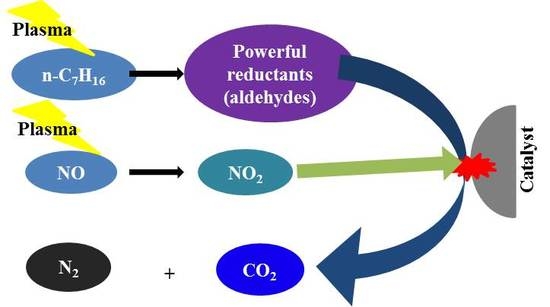
Consideration of the Role of Plasma in a Plasma-Coupled Selective Catalytic Reduction of Nitrogen Oxides with a Hydrocarbon Reducing Agent
Abstract
1. Introduction
2. Results and Discussion
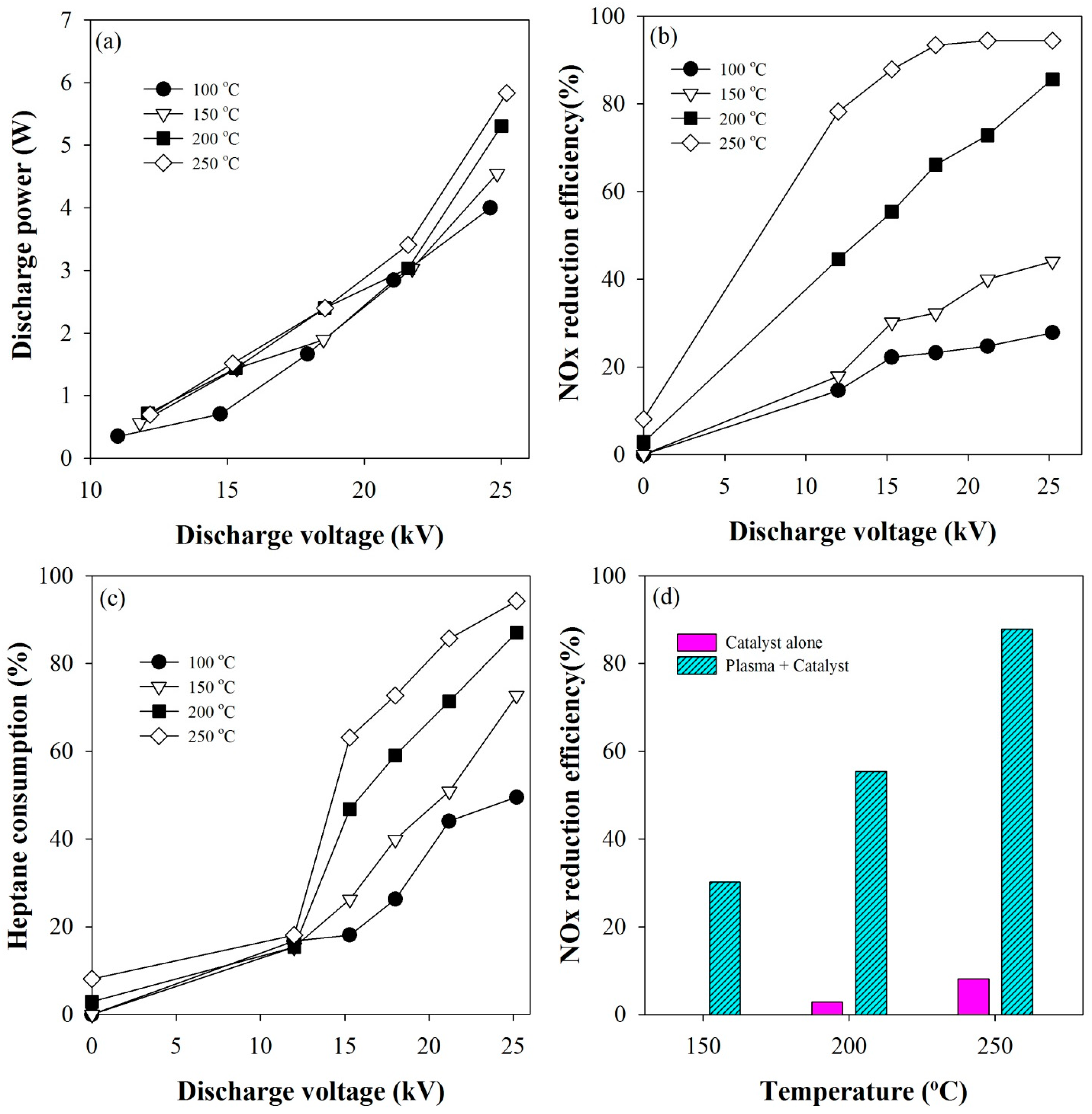
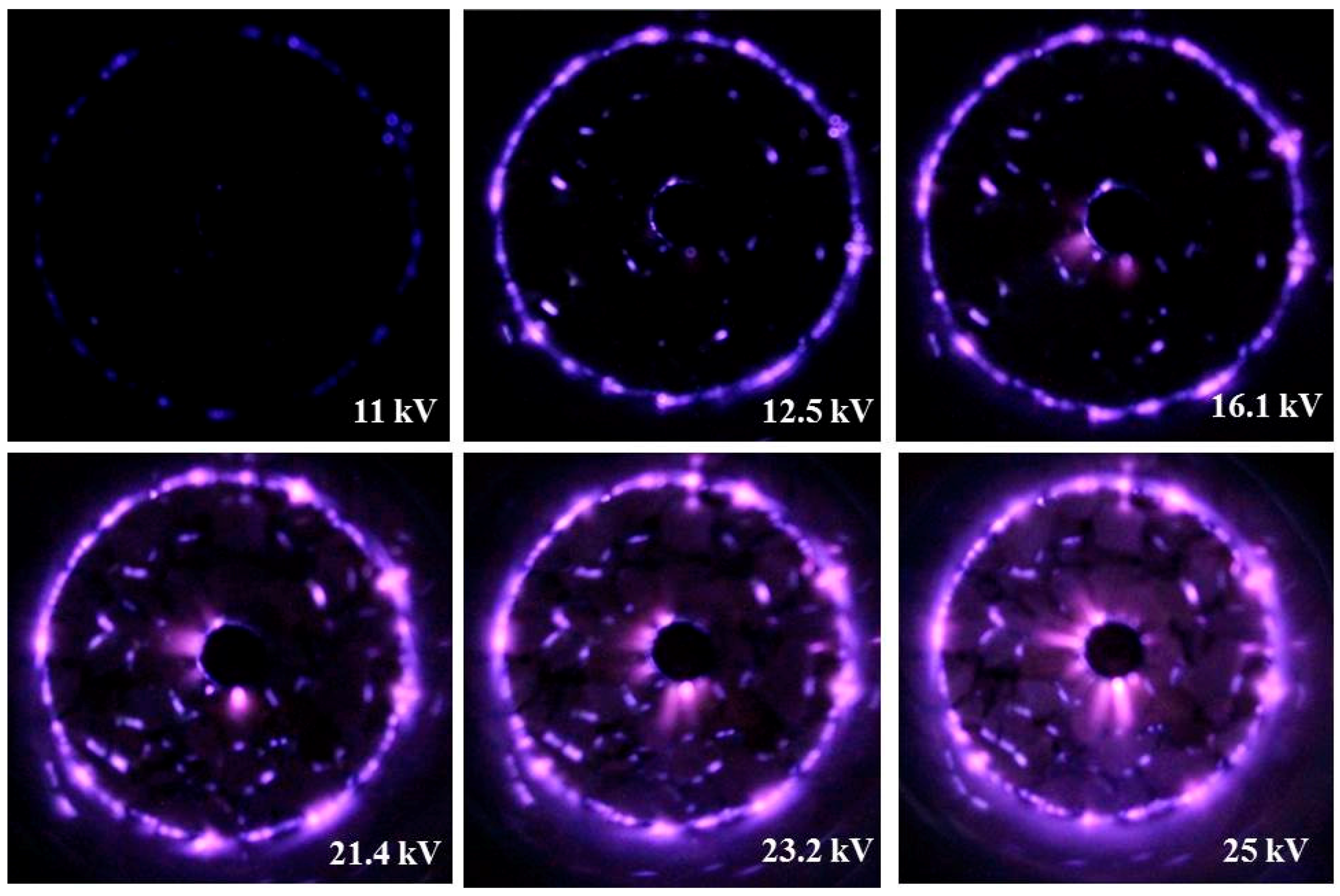
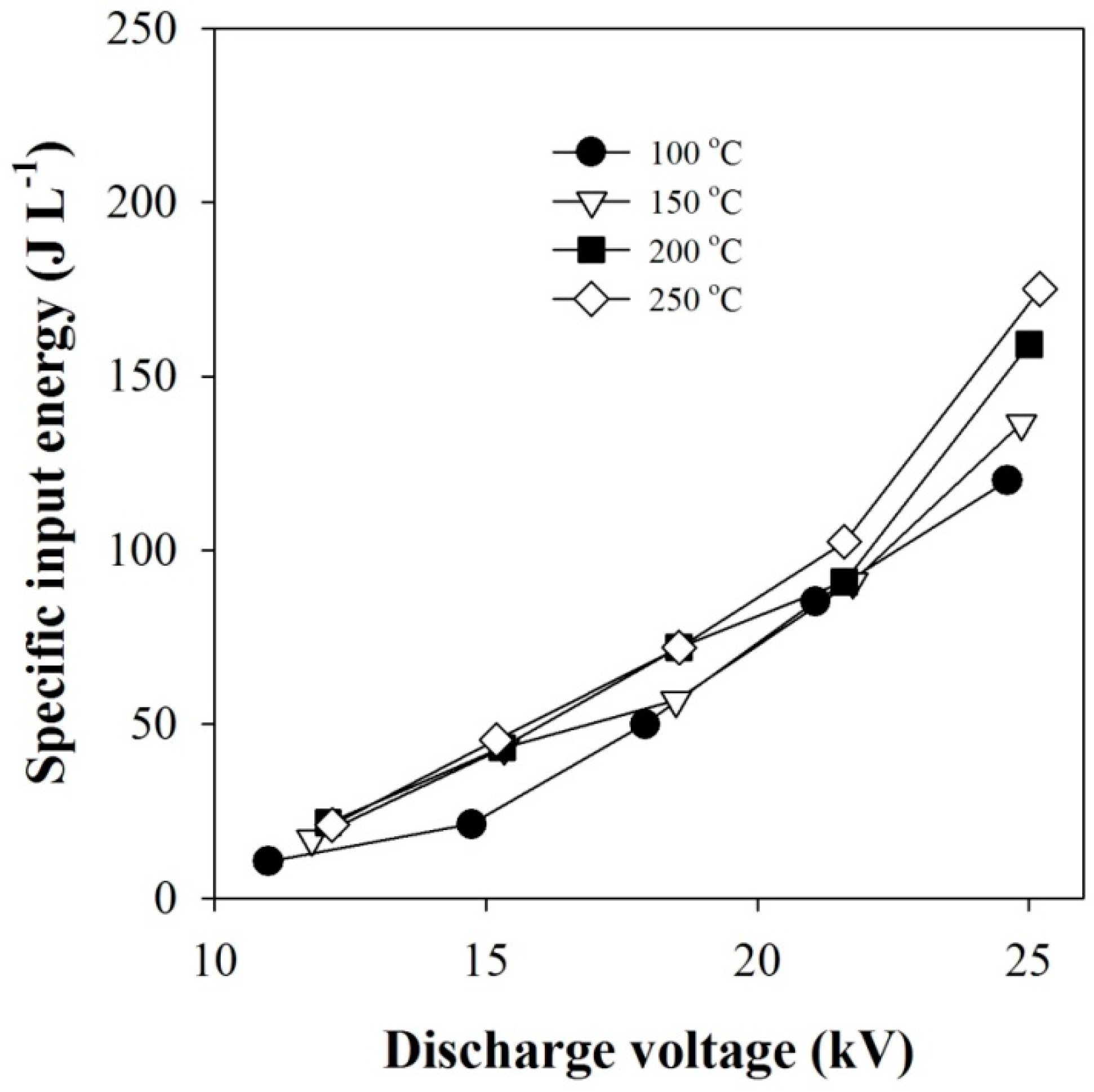
2.1. Plasma-Coupled SCR of NOx
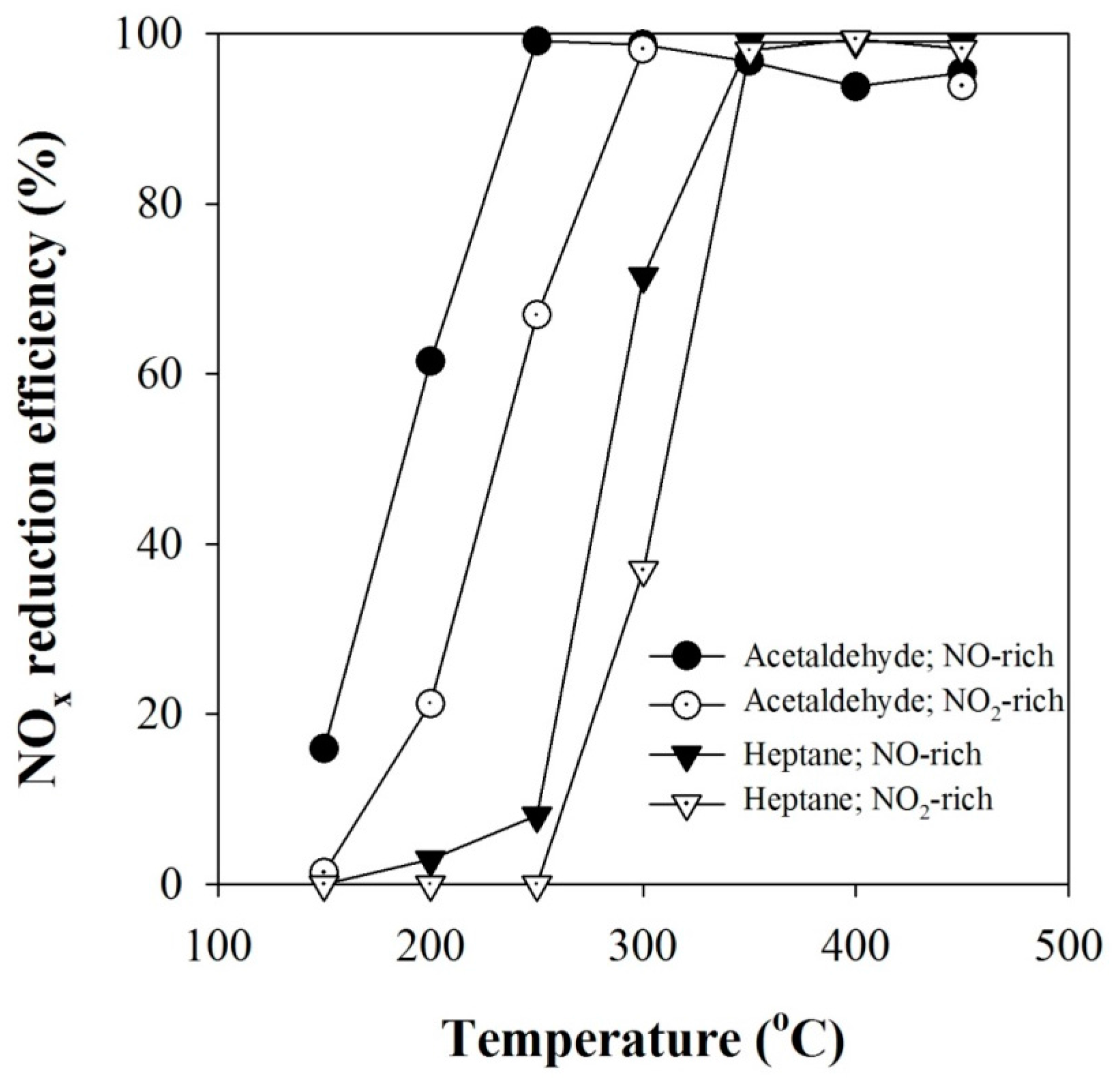
2.2. Effect of NO2 Fraction on the Catalytic Reduction of NOx
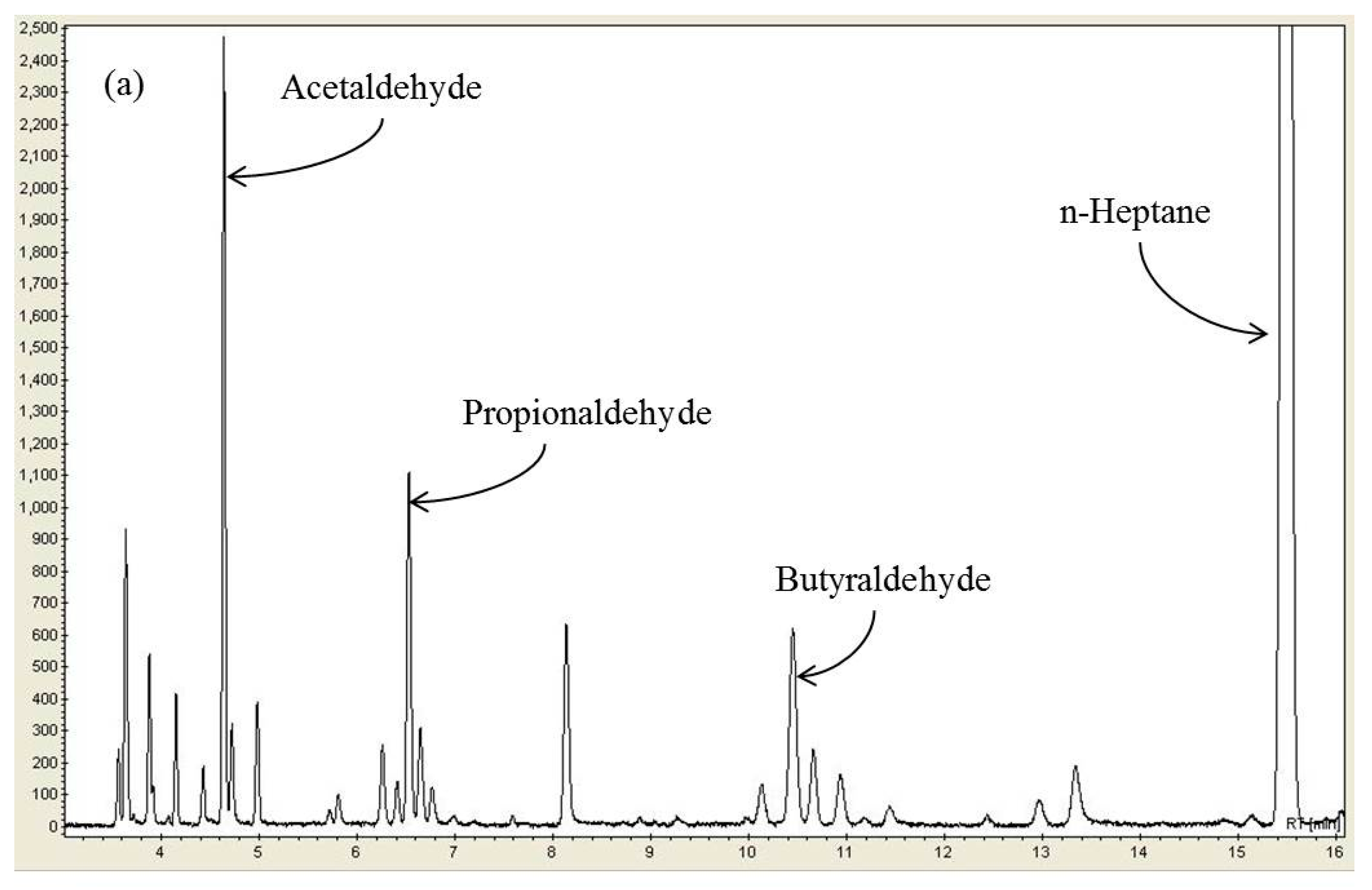
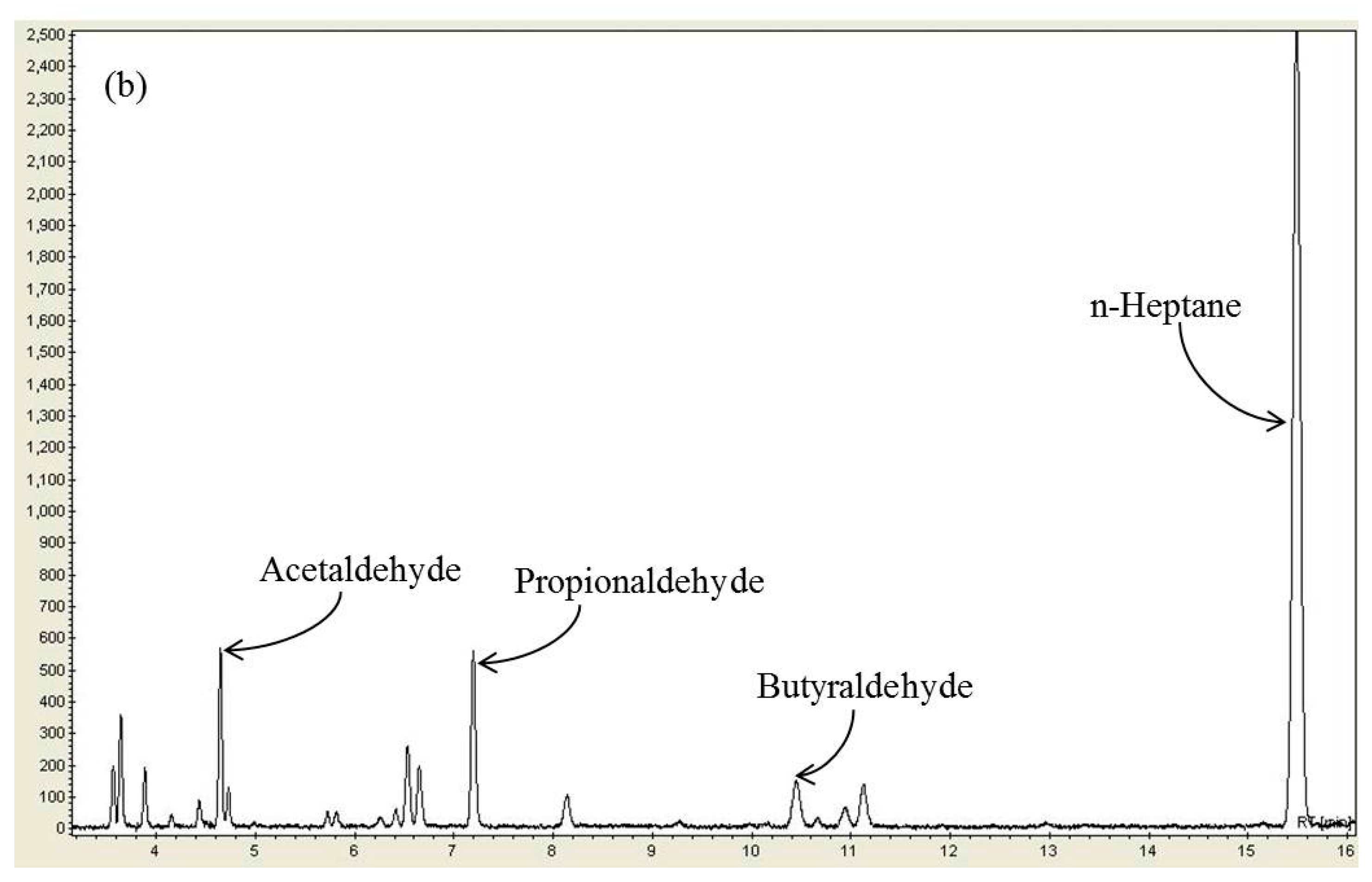
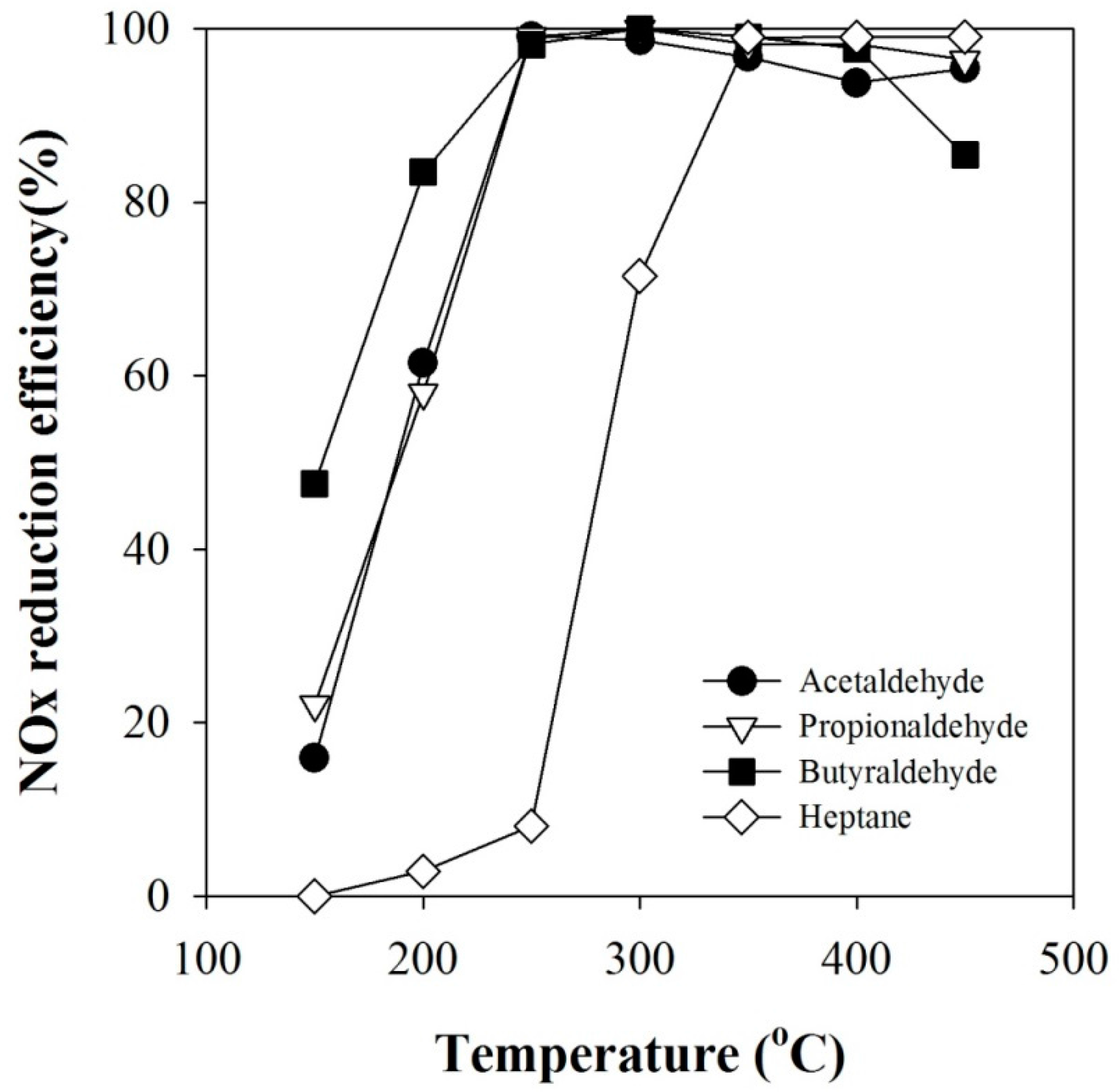
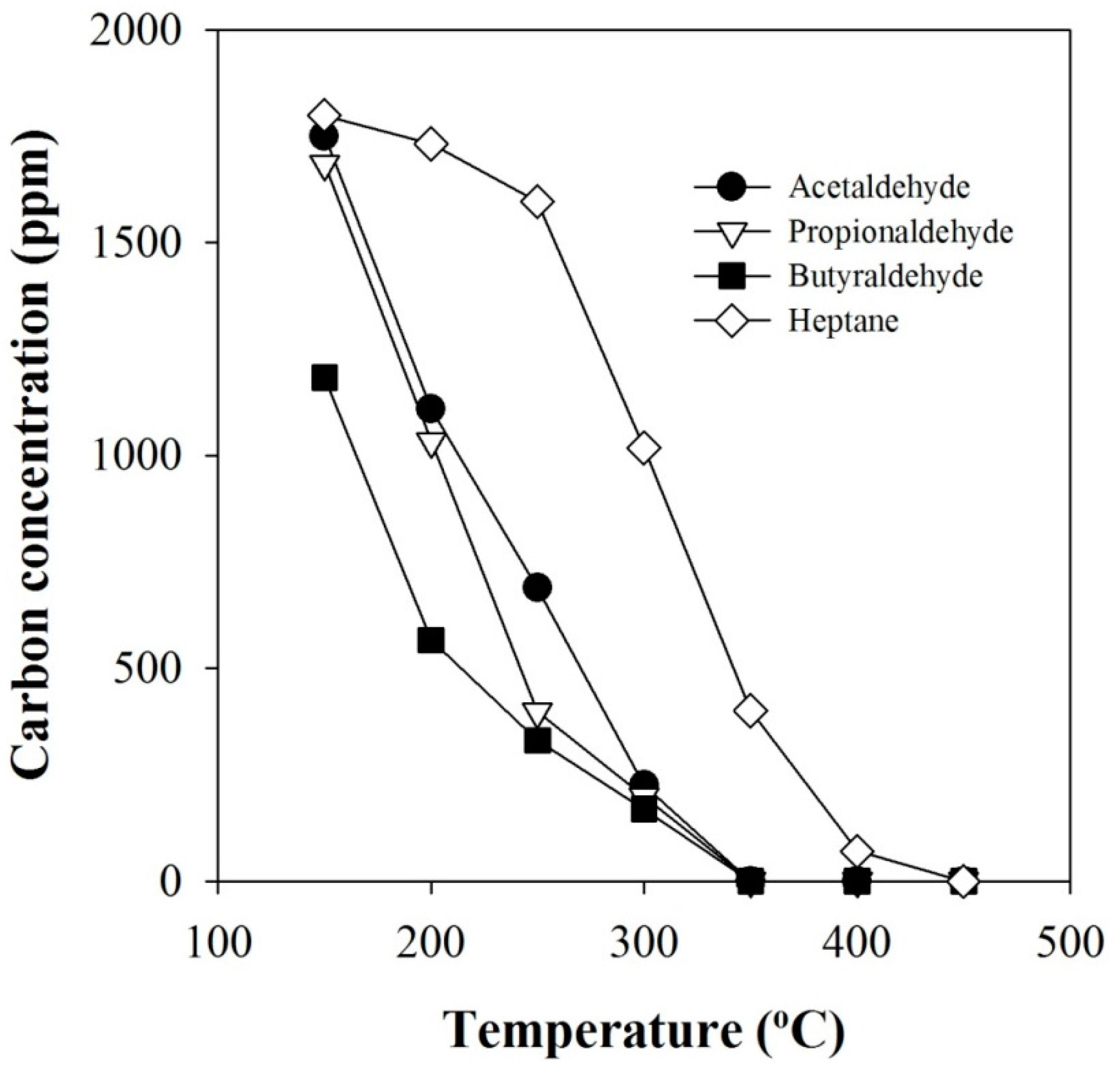
2.3. Effect of n-Heptane Decomposition Products on the Catalytic Reduction of NOx
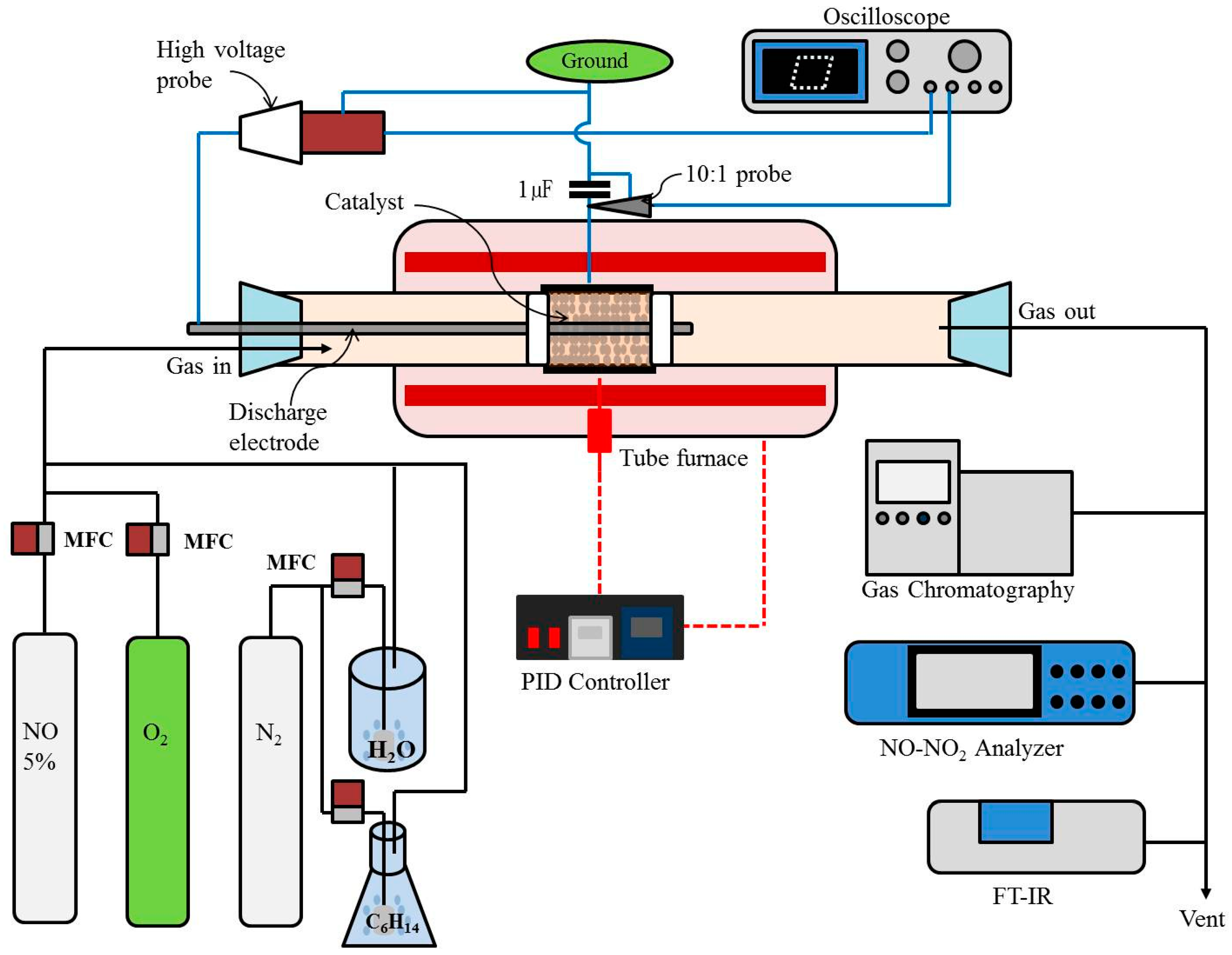
3. Experimental Section
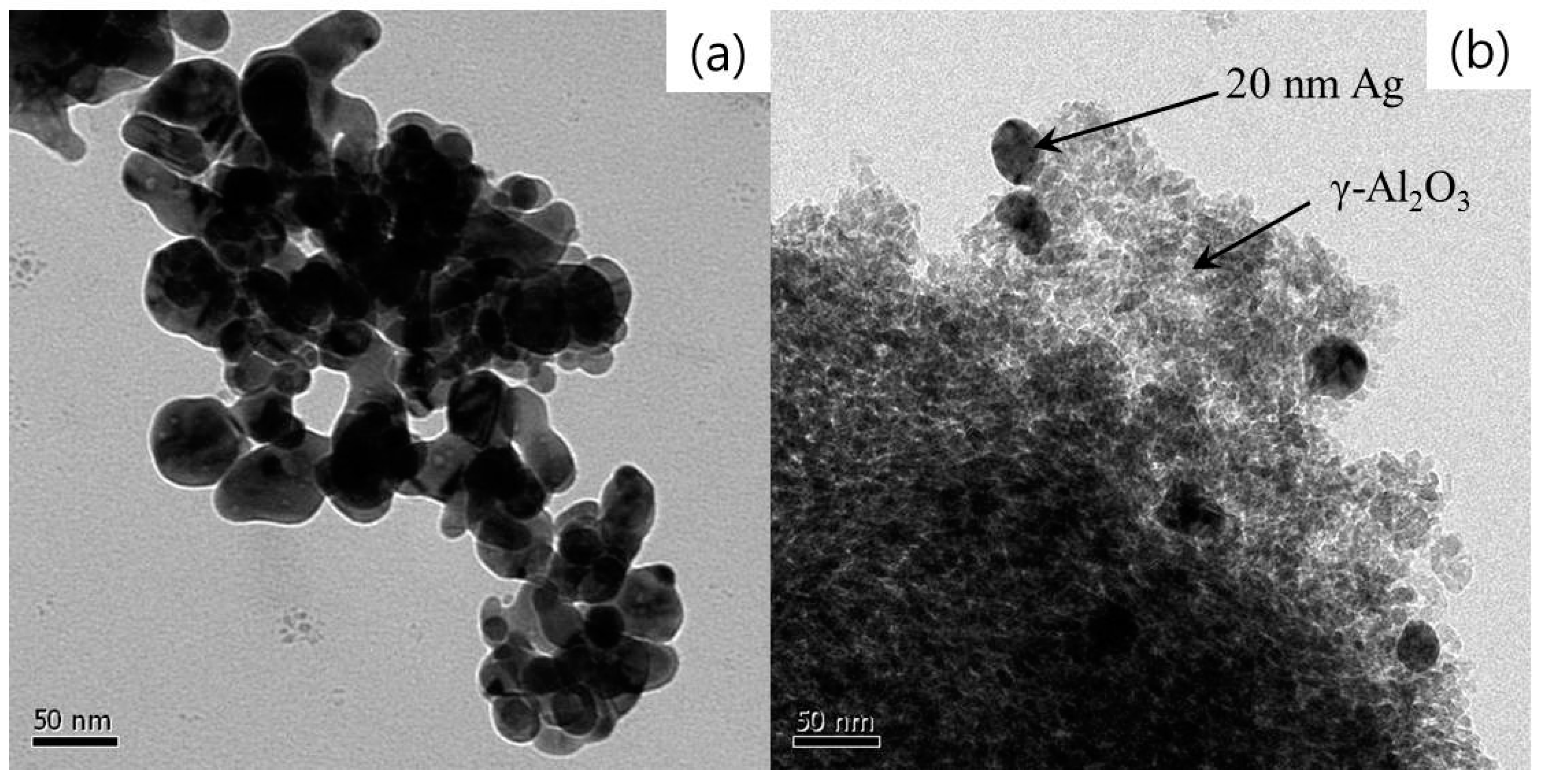
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacobson, M.Z. Air Pollution and Global Warming: History, Science, and Solutions, 2nd ed.; Stanford University: Stanford, CA, USA, 2012; ISBN 9781107691155. [Google Scholar]
- Schill, L.; Putluru, S.S.R.; Fehrmann, R.; Jensen, A.D. Low-temperature NH3–SCR of NO on mesoporous Mn0.6Fe0.4/TiO2 prepared by a hydrothermal method. Catal. Lett. 2014, 144, 395–402. [Google Scholar] [CrossRef]
- Cheng, X.; Bi, X.T. A review of recent advances in selective catalytic NOx reduction reactor technologies. Particuology 2014, 16, 1–18. [Google Scholar] [CrossRef]
- Brandenberger, S.; Krocher, O.; Casapu, M.; Tissler, A.; Althoff, R. Hydrothermal deactivation of Fe-ZSM-5 catalysts for the selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2011, 101, 649–659. [Google Scholar] [CrossRef]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Experimental and kinetic modeling study of NH3-SCR of NOx on Fe-ZSM-5, Cu-chabazite and combined Fe- and Cu-zeolite monolithic catalysts. Chem. Eng. Sci. 2013, 87, 51–66. [Google Scholar] [CrossRef]
- Eranen, K.; Lindfors, L.E.; Klingstedt, F.; Murzin, D.Y. Continuous reduction of NO with octane over a silver/alumina catalyst in oxygen-rich exhaust gases: Combined heterogeneous and surface-mediated homogeneous reactions. J. Catal. 2003, 219, 25–40. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, H.J.; Heo, I.; Nam, I.-S.; Cho, B.K.; Choung, J.W.; Cha, M.-S.; Yeo, G.K. Mn-Fe/ZSM5 as a low-temperature SCR catalyst to remove NOx from diesel engine exhaust. Appl. Catal. B Environ. 2012, 126, 9–21. [Google Scholar] [CrossRef]
- Yang, T.T.; Bi, H.T.; Cheng, X.X. Effects of O2, CO2 and H2O on NOx adsorption and selective catalytic reduction over Fe/ZSM-5. Appl. Catal. B Environ. 2011, 102, 163–171. [Google Scholar] [CrossRef]
- Zhang, L.; Sha, X.-L.; Zhang, L.; He, H.; Ma, Z.; Wang, L.; Wang, Y.; She, L. Synergistic catalytic removal NOx and the mechanism of plasma and hydrocarbon gas. AIP Adv. 2016, 6. [Google Scholar] [CrossRef]
- Lee, T.Y.; Bai, H. Low temperature selective catalytic reduction of NOx with NH3 over Mn-based catalyst: A review. AIMS Environ. Sci. 2016, 3, 261–289. [Google Scholar] [CrossRef]
- Ciardelli, C.; Nova, I.; Tronconi, E.; Chatterjee, D.; Bandl-Konrad, B. A “nitrate route” for the low temperature “fast SCR” reaction over a V2O5–WO3/TiO2 commercial catalyst. Chem. Commun. 2004, 23, 2718–2719. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Shinjoh, H. A comparative study of “standard”, “fast” and “NO2” SCR reactions over Fe/zeolite catalyst. Appl. Catal. A Gen. 2010, 390, 71–77. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Grossale, A.; Nova, I.; Tronconi, E.; Chatterjee, D.; Weibel, M. The chemistry of the NO/NO2–NH3 “fast” SCR reaction over Fe-ZSM5 investigated by transient reaction analysis. J. Catal. 2008, 256, 312–322. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Fino, D.; Russo, N. Catalysis in diesel engine NOx after treatment: A review. Catal. Struct. React. 2015, 1, 155–173. [Google Scholar] [CrossRef]
- Tonkyn, R.G.; Barlowa, S.E.; Hoard, J.W. Reduction of NOx in synthetic diesel exhaust via two-step plasma-catalysis treatment. Appl. Catal. B Environ. 2003, 40, 207–217. [Google Scholar] [CrossRef]
- Pan, H.; Guo, Y.; Jian, Y.; He, C. Synergistic effect of non-thermal plasma on NOx Reduction by CH4 over an In/H-BEA catalyst at low temperatures. Energy Fuels 2015, 29, 5282–5289. [Google Scholar] [CrossRef]
- Chen, H.L.; Lee, H.M.; Chen, S.H.; Chang, M.B.; Yu, S.J.; Li, S.N. Removal of volatile organic compounds by single-stage and two-stage plasma catalysis systems: A review of the performance enhancement mechanisms, current status, and suitable applications. Environ. Sci. Technol. 2009, 43, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Trinh, Q.H.; Mok, Y.S. Environmental plasma-catalysis for the energy-efficient treatment of volatile organic compounds. Korean J. Chem. Eng. 2016, 33, 735–748. [Google Scholar] [CrossRef]
- Whitehead, J.C. Plasma–catalysis: The known knowns, the known unknowns and the unknown unknowns. J. Phys. D Appl. Phys. 2016, 49, 243001. [Google Scholar] [CrossRef]
- Stere, C.E.; Adress, W.; Burch, R.; Chansai, S.; Goguet, A.; Graham, W.G.; De Rosa, F.; Palma, V.; Hardacre, C. Ambient temperature hydrocarbon selective catalytic reduction of NOx using atmospheric pressure non-thermal plasma activation of a Ag/Al2O3 catalyst. ACS Catal. 2014, 4, 666–673. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, T.; Wang, H.; Xiao, L.; Chen, M.; Jiang, X.; Zheng, X. Cold plasma-assisted selective catalytic reduction of NO over B2O3/γ-Al2O3. Chin. J. Catal. 2012, 33, 783–789. [Google Scholar] [CrossRef]
- Jiang, N.; Shang, K.-F.; Lu, N.; Li, H.; Li, J.; Wu, Y. High-efficiency removal of NOx from flue gas by multitooth wheel-cylinder corona discharge plasma facilitated selective catalytic reduction process. IEEE Trans. Plasma Sci. 2016, 44, 2738–2744. [Google Scholar] [CrossRef]
- Guan, B.; Lin, H.; Cheng, Q.; Huang, Z. Removal of NOx with selective catalytic reduction based on nonthermal plasma preoxidation. Ind. Eng. Chem. Res. 2011, 50, 5401–5413. [Google Scholar] [CrossRef]
- Talebizadeh, P.; Babaie, M.; Brown, R.; Rahimzadeh, H.; Ristovski, Z.; Arai, M. The role of non-thermal plasma technique in NOx treatment: A review. Renew. Sustain. Energy Rev. 2014, 40, 886–901. [Google Scholar] [CrossRef]
- Wang, J.; He, T.; Li, C. Majorization of working parameters for non-thermal plasma reactor and impact on no oxidation of diesel engine. Int. J. Automot. Technol. 2017, 18, 229–233. [Google Scholar] [CrossRef]
- Pan, H.; Qiang, Y. Promotion of non-thermal plasma on catalytic reduction of NOx by C3H8 over Co/BEA catalyst at low temperature. Plasma Chem. Plasma Process. 2014, 34, 811–824. [Google Scholar] [CrossRef]
- Cho, B.K.; Lee, J.-H.; Crellin, C.C.; Olson, K.L.; Hilden, D.L.; Kim, M.K.; Kim, P.S.; Heo, I.; Oh, S.H.; Nam, I.-S. Selective catalytic reduction of NOx by diesel fuel: Plasma-assisted HC/SCR system. Catal. Today 2012, 191, 20–24. [Google Scholar] [CrossRef]
- Miessner, H.; Francke, K.; Rudolph, R. Plasma-enhanced HC-SCR of NOx in the presence of excess oxygen. Appl. Catal. B Environ. 2002, 36, 53–62. [Google Scholar] [CrossRef]
- Mok, Y.S.; Nam, I.-S. Reduction of nitrogen oxides by ozonization-catalysis hybrid process. Korean J. Chem. Eng. 2004, 21, 976–982. [Google Scholar] [CrossRef]
- Jogi, I.; Stamate, E.; Irimiea, C.; Schmidt, M.; Brandenburg, R.; Hołub, M.; Bonisławski, M.; Jakubowski, T.; Kaariainen, M.-L.; Cameron, D.C. Comparison of direct and indirect plasma oxidation of NO combined with oxidation by catalyst. Fuel 2015, 144, 137–144. [Google Scholar] [CrossRef]
- Jogi, I.; Levoll, E.; Raud, J. Plasma oxidation of NO in O2:N2 mixtures: The importance of back-reaction. Chem. Eng. J. 2016, 301, 149–157. [Google Scholar] [CrossRef]
- Bao, X.Y.; Malik, M.A.; Norton, D.G.; Neculaes, V.B.; Schoenbach, K.H.; Heller, R.; Siclovan, O.P.; Corah, S.E.; Caiafa, A.; Inzinna, L.P.; et al. Shielded sliding discharge-assisted hydrocarbon selective catalytic reduction of NOx over Ag/Al2O3 catalysts using diesel as a reductant. Plasma Chem. Plasma Process. 2014, 34, 825–836. [Google Scholar] [CrossRef]
- Mhadeshwar, A.B.; Winkler, B.H.; Eiteneer, B.; Hancu, D. Microkinetic modeling for hydrocarbon (HC)-based selective catalytic reduction (SCR) of NOx on a silver-based catalyst. Appl. Catal. B Environ. 2009, 89, 229–238. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.; Chen, L. Selective catalytic reduction of NO with methane. J. Nat. Gas Chem. 2003, 12, 264–270. [Google Scholar]
- Yeom, Y.H.; Li, M.; Sachtler, W.M.H.; Weitz, E. A study of the mechanism for NOx reduction with ethanol on γ-alumina supported silver. J. Catal. 2006, 238, 100–110. [Google Scholar] [CrossRef]
- Furusawa, T.; Seshan, K.; Lercher, J.A.; Lefferts, L.; Aika, K. Selective reduction of NO to N2 in the presence of oxygen over supported silver catalysts. Appl. Catal. B Environ. 2002, 37, 205–216. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Zhang, X.; Yu, Y.; Zhang, C. Precipitable silver compound catalysts for the selective catalytic reduction of NOx by ethanol. Appl. Catal. A Gen. 2010, 375, 258–264. [Google Scholar] [CrossRef]
- Ihm, T.H.; Jo, J.O.; Hyun, Y.J.; Mok, Y.S. Removal of nitrogen oxides using hydrocarbon selective catalytic reduction coupled with plasma. Appl. Chem. Eng. 2016, 27, 92–100. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.J.; Kang, H.-C.; Jo, J.O.; Mok, Y.S. Consideration of the Role of Plasma in a Plasma-Coupled Selective Catalytic Reduction of Nitrogen Oxides with a Hydrocarbon Reducing Agent. Catalysts 2017, 7, 325. https://doi.org/10.3390/catal7110325
Lee BJ, Kang H-C, Jo JO, Mok YS. Consideration of the Role of Plasma in a Plasma-Coupled Selective Catalytic Reduction of Nitrogen Oxides with a Hydrocarbon Reducing Agent. Catalysts. 2017; 7(11):325. https://doi.org/10.3390/catal7110325
Chicago/Turabian StyleLee, Byeong Ju, Ho-Chul Kang, Jin Oh Jo, and Young Sun Mok. 2017. "Consideration of the Role of Plasma in a Plasma-Coupled Selective Catalytic Reduction of Nitrogen Oxides with a Hydrocarbon Reducing Agent" Catalysts 7, no. 11: 325. https://doi.org/10.3390/catal7110325
APA StyleLee, B. J., Kang, H.-C., Jo, J. O., & Mok, Y. S. (2017). Consideration of the Role of Plasma in a Plasma-Coupled Selective Catalytic Reduction of Nitrogen Oxides with a Hydrocarbon Reducing Agent. Catalysts, 7(11), 325. https://doi.org/10.3390/catal7110325