyst for oxygen reduction reaction. Chem. Commun. 2014, 50, 10672–10675. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin Iron-Cobalt Oxide Nanosheets with Abundant Oxygen Vacancies for the Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ren, Z.; Chen, S.; Meng, Y.; Biswas, S.; Nandi, P.; Elsen, H.A.; Gao, P.; Suib, S.L. Ni- and Mn-Promoted Mesoporous Co3O4: A Stable Bifunctional Catalyst with Surface-Structure-Dependent Activity for Oxygen Reduction Reaction and Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 20802–20813. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jiang, L.; Yang, H. Ultrathin nanosheets constructed CoMoO4 porous flowers with high activity for electrocatalytic oxygen evolution. Chem. Commun. 2015, 51, 14361–14364. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, S.; Wu, X.; Liu, J.; Tian, W.; Chen, J. Self-assembly of ultrathin mesoporous CoMoO4 nanosheet networks on flexible carbon fabric as a binder-free anode for lithium-ion batteries. New J. Chem. 2016, 40, 2259–2267. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, R.; Liao, H.; Hou, Y. Synthesis of amino-functionalized graphene as metal–free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2013, 2, 88–97. [Google Scholar] [CrossRef]
- Baraldi, A.; Brena, B.; Cocco, D.; Comelli, G.; Lizzit, S.; Paolucci, G.; Baumann, P.; Scheuch, V.; Uebing, C. The structure of the MoN surface compound on Fe-3.5%Mo-N (100) studied by X-ray photoelectron diffraction: First results from ELElTRA. Vacuum 1997, 48, 351–355. [Google Scholar] [CrossRef]
- Gao, S.; Geng, K.; Liu, H.; Wei, X.; Zhang, M.; Wang, P.; Wang, J. Transforming organic-rich amaranthus waste into nitrogen-doped carbon with superior performance of the oxygen reduction reaction. Energy Environ. Sci. 2015, 8, 221–229. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Wang, Y.; Dou, S.; Yan, D.; Liu, D.; Xia, Z.; Wang, S. In Situ Exfoliated, Edge-Rich, Oxygen-Functionalized Graphene from Carbon Fibers for Oxygen Electrocatalysis. Adv. Mater. 2017, 29, 1606207. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.D.; Fang, T.T. Effects of Molar Ratio of Citric Acid to Cations and of pH Value on the Formation and Thermal-Decomposition Behavior of Barium Titanium Citrate. J. Am. Ceram. Soc. 1999, 82, 1409–1415. [Google Scholar] [CrossRef]
- Moura, A.P.D.; Oliveira, L.H.D.; Pereira, P.F.S.; Rosa, I.L.V.; Li, M.S.; Longo, E.; Varela, J.A. Photoluminescent Properties of CoMoO4 Nanorods Quickly Synthesized and Annealed in a Domestic Microwave Oven. Adv. Chem. Eng. Sci. 2012, 2, 465–473. [Google Scholar] [CrossRef]
- Sieber, K.; Kershaw, R.; Dwight, K.; Wold, A. Dependence of Magnetic Properties on Structure in the Systems NiMoO4 and CoMoO4. Inorg. Chem. 1983, 22, 2667–2669. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskill, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Meng, J.-L.; Wei, M.-J.; Zang, H.-Y.; Tan, H.-Q.; Wang, Y.-H.; Miras, H.N.; Li, Y.-G. Cross-Linked CoMoO4/rGO Nanosheets as Oxygen Reduction Catalyst. Catalysts 2017, 7, 375. https://doi.org/10.3390/catal7120375
Fu J, Meng J-L, Wei M-J, Zang H-Y, Tan H-Q, Wang Y-H, Miras HN, Li Y-G. Cross-Linked CoMoO4/rGO Nanosheets as Oxygen Reduction Catalyst. Catalysts. 2017; 7(12):375. https://doi.org/10.3390/catal7120375
Chicago/Turabian StyleFu, Jiaqi, Jiang-Li Meng, Mei-Jie Wei, Hong-Ying Zang, Hua-Qiao Tan, Yong-Hui Wang, Haralampos N. Miras, and Yang-Guang Li. 2017. "Cross-Linked CoMoO4/rGO Nanosheets as Oxygen Reduction Catalyst" Catalysts 7, no. 12: 375. https://doi.org/10.3390/catal7120375
APA StyleFu, J., Meng, J.-L., Wei, M.-J., Zang, H.-Y., Tan, H.-Q., Wang, Y.-H., Miras, H. N., & Li, Y.-G. (2017). Cross-Linked CoMoO4/rGO Nanosheets as Oxygen Reduction Catalyst. Catalysts, 7(12), 375. https://doi.org/10.3390/catal7120375