Polyelectrolyte Complex Beads by Novel Two-Step Process for Improved Performance of Viable Whole-Cell Baeyer-Villiger Monoxygenase by Immobilization
Abstract
:1. Introduction
2. Results and Discussion
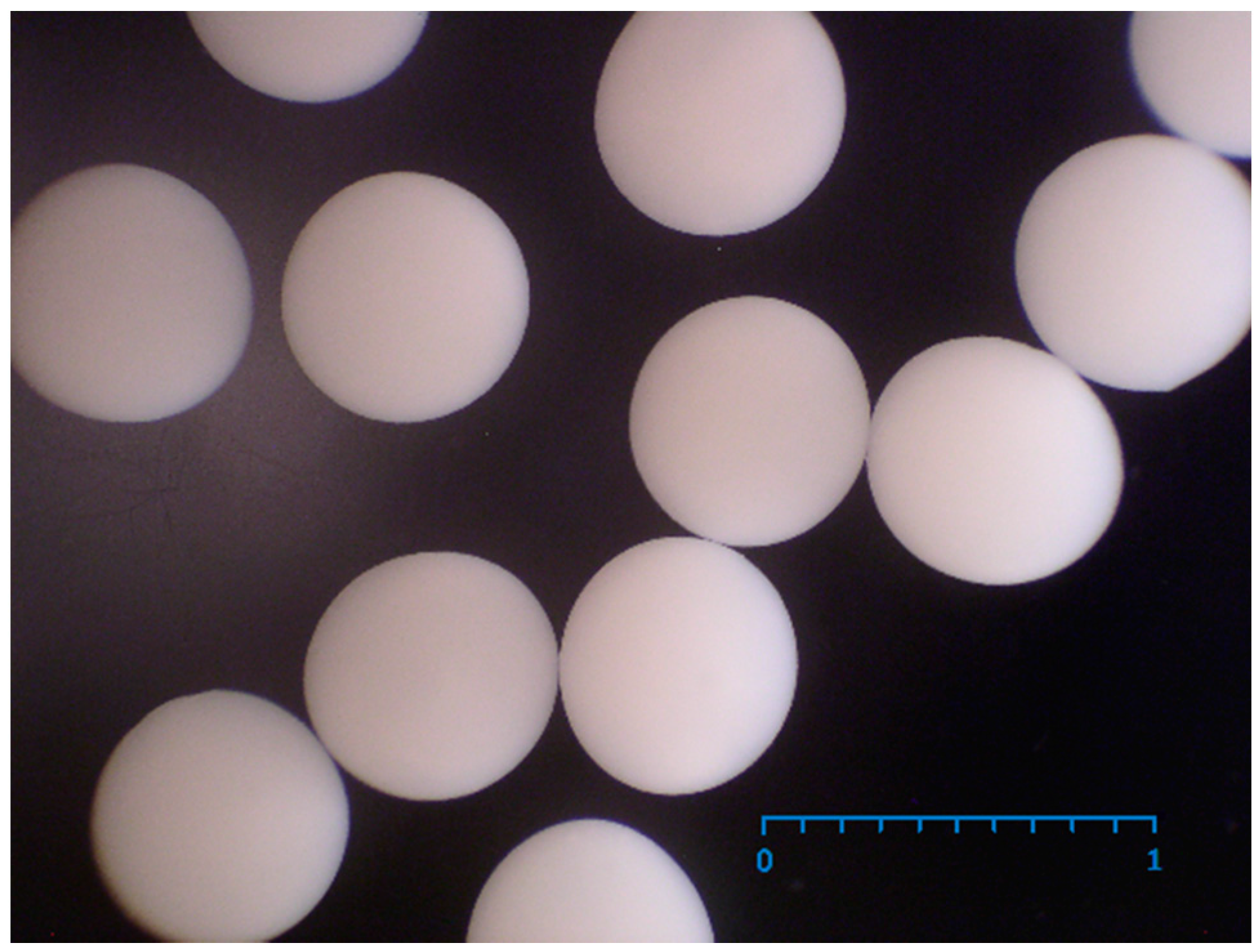
2.1. Production of PEC Bads with Immobilized E. coli Containing CHMO
2.2. Suitability of Cellulose Sulphate
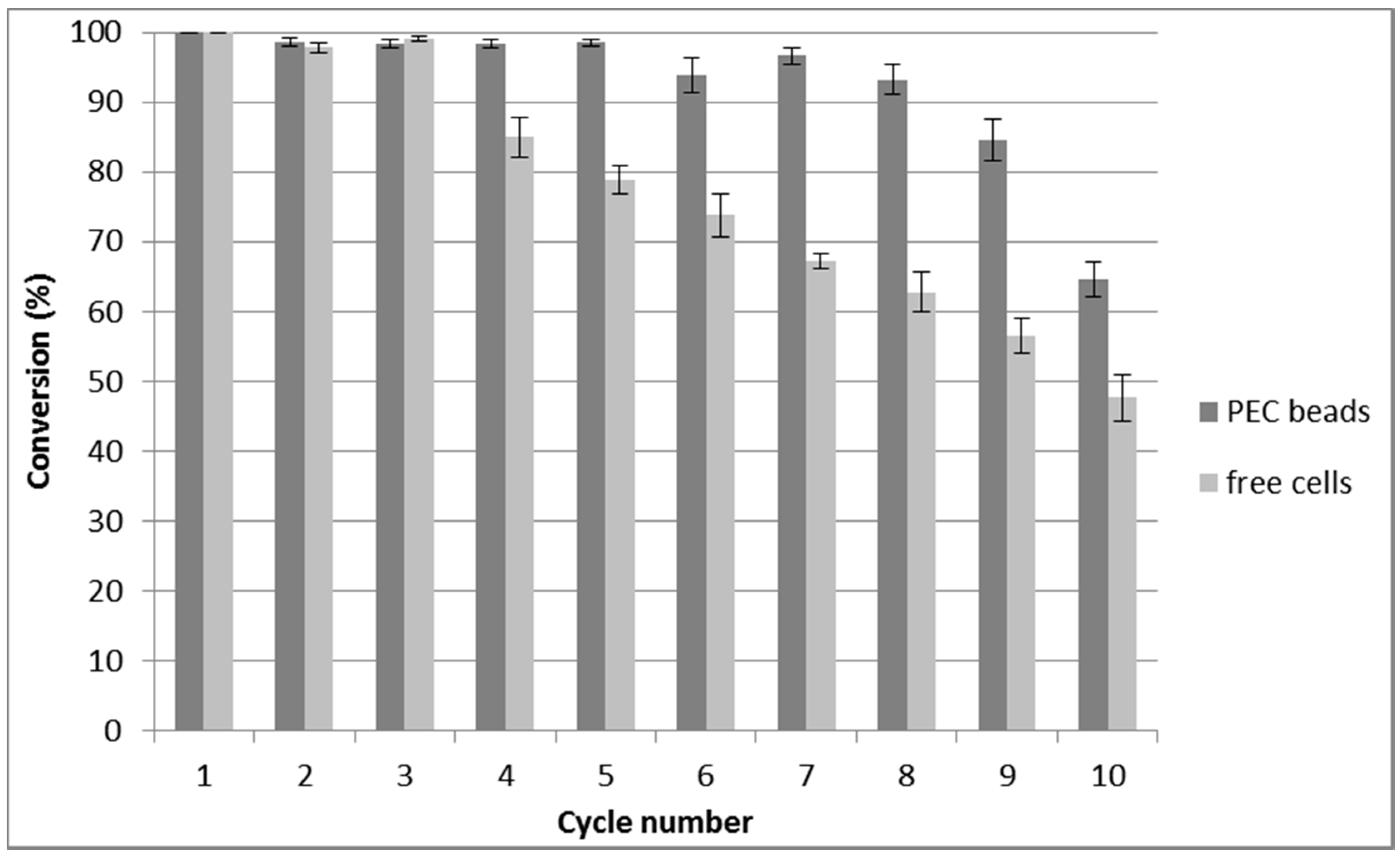
2.3. Repeated Biotransformations Using Immobilized Cells
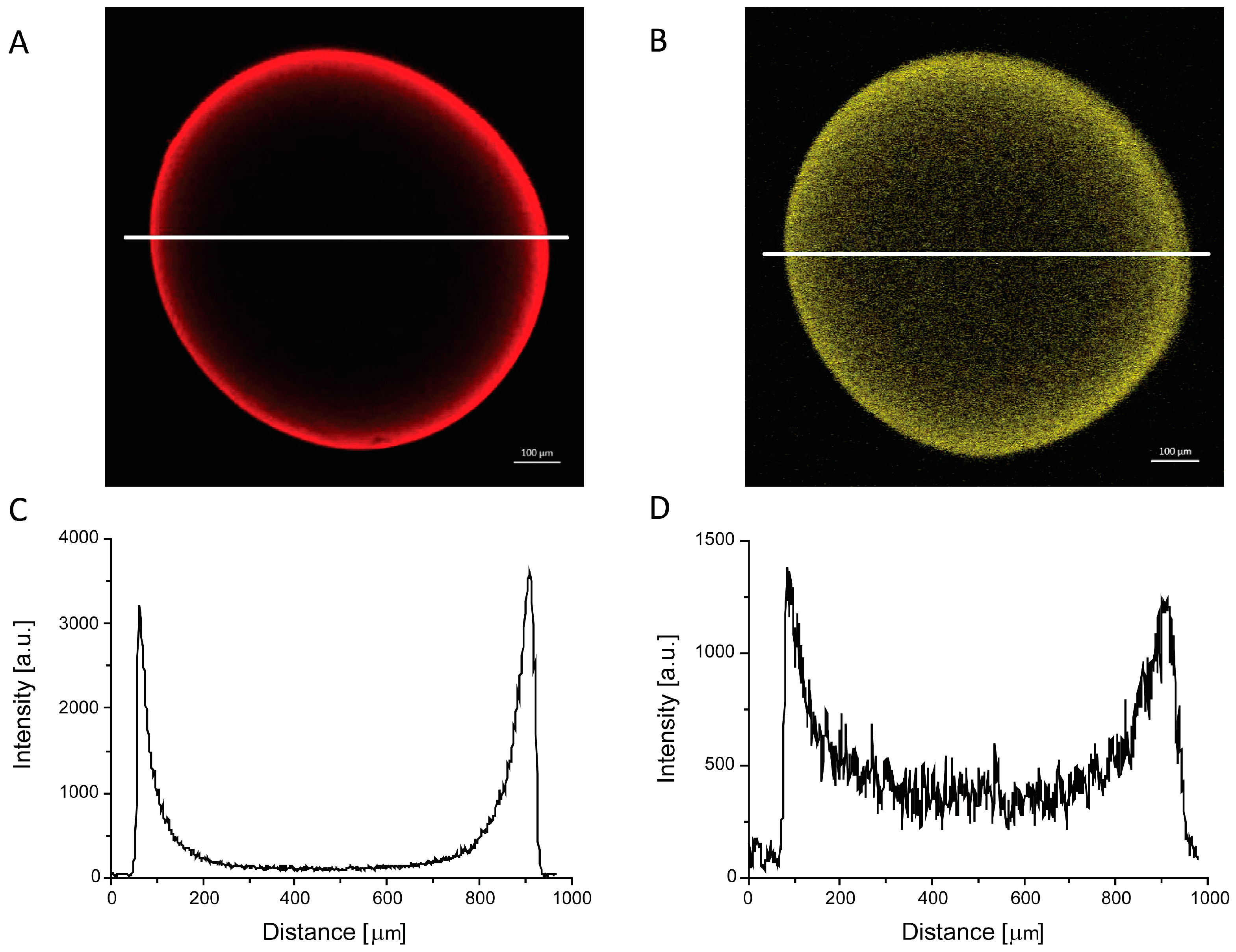
2.4. Morphology of PEC Beads
2.5. Cell Viability
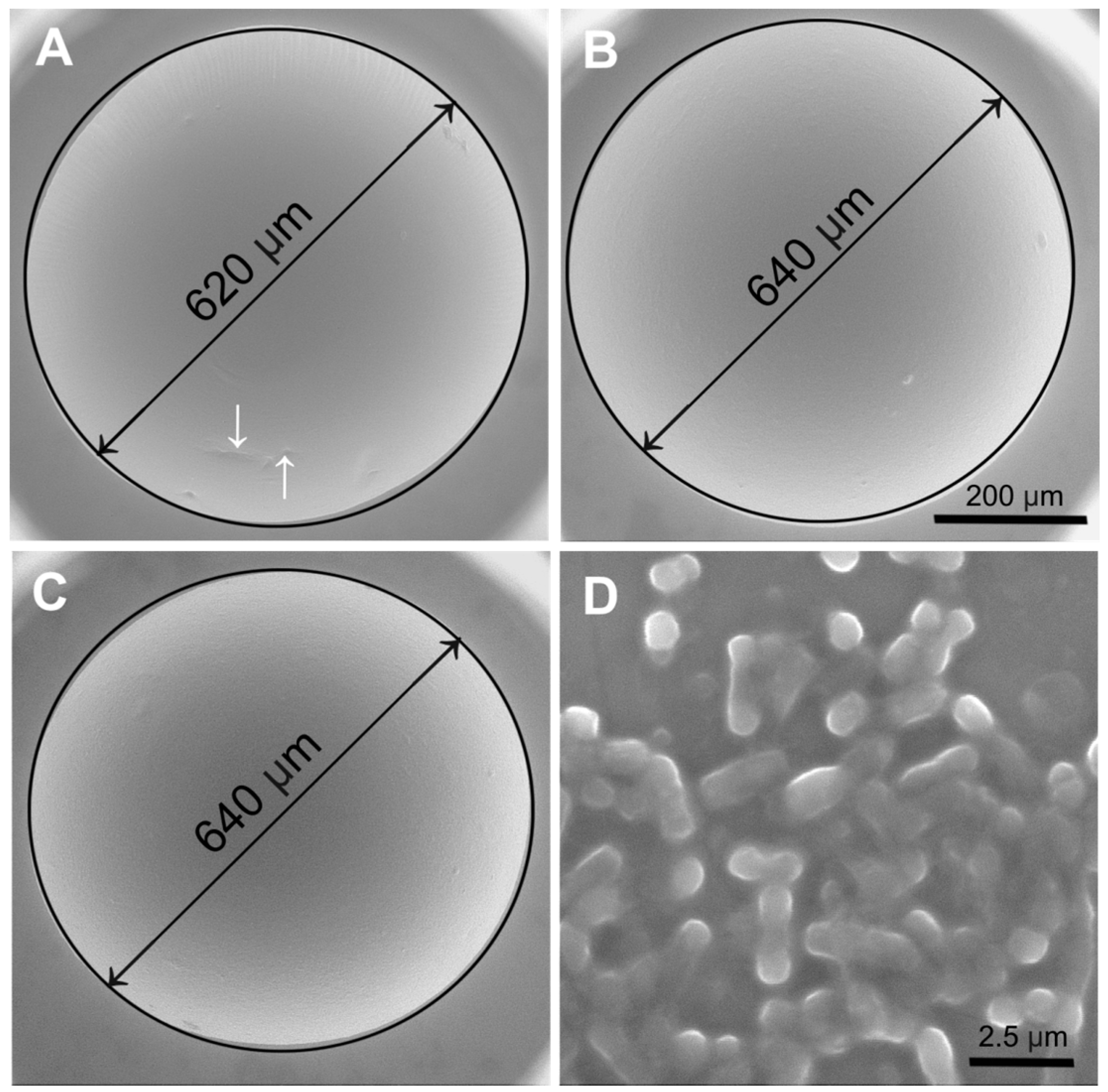
2.6. Environmental Scanning Electron Microscopy (ESEM)
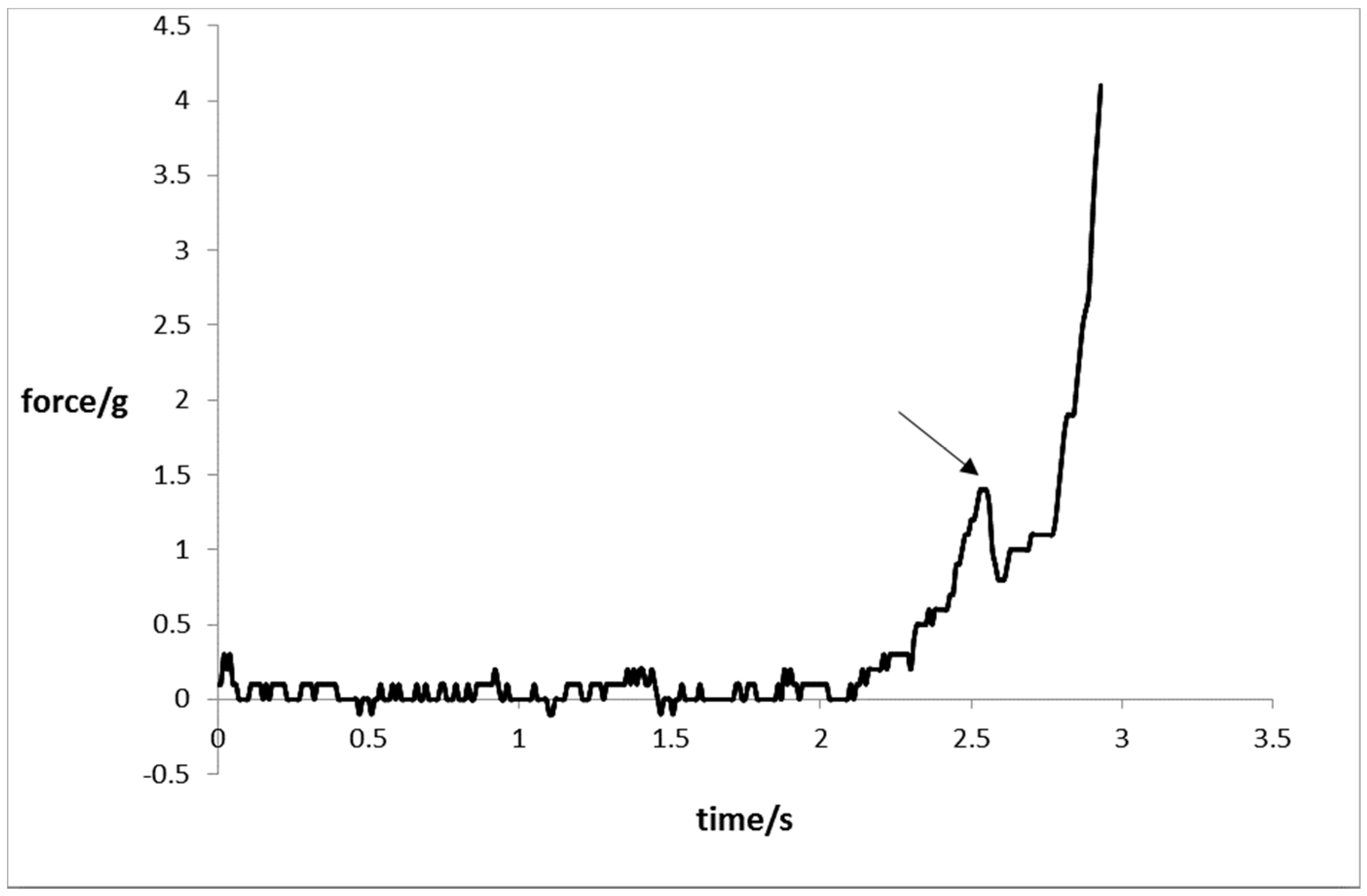
2.7. Mechanical Resistance
3. Materials and Methods
3.1. Characterisation Polymers
3.2. Cultivation of Cells
3.3. Preparation of Polyelectrolyte Complex Beads
3.4. Repeated Biotransformations
3.5. Confocal Laser Scanning Microscopy
3.6. Cell Viability
3.7. Environmental Scanning Electron Microscopy
3.8. Mechanical Strength
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kadisch, M.; Willrodt, C.; Hillen, M.; Bühler, B.; Schmid, A. Maximizing the stability of metabolic engineering-derived whole-cell biocatalysts. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Biotechnol. 2016, 42, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Bučko, M.; Vikartovská, A.; Lacík, I.; Kolláriková, G.; Gemeiner, P.; Pätoprstý, V.; Brygin, M. Immobilization of a whole-cell epoxide-hydrolyzing biocatalyst in sodium alginate-cellulose sulfate-poly(methylene-co-guanidine) capsules using a controlled encapsulation process. Enzyme Microb. Technol. 2005, 36, 118–126. [Google Scholar] [CrossRef]
- Hucík, M.; Bučko, M.; Gemeiner, P.; Štefuca, V.; Vikartovská, A.; Mihovilovic, M.D.; Rudroff, F.; Iqbal, N.; Chorvát, D., Jr.; Lacík, I. Encapsulation of recombinant E. coli expressing cyclopentanone monooxygenase in polyelectrolyte complex capsules for Baeyer-Villiger biooxidation of 8-oxabicyclo[3.2.1]oct-6-en-3-one. Biotechnol. Lett. 2010, 32, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Bertóková, A.; Vikartovská, A.; Bučko, M.; Gemeiner, P.; Tkáč, J.; Chorvát, D.; Štefuca, V.; Neděla, V. Biooxidation of 2-phenylethanol to phenylacetic acid by whole-cell Gluconobacter oxydans biocatalyst immobilized in polyelectrolyte complex capsules. Biocatal. Biotransform. 2015, 33, 111–120. [Google Scholar] [CrossRef]
- Lacík, I.; Briššová, M.; Anilkumar, A.V.; Powers, A.C.; Wang, T. New capsule with tailored properties for the encapsulation of living cells. J. Biomed. Mater. Res. 1998, 39, 52–60. [Google Scholar] [CrossRef]
- Anilkumar, A.V.; Lacik, I.; Wang, T.G. A novel reactor for making uniform capsules. Biotechnol. Bioeng. 2001, 75, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Schenkmayerová, A.; Bučko, M.; Gemeiner, P.; Chorvát, D., Jr.; Lacík, I. Viability of free and encapsulated Escherichia coli overexpressing cyclopentanone monooxygenase monitored during model Baeyer–Villiger biooxidation by confocal laser scanning microscopy. Biotechnol. Lett. 2012, 34, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bučko, M.; Schenkmayerová, A.; Gemeiner, P.; Vikartovská, A.; Mihovilovič, M.D.; Lacík, I. Continuous testing system for Baeyer-Villiger biooxidation using recombinant Escherichia coli expressing cyclohexanone monooxygenase encapsulated in polyelectrolyte complex capsules. Enzyme Microb. Technol. 2011, 49, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Schenkmayerová, A.; Bučko, M.; Gemeiner, P.; Treľová, D.; Lacík, I.; Chorvát, D., Jr.; Ačai, P.; Polakovič, M.; Lipták, L.; Rebroš, M.; et al. Physical and bioengineering properties of polyvinyl alcohol lens-shaped particles versus spherical polyelectrolyte complex microcapsules as immobilization matrices for a whole-cell Baeyer-Villiger monooxygenase. Appl. Biochem. Biotechnol. 2014, 174, 1834–1849. [Google Scholar] [CrossRef]
- De Vos, P.; Bučko, M.; Gemeiner, P.; Navrátil, M.; Švitel, J.; Faas, M.; Strand, B.L.; Skjak-Braek, G.; Morch, Y.A.; Vikartovská, A.; et al. Multiscale requirements for bioencapsulation in medicine and biotechnology. Biomaterials 2009, 30, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Rokstad, A.M.A.; Lacík, I.; de Vos, P.; Strand, B.L. Advances in biocompatibility and physico-chemical characterization of micorspheres for cell encapsulation. Adv. Drug Deliv. Rev. 2014, 67–68, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Polakovič, M.; Švitel, J.; Bučko, M.; Filip, J.; Neděla, V.; Ansorge-Schumacher, M.B.; Gemeiner, P. Progress in biocatalysis with immobilized viable whole cells: Systems development, reaction engineering and applications. Biotechnol. Lett. 2017, 39, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Seelbach, K.; Buchholz, A.; Haberland, J. Processes. In Industrial Biotransformations, 2nd ed.; Liese, A., Seelbach, K., Wandrey, C., Eds.; Wiley-VCH Verlag GmbH&Co.: Weinheim, Germany, 2006; pp. 147–513. ISBN 3-527-31001-0. [Google Scholar]
- Gericke, M.; Liebert, T.; Heinze, T. Interaction of ionic liquids with polysaccharides, 8–synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol. Biosci. 2009, 9, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Bučko, M.; Gemeiner, P.; Schenkmayerová, A.; Krajčovič, T.; Rudroff, F.; Mihovilovič, M.D. Baeyer-Villiger oxidations: Biotechnological approach. Appl. Microbiol. Biotechnol. 2016, 100, 6585–6599. [Google Scholar] [CrossRef] [PubMed]
- Leisch, H.; Morley, K.; Lau, C.K. Baeyer-Villiger Monooxygenases: More Than Just Green Chemistry. Chem. Rev. 2011, 111, 4165–4222. [Google Scholar] [CrossRef] [PubMed]
- Balke, K.; Kadow, M.; Mallin, H.; Saß, S.; Bornscheuer, U.T. Discovery, application and protein engineering of Baeyer–Villiger monooxygenases for organic synthesis. Org. Biomol. Chem. 2012, 10, 6249–6265. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, F.; Fink, M.J.; Pydi, R.; Bornscheuer, U.T.; Mihovilovic, M.D. First chemo-enzymatic synthesis of the (R)-Taniguchi lactone and substrate profiles of CAMO and OTEMO, two new Baeyer-Villiger monooxygenases. Monatsh. Chem. 2017, 148, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohyd. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Podskočová, J.; Chorvát, D., Jr.; Kolláriková, G.; Lacík, I. Characterization of polyelectrolyte microcapsules by confocal laser scanning microscopy and atomic force microscopy. Laser Phys. 2005, 15, 545–551. [Google Scholar]
- Zhang, L.Y.; Yao, S.J.; Guan, Y.X. Effects of poly(methylene-co-guanidine) in microbial growth in an alginate/cellulose sulphate-CaCl2/poly(methylene-co-guanidine) capsule system. Process Biochem. 2005, 40, 189–193. [Google Scholar] [CrossRef]
- Neděla, V.; Tihlaříková, E.; Runštuk, J.; Hudec, J. High-efficiency detector of secondary and backscattered electrons for low-dose imaging in the ESEM. Ultramicroscopy 2018, 184, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tihlaříková, E.; Neděla, V.; Shiojiri, M. In Situ study of live specimens in an environmental scanning electron microscope. Microsc. Microanal. 2013, 19, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Bučko, M.; Vikartovská, A.; Gemeiner, P.; Lacík, I.; Kolláriková, G.; Marison, I.W. Nocardia tartaricans cells immobilized in sodium alginate-cellulose sulfate-poly(methylene-co-guanidine) capsules: Mechanical resistance and operational stability. J. Chem. Technol. Biotechnol. 2006, 81, 500–504. [Google Scholar] [CrossRef]
- Kishino, K.; Kawai, T.; Nose, M.; Saitoh, M.; Kamide, K. Dilute Solution Properties of sodium cellulose disulphate. Eur. Polym. J. 1981, 17, 623–630. [Google Scholar] [CrossRef]
- Mihovilovic, M.D.; Rudroff, F.; Grötzl, B.; Kapitan, P.; Snajdrova, R.; Rydz, J.; Mach, R. Family clustering of Baeyer–Villiger monooxygenases based on protein sequence and stereopreference. Angew. Chem. Int. Ed. 2005, 44, 3609–3613. [Google Scholar] [CrossRef] [PubMed]
- Neděla, V.; Konvalina, I.; Lencová, B.; Zlámal, J. Comparison of calculated, simulated and measured signal amplification in a variable pressure SEM. Nucl. Instrum. Meth. A 2011, 645, 79–83. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajčovič, T.; Bučko, M.; Vikartovská, A.; Lacík, I.; Uhelská, L.; Chorvát, D.; Neděla, V.; Tihlaříková, E.; Gericke, M.; Heinze, T.; et al. Polyelectrolyte Complex Beads by Novel Two-Step Process for Improved Performance of Viable Whole-Cell Baeyer-Villiger Monoxygenase by Immobilization. Catalysts 2017, 7, 353. https://doi.org/10.3390/catal7110353
Krajčovič T, Bučko M, Vikartovská A, Lacík I, Uhelská L, Chorvát D, Neděla V, Tihlaříková E, Gericke M, Heinze T, et al. Polyelectrolyte Complex Beads by Novel Two-Step Process for Improved Performance of Viable Whole-Cell Baeyer-Villiger Monoxygenase by Immobilization. Catalysts. 2017; 7(11):353. https://doi.org/10.3390/catal7110353
Chicago/Turabian StyleKrajčovič, Tomáš, Marek Bučko, Alica Vikartovská, Igor Lacík, Lucia Uhelská, Dušan Chorvát, Vilém Neděla, Eva Tihlaříková, Martin Gericke, Thomas Heinze, and et al. 2017. "Polyelectrolyte Complex Beads by Novel Two-Step Process for Improved Performance of Viable Whole-Cell Baeyer-Villiger Monoxygenase by Immobilization" Catalysts 7, no. 11: 353. https://doi.org/10.3390/catal7110353
APA StyleKrajčovič, T., Bučko, M., Vikartovská, A., Lacík, I., Uhelská, L., Chorvát, D., Neděla, V., Tihlaříková, E., Gericke, M., Heinze, T., & Gemeiner, P. (2017). Polyelectrolyte Complex Beads by Novel Two-Step Process for Improved Performance of Viable Whole-Cell Baeyer-Villiger Monoxygenase by Immobilization. Catalysts, 7(11), 353. https://doi.org/10.3390/catal7110353





