YCl3-Catalyzed Highly Selective Ring Opening of Epoxides by Amines at Room Temperature and under Solvent-Free Conditions
Abstract
:1. Introduction
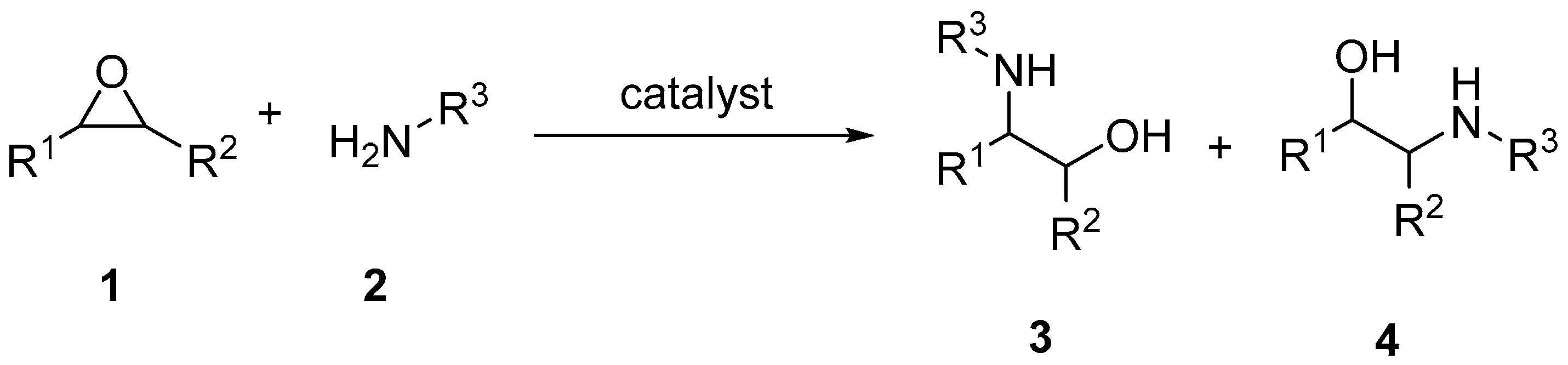
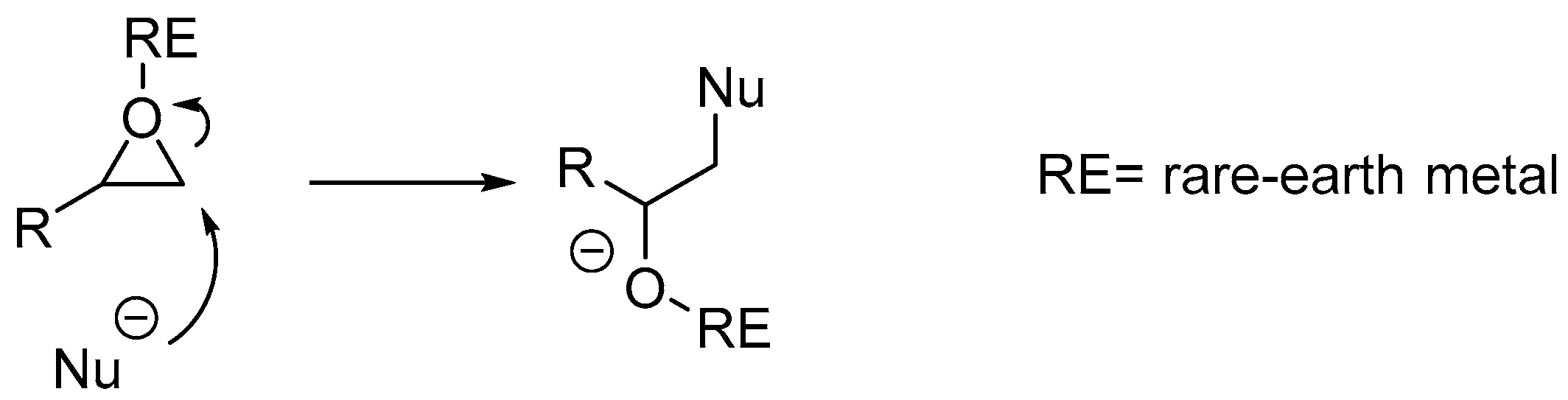
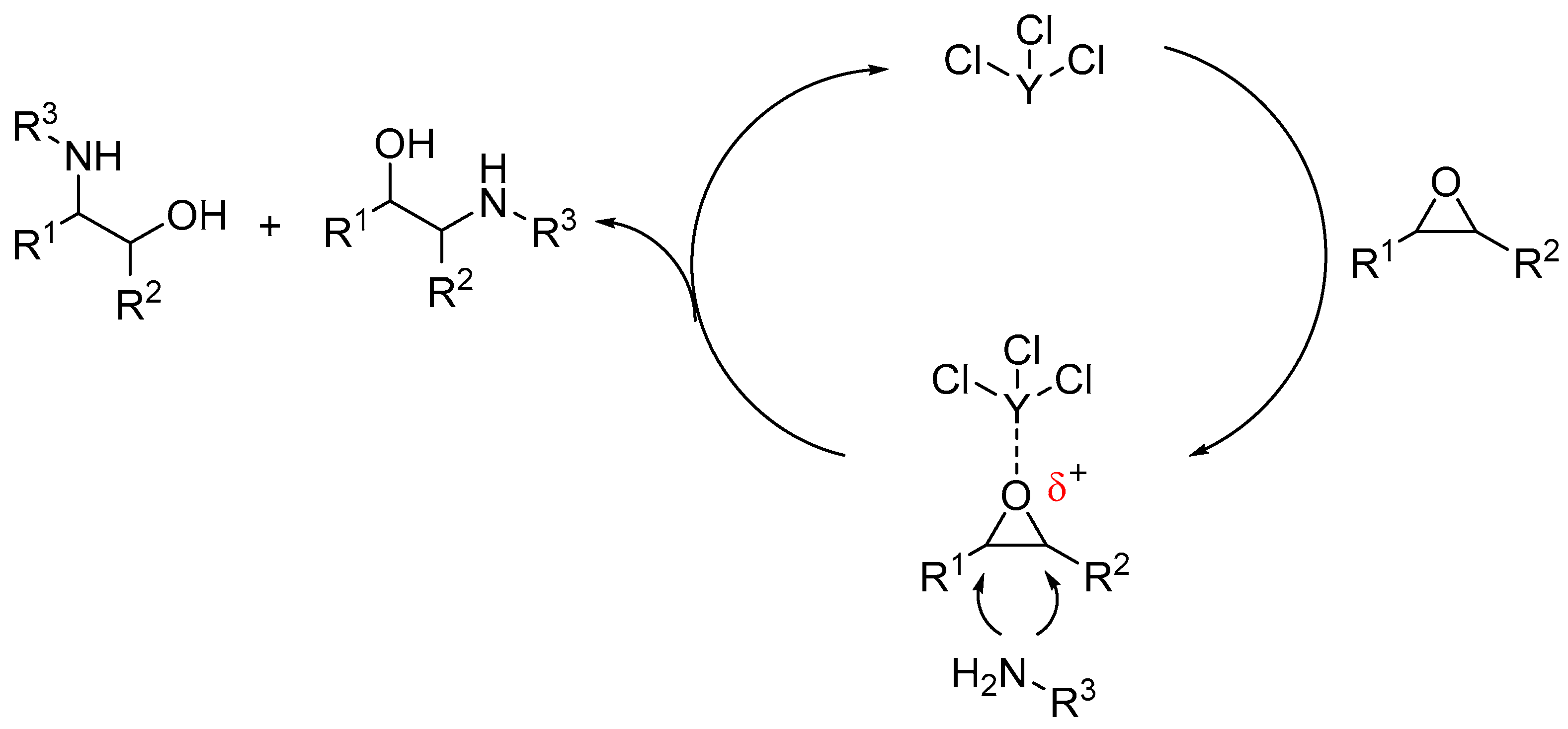
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure
3.2. Spectroscopic Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Erhardt, P.W.; Woo, C.M.; Gorczynski, R.J.; Anderson, W.G. Ultra-short-acting Beta-adrenergic receptor blocking agents. 1. (aryloxy)propanolamines containing esters in the nitrogen substituent. J. Med. Chem. 1982, 25, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Gregory, T.F.; Heffner, T.G.; MacKenzie, R.G.; Pugsley, T.A.; Meulen, S.V.; Wise, L.D. Discovery of selective dopamine D4 receptor antagonists: 1-aryloxy-3-(4-aryloxypiperidinyl)-2-propanols. Bioorg. Med. Chem. Lett. 1997, 7, 1377–1380. [Google Scholar] [CrossRef]
- Klingler, F.D. Asymmetric hydrogenation of prochiral amino ketones to amino alcohols for pharmaceutical use. Acc. Chem. Res. 2007, 40, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Breuer, M.; Ditrich, K.; Habicher, T.; Hauer, B.; Kesseler, M.; Sturmer, R.; Zelinski, T. Industrial methods for the production of optically active intermediates. Angew. Chem. Int. Ed. 2004, 43, 788–824. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.S.; Fernandes, M.A.; Padayachy, K.; Marques, H.M. Amino-alcohol ligands: Synthesis and structure of N,N′-bis(2-hydroxycyclopentyl)ethane-1,2-diamine and its salts, and an assessment of its fitness and that of related ligands for complexing metal ions. Inorg. Chem. 2010, 49, 8003–8011. [Google Scholar] [CrossRef] [PubMed]
- González-Bobes, F.; Fu, G.C. Amino alcohols as ligands for nickel-catalyzed suzuki reactions of unactivated alkyl halides, including secondary alkyl chlorides, with arylboronic acids. J. Am. Chem. Soc. 2006, 128, 5360–5361. [Google Scholar] [CrossRef] [PubMed]
- Saddique, F.A.; Zahoor, A.F.; Faiz, S.; Naqvi, S.A.R.; Usman, M.; Ahmad, M. Recent trends in ring opening of epoxides by amines as nucleophiles. Synth. Commun. 2016, 46, 831–868. [Google Scholar] [CrossRef]
- Harrak, Y.; Pujol, M.D. Mild cleavage of aliphatic epoxides with substituted anilines on alumina. Tetrahedron Lett. 2002, 43, 819–822. [Google Scholar] [CrossRef]
- Kumar, S.R.; Leelavathi, P. Phosphomolybdic acid-Al2O3: A mild, efficient, heterogeneous and reusable catalyst for regioselective opening of oxiranes with amines to β-amino alcohols. J. Mol. Catal. A Chem. 2007, 266, 65–68. [Google Scholar] [CrossRef]
- Posner, G.H.; Rogers, D.Z. Organic-reactions at alumina surfaces—Mild and selective opening of epoxides by alcohols, thiols, benzeneselenol, amines, and acetic-acid. J. Am. Chem. Soc. 1977, 99, 8208–8214. [Google Scholar] [CrossRef]
- Khaksar, S.; Heydari, A.; Tajbaksh, M.; Bijanzadeh, H.R. A facile and efficient synthesis of β-amino alcohols using 2,2,2-trifluoroethanol as a metal-free and reusable medium. J. Fluorine Chem. 2010, 131, 106–110. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Singh, S.; Khan, N.U.H.; Abdi, S.H.R.; Suresh, E.; Jasra, R.V. Efficient method for ring opening of epoxides with amines by nay zeolite under solvent-free conditions. J. Mol. Catal. A-Chem. 2007, 264, 162–169. [Google Scholar] [CrossRef]
- Acocella, M.R.; D’Urso, L.; Maggio, M.; Guerra, G. Green regio- and enantioselective aminolysis catalyzed by graphite and graphene oxide under solvent-free conditions. ChemCatChem 2016, 8, 1915–1920. [Google Scholar] [CrossRef]
- Shinde, S.S.; Said, M.S.; Surwase, T.B.; Kumar, P. Mild regiospecific alcoholysis and aminolysis of epoxides catalyzed by zirconium(IV) oxynitrate. Tetrahedron Lett. 2015, 56, 5916–5919. [Google Scholar] [CrossRef]
- Shah, A.K.; Kumar, M.; Abdi, S.H.R.; Kureshy, R.I.; Khan, N.U.H.; Bajaj, H.C. Solvent-free aminolysis of aliphatic and aryloxy epoxides with sulfated zirconia as solid acid catalyst. Appl. Catal. A-Gen. 2014, 486, 105–114. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Hosseinzadeh, R.; Rezaee, P.; Alinezhad, H. Regioselective ring opening of epoxides with amines using silica-bonded S-sulfonic acid under solvent-free conditions. J. Mex. Chem. Soc. 2012, 56, 402–407. [Google Scholar]
- Negron-Silva, G.; Hernandez-Reyes, C.X.; Angeles-Beltran, D.; Lomas-Romero, L.; Gonzalez-Zamora, E.; Mendez-Vivar, J. Comparative study of the regioselective synthesis of β-aminoalcohols under solventless conditions catalyzed by sulfated zirconia and SZ/MCM-41. Molecules 2007, 12, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Kamrani, P.; Saadat, M. A magnetic nanoparticle-catalyzed regioselective ring opening of epoxides by aromatic amines. Appl. Organomet. Chem. 2016, 30, 431–434. [Google Scholar] [CrossRef]
- Islam, M.M.; Bhanja, P.; Halder, M.; Kundu, S.K.; Bhaumik, A.; Islam, S.M. Chiral Co(III)-salen complex supported over highly ordered functionalized mesoporous silica for enantioselective aminolysis of racemic epoxides. RSC Adv. 2016, 6, 109315–109321. [Google Scholar] [CrossRef]
- Halder, M.; Bhanja, P.; Roy, S.; Ghosh, S.; Kundu, S.; Islam, M.M.; Islam, S.M. A new recyclable functionalized mesoporous SBA-15 catalyst grafted with chiral Fe(III) sites for the enantioselective aminolysis of racemic epoxides under solvent free conditions. RSC Adv. 2016, 6, 97599–97605. [Google Scholar] [CrossRef]
- Aghapoor, K.; Amini, M.M.; Jadidi, K.; Darabi, H.R. N-functionalized l-proline anchored MCM-41: A novel organic-inorganic hybrid material for solvent-free aminolysis of styrene oxide under microwave irradiation. Acta Chim. Slov. 2015, 62, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srinivas, D. Aminolysis of epoxides catalyzed by three-dimensional, mesoporous titanosilicates, Ti-SBA-12 and Ti-SBA-16. J. Catal. 2012, 293, 126–140. [Google Scholar] [CrossRef]
- Chakravarti, R.; Oveisi, H.; Kalita, P.; Pal, R.R.; Halligudi, S.B.; Kantam, M.L.; Vinu, A. Three-dimensional mesoporous cage type aluminosilicate: An efficient catalyst for ring opening of epoxides with aromatic and aliphatic amines. Micropor. Mesopor. Mater. 2009, 123, 338–344. [Google Scholar] [CrossRef]
- Saikia, L.; Satyarthi, J.K.; Srinivas, D.; Ratnasamy, P. Activation and reactivity of epoxides on solid acid catalysts. J. Catal. 2007, 252, 148–160. [Google Scholar] [CrossRef]
- Sreedhar, B.; Radhika, P.; Neelima, B.; Hebalkar, N. Regioselective ring opening of epoxides with amines using monodispersed silica nanoparticles in water. J. Mol. Catal. A-Chem. 2007, 272, 159–163. [Google Scholar] [CrossRef]
- Chakraborti, A.K.; Rudrawar, S.; Kondaskar, A. An efficient synthesis of 2-amino alcohols by silica gel catalysed opening of epoxide rings by amines. Org. Biomol. Chem. 2004, 2, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Gupte, S.; Shivarkar, A. Synthesis of β-amino alcohols from aromatic amines and alkylene carbonates using Na-Y zeolite catalyst. Synlett 2006, 9, 1374–1378. [Google Scholar]
- Shivarkar, A.B.; Gupte, S.P.; Chaudhari, R.V. Tandem synthesis of β-amino alcohols from aniline, dialkyl carbonate, and ethylene glycol. Ind. Eng. Chem. Res. 2008, 47, 2484–2494. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sugita, K.; Oyamada, H. Scandium triflate catalyzed allylation reactions of benzoylhydrazones with tetraallyltin. An efficient catalytic route to homoallylic amines. Synlett 1999, 1999, 138–140. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, S.F.; Yang, W.H.; Hao, X.; Glaser, R.; Sun, W.H. Chloroyttrium 2-(1-(arylimino)alkyl)quinolin-8-olate complexes: Synthesis, characterization, and catalysis of the ring-opening polymerization of ε-caprolactone. Organometallics 2012, 31, 8178–8188. [Google Scholar] [CrossRef]
- Bu, X.L.; Zhang, Z.X.; Zhou, X.G. Switching from dimerization to cyclotrimerization selectivity by FeCl3 in the Y[N(TMS)2]3-catalyzed transformation of terminal alkynes: A new strategy for controlling the selectivity of organolanthanide-based catalysis. Organometallics 2010, 29, 3530–3534. [Google Scholar] [CrossRef]
- Woodman, T.J.; Schormann, M.; Bochmann, M. Synthesis, characterization, and reactivity of lanthanide complexes with bulky silylallyl ligands. Isr. J. Chem. 2002, 42, 283–293. [Google Scholar] [CrossRef]
- Likhar, P.R.; Bandyopadhyay, A.K. YCl3-catalyzed highly selective conversion of arylglyoxal to α-aryl-α-hydroxyacetic ester: Dramatic influence of base. Synlett 2000, 2000, 538–540. [Google Scholar] [CrossRef]
- Schumann, H.; Erbstein, F.; Weimann, R.; Demtschuk, J. Organometallic compounds of the lanthanides.115. donor substituted chiral Ansa-lanthanidocenes: Synthesis of dimethyl(dimethylaminoethylcyclopentadienyl)(tetramethylcyclopentadienl)silane dipotassium and of some chiral Ansa-metallocene derivatives of Y, Sm, Ho, Er and Lu. J. Organomet. Chem. 1997, 536, 541–547. [Google Scholar]
- Robinson, J.R.; Fan, X.Y.; Yadav, J.; Carroll, P.J.; Wooten, A.J.; Pericas, M.A.; Schelter, E.J.; Walsh, P.J. Air- and water-tolerant rare earth guanidinium binolate complexes as practical precatalysts in multifunctional asymmetric catalysis. J. Am. Chem. Soc. 2014, 136, 8034–8041. [Google Scholar] [CrossRef] [PubMed]
- Clapsaddle, B.J.; Neumann, B.; Wittstock, A.; Sprehn, D.W.; Gash, A.E.; Satcher, J.H.; Simpson, R.L.; Baumer, M. A sol-gel methodology for the preparation of lanthanide-oxide aerogels: Preparation and characterization. J. Sol-Gel Sci. Tech. 2012, 64, 381–389. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Cui, D.M.; Liu, X.L. Alternating copolymerization of cyclohexene oxide and carbon dioxide catalyzed by noncyclopentadienyl rare-earth metal bis(alkyl) complexes. J. Polym. Sci. Pol. Chem. 2008, 46, 6810–6818. [Google Scholar] [CrossRef]
- Barthel, A.; Saih, Y.; Gimenez, M.; Pelletier, J.D.A.; Kuhn, F.E.; D’Elia, V.; Basset, J.M. Highly integrated CO2 capture and conversion: Direct synthesis of cyclic carbonates from industrial flue gas. Green Chem. 2016, 18, 3116–3123. [Google Scholar] [CrossRef]
- Palmieri, A.; Petrini, M.; Shaikh, R.R. Synthesis of 3-substituted indoles via reactive alkylideneindolenine intermediates. Org. Biomol. Chem. 2010, 8, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Shaikh, R.R. A ‘click’ approach to the synthesis of 3-[2-(1-alkyltriazol-4-yl)ethyl]indoles. Synthesis 2009, 2009, 3143–3149. [Google Scholar] [CrossRef]
- Petrini, M.; Shaikh, R.R. Synthesis of indolylalkylphosphonates and 3-(1-diphenylphosphinoalkyl) indoles by reaction of 3-(1-arylsulfonlyalkyl) indoles with phosphorus derivatives. Tetrahedron Lett. 2008, 49, 5645–5648. [Google Scholar] [CrossRef]
- Palmieri, A.; Petrini, M.; Shaikh, R.R. Double functionalization of N-Boc-3-(tosylmethyl)indole exploiting the activating properties of the tosyl group. Synlett 2008, 2008, 1845–1851. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Mazzanti, A.; Petrini, M.; Bartoli, G.; Melchiorre, P. Proline-catalyzed asymmetric formal α-alkylation of aldehydes via vinylogous iminium ion intermediates generated from arylsulfonyl indoles. Angew. Chem. Int. Ed. 2008, 47, 8707–8710. [Google Scholar] [CrossRef] [PubMed]
- Ballini, R.; Palmieri, A.; Petrini, M.; Shaikh, R.R. Reaction of 3-(1-arylsulfonylalkyl)-indoles with easily enolisable derivatives promoted by potassium fluoride on basic alumina. Adv. Synth. Catal. 2008, 350, 129–134. [Google Scholar] [CrossRef]
- Yoshida, Y.; Endo, T. Radical polymerization behavior and thermal properties of vinyl ethylene carbonate derivatives bearing aromatic moieties. Polymer 2016, 102, 167–175. [Google Scholar] [CrossRef]
- Aoyagi, N.; Furusho, Y.; Endo, T. Convenient synthesis of cyclic carbonates from CO2 and epoxides by simple secondary and primary ammonium iodides as metal-free catalysts under mild conditions and its application to synthesis of polymer bearing cyclic carbonate moiety. J. Polym. Sci. Part A 2013, 51, 1230–1242. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, S.; Gupta, R. Manganese complexes of pyrrole- and indolecarboxamide ligands: Synthesis, structure, electrochemistry, and applications in oxidative and lewis-acidassisted catalysis. Eur. J. Inorg. Chem. 2015, 2015, 5534–5544. [Google Scholar] [CrossRef]
- Bansal, D.; Hundal, G.; Gupta, R. A metalloligand appended with thiazole rings: Heterometallic {Co3+-Zn2+} and {Co3+-Cd2+} complexes and their heterogeneous catalytic applications. Eur. J. Inorg. Chem. 2015, 2015, 1022–1032. [Google Scholar] [CrossRef]
- Bansal, D.; Kumar, G.; Hundal, G.; Gupta, R. Mononuclear complexes of amide-based ligands containing appended functional groups: Role of secondary coordination spheres on catalysis. Dalton Trans. 2014, 43, 14865–14875. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Kumar, A.; Parella, R. Magnetic nano Fe3O4 catalyzed solvent-free stereo- and regioselectiveaminolysis of epoxides by amines; a green method for the synthesis of β-amino alcohols. Synlett 2014, 25, 835–842. [Google Scholar] [CrossRef]


| Sr. No. | Catalyst | Conversion (%) | 3aa:4aa Ratio (%) |
|---|---|---|---|
| 1 | None | NR | - |
| 2 | YCl3 | 90 | 93:7 |
| 3 | ScCl3 | 82 | 92:8 |
| 4 a | YCl3 | 100 | 93:7 |
| 5 b | ScCl3 | 100 | 92:8 |
| 6 c | YCl3 | 100 | 93:7 |
| 7 d | YCl3 | 50 | 94:6 |
| 8 e | YCl3 | 87 | 94:6 |
| 9 f | ScCl3 | 88 | 93:7 |
| 10 | NbCl5 | 82 | 87:13 |
| 11 | ZrCl4 | 75 | 87:13 |
| 12 | ZnCl2 | traces | nd |
| 13 | Nb(OEt)5 | 85 | 82:18 |

| Epoxide | Amine | Epoxide Conversion a and 3:4 Ratio b (%) | |||
|---|---|---|---|---|---|
| Sr. | |||||
| No. | 1 | 2 | 3 | 4 | |
| 1 | 1a | 2a |  |  | 100 93:7 |
| 2 | 1a | 2b |  |  | 100 >99:<1 |
| 3 | 1a | 2c |  |  | 100 98:2 |
| 4 | 1a | 2d |  |  | 13 50:50 |
| 5 | 1b | 2a |  |  | 100 19:81 |
| 6 | 1b | 2b |  |  | 70 <1:>99 |
| 7 | 1b | 2c |  |  | 100 2:98 |
| 8 | 1c | 2a |  |  | 100 70:30 |
| 9 | 1d | 2a |  | 100 - | |
| 10 | 1a | 2e |  |  | 30 93:7 |
| 11 | 1e | 2a |  |  | 75 83:17 |
| 12 | 1e | 2f |  |  | 100 82:18 |
| 13 | 1f | 2a |  |  | 100 0/100 |
| Sr. No. | Catalyst | Cat. Loading (mol %), Temperature (°C)/Time (h) | Conversion (%) | Ref. |
|---|---|---|---|---|
| 1 | (Et4N)2[MnL2Cl] | 1 25/4 | 99 | [47] |
| 2 | [Co(L1)Cd(OH2)2(NO3)]·H2O | 1 r.t./4 | 98 | [48] |
| 3 | [(L2(H2))Zn(NO3)2] | 2 30/4 | 98 | [49] |
| 4 | Nano Fe3O4 | 10 r.t./20 | 83 | [50] |
| 5 | YCl3 | 1 25/3 | 100 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natongchai, W.; Khan, R.A.; Alsalme, A.; Shaikh, R.R. YCl3-Catalyzed Highly Selective Ring Opening of Epoxides by Amines at Room Temperature and under Solvent-Free Conditions. Catalysts 2017, 7, 340. https://doi.org/10.3390/catal7110340
Natongchai W, Khan RA, Alsalme A, Shaikh RR. YCl3-Catalyzed Highly Selective Ring Opening of Epoxides by Amines at Room Temperature and under Solvent-Free Conditions. Catalysts. 2017; 7(11):340. https://doi.org/10.3390/catal7110340
Chicago/Turabian StyleNatongchai, Wuttichai, Rais Ahmad Khan, Ali Alsalme, and Rafik Rajjak Shaikh. 2017. "YCl3-Catalyzed Highly Selective Ring Opening of Epoxides by Amines at Room Temperature and under Solvent-Free Conditions" Catalysts 7, no. 11: 340. https://doi.org/10.3390/catal7110340
APA StyleNatongchai, W., Khan, R. A., Alsalme, A., & Shaikh, R. R. (2017). YCl3-Catalyzed Highly Selective Ring Opening of Epoxides by Amines at Room Temperature and under Solvent-Free Conditions. Catalysts, 7(11), 340. https://doi.org/10.3390/catal7110340