Core-Shell Structured Ni@SiO2 Catalysts Exhibiting Excellent Catalytic Performance for Syngas Methanation Reactions
Abstract
:1. Introduction
2. Results and Discussion
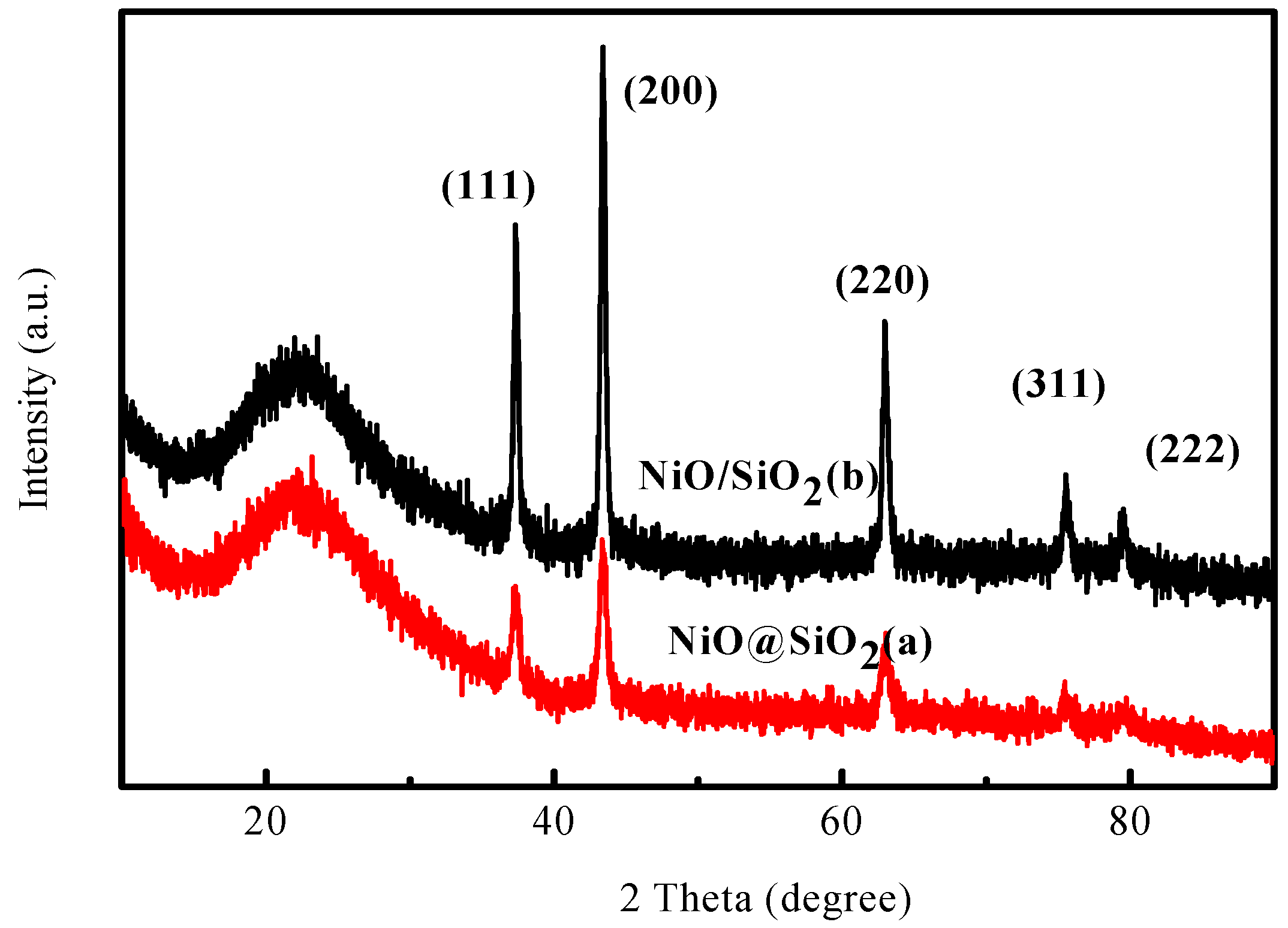
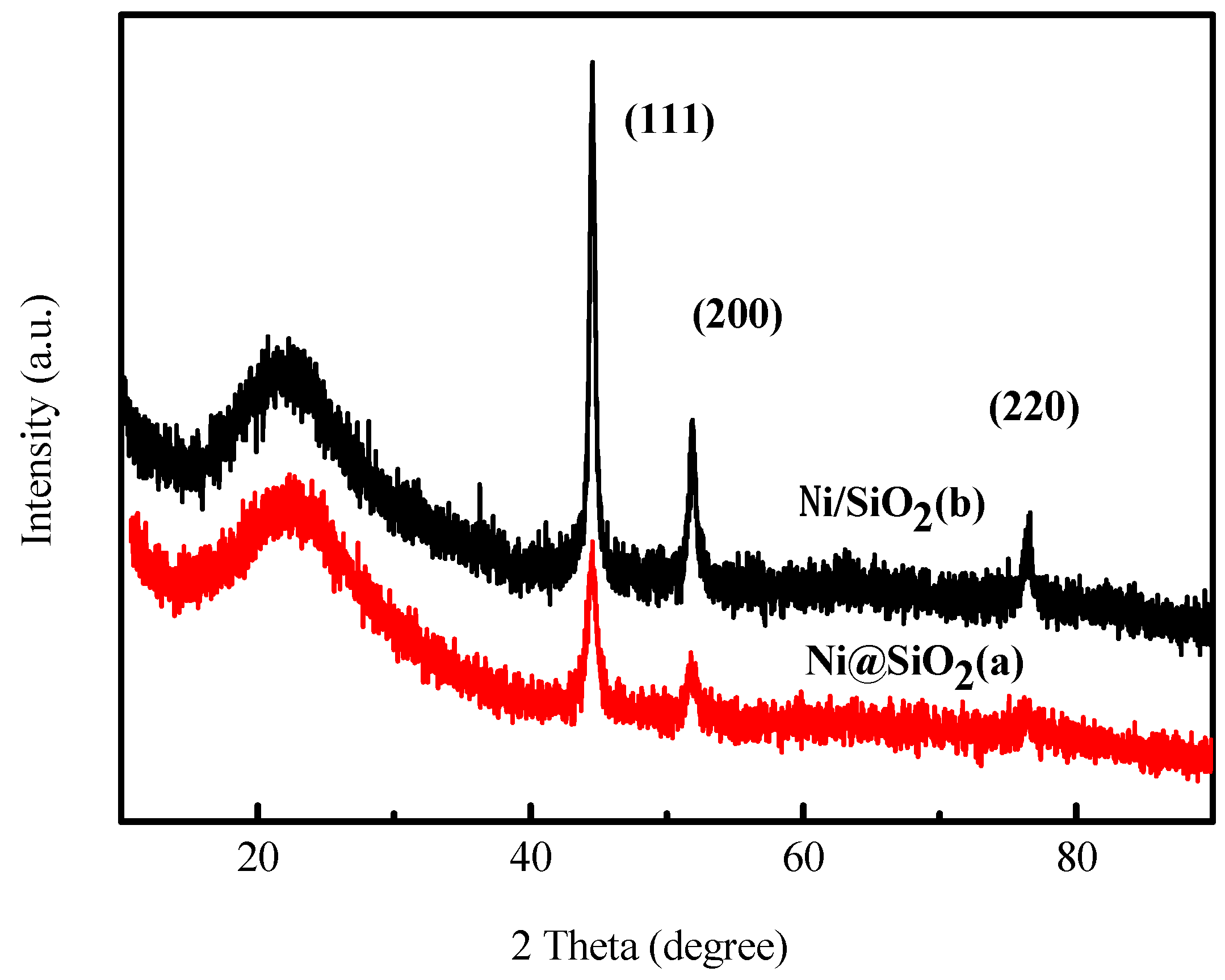
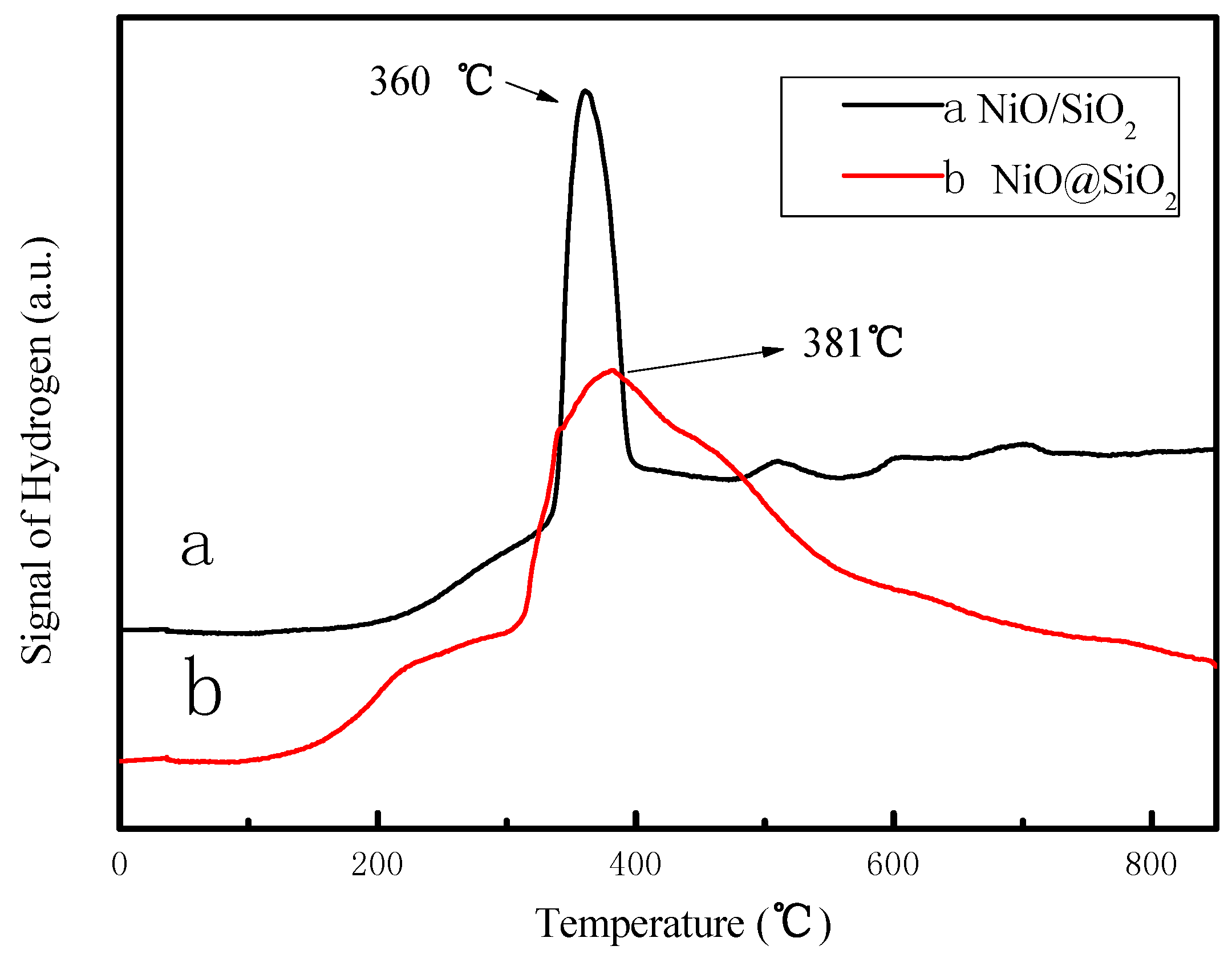
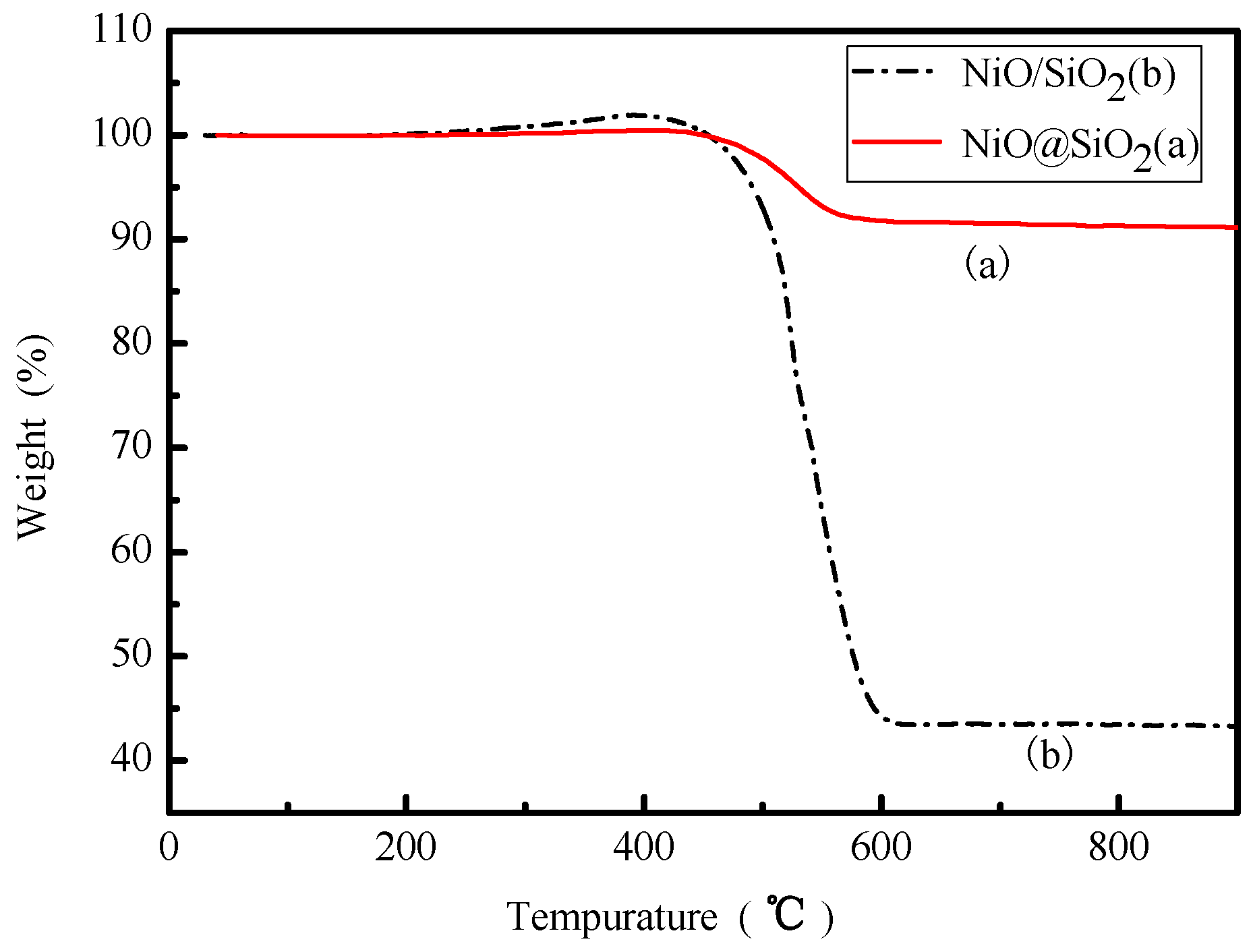
2.1. Characterization of the Catalysts
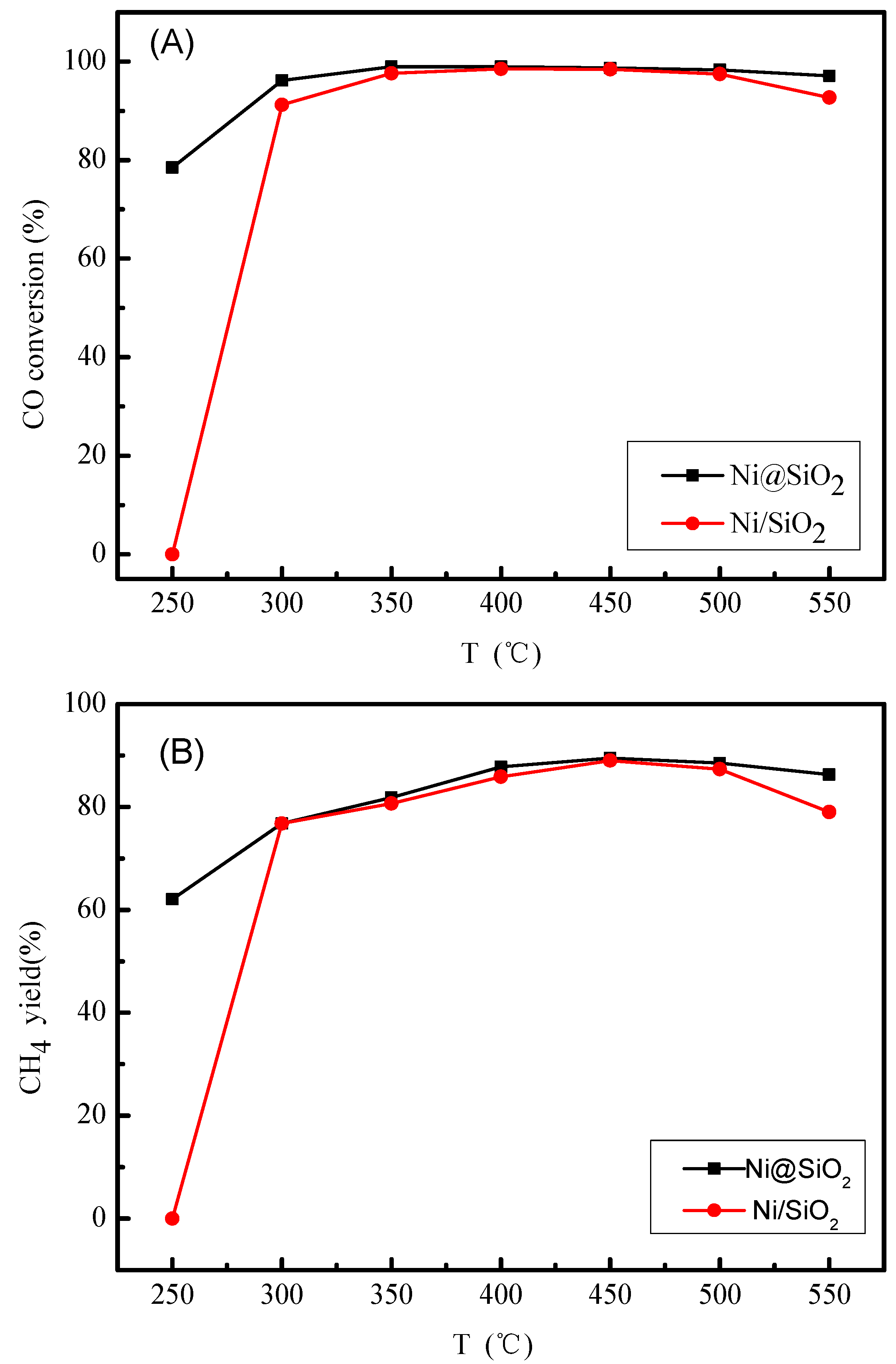
2.2. Catalytic Performances of the Catalysts
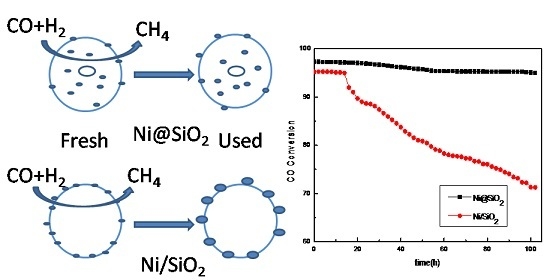
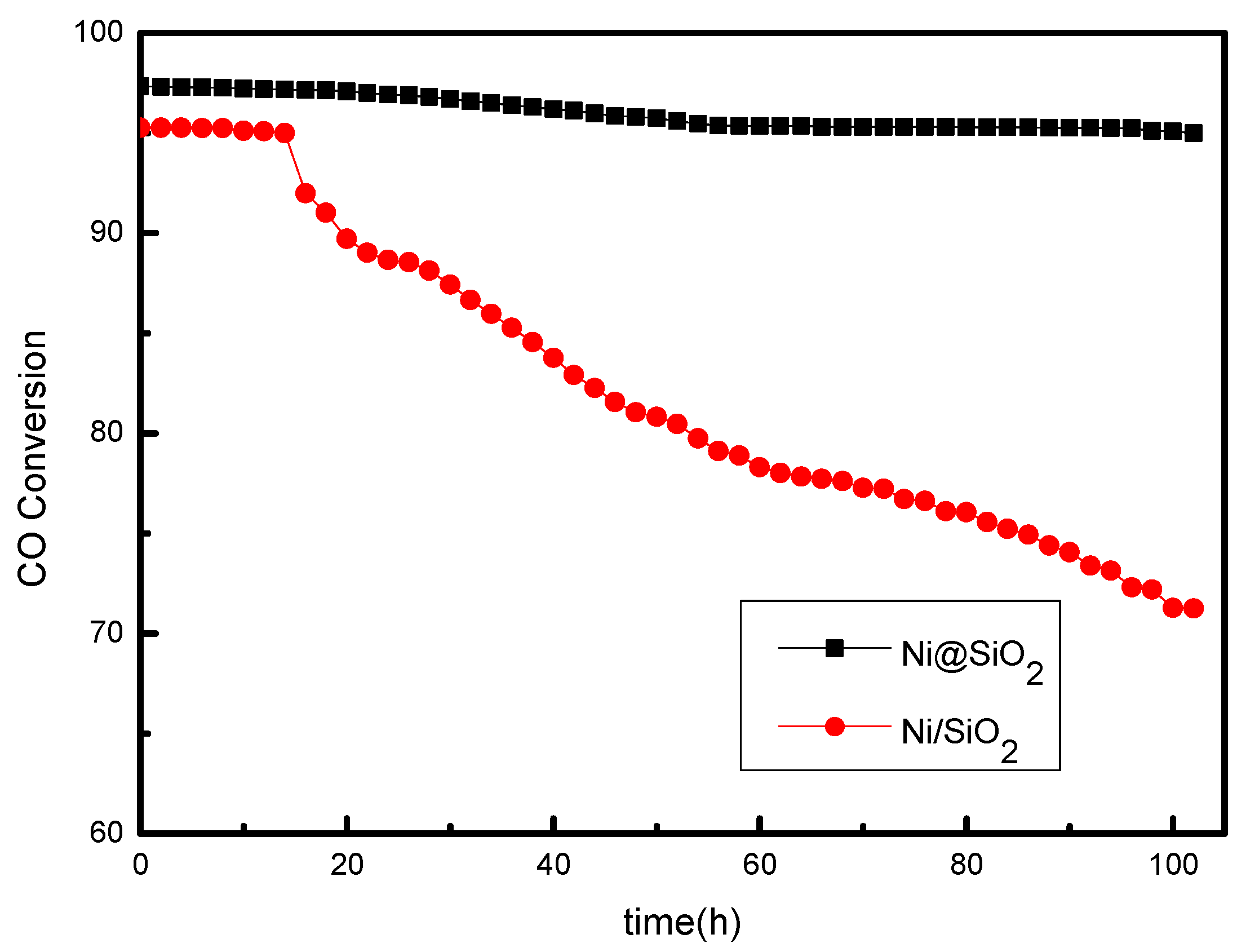
2.3. Stability of the Catalysts
2.4. Characterization of the Used Catalysts
3. Experimental
3.1. Catalyst Preparation
3.1.1. Preparation of Ni@SiO2 Catalyst
3.1.2. Preparation of Ni/SiO2 Catalyst
3.2. Catalyst Characterization
3.3. Evaluation of the Catalyst Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dai, B.; Wen, B.; Zhu, M.Y.; Kang, L.H.; Yu, F. Nickel catalysts supported on aminofunctionalized MCM-41 for syngas methanation. RSC Adv. 2016, 6, 66957–66962. [Google Scholar] [CrossRef]
- Delgado, K.H.; Maier, L.; Tischer, S.; Zellner, A.; Stotz, H.; Deutschmann, O. Surface reaction kinetics of steam-and CO2-reforming as well as oxidation of methane over nickel-based catalysts. Catalysts 2015, 5, 871–904. [Google Scholar] [CrossRef]
- Tao, M.; Meng, X.; Lv, Y.H.; Bian, Z.C.; Xin, Z. Effect of impregnation solvent on Ni dispersion and catalytic properties of Ni/SBA-15 for CO methanation reaction. Fuel 2016, 165, 289–297. [Google Scholar] [CrossRef]
- Jin, G.J.; Gu, F.N.; Liu, Q.; Wang, X.Y.; Jia, L.H.; Xu, G.W.; Zhong, Z.Y.; Su, F.B. Highly stable Ni/SiC catalyst modified by Al2O3 for CO methanation reaction. RSC Adv. 2016, 6, 9631–9639. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xin, Z.; Meng, X.; Tao, M. Synthesis, characterization and properties of anti-sintering nickel incorporated MCM-41 methanation catalysts. Fuel 2013, 109, 693–701. [Google Scholar] [CrossRef]
- Bian, L.; Wang, W.H.; Xia, R.; Li, Z.H. Ni-based catalyst derived from Ni/Al hydrotalcitelike compounds by the urea hydrolysis method for CO methanation. RSC Adv. 2016, 6, 677–686. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.Y.; Gu, F.N.; Wang, X.Y.; Lu, X.P.; Li, H.F.; Xu, G.W.; Su, F.B. CO methanation on ordered mesoporous Ni–Cr–Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, F.H.; Ji, K.M.; Song, Y.; Li, Z. Slurry phase methanation of carbon monoxide over nanosized Ni–Al2O3 catalysts prepared by microwave-assisted solution combustion. Appl. Catal. A 2016, 510, 74–83. [Google Scholar] [CrossRef]
- Li, S.; Jin, H.G.; Gao, L.; Zhang, X.S. Exergy analysis and the energy saving mechanism for coal to synthetic/substitute natural gas and power cogeneration system without and with CO2 capture. Appl. Energy 2014, 130, 552–561. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Li, Y.K.; Zhang, Q.F.; Chai, R.J.; Zhao, G.F.; Cao, F.H.; Liu, Y.; Lu, Y. Metal-foam-structured Ni–Al2O3 catalysts: Wet chemical etchingpreparation and syngas methanation performance. Appl. Catal. A 2016, 510, 216–226. [Google Scholar] [CrossRef]
- Lucchini, M.A.; Testino, A.; Kambolis, A.; Proff, C.; Ludwig, C. Sintering and coking resistant core-shell microporous silica-nickel nanoparticles for CO methanation: Towards advanced catalysts production. Appl. Catal. B 2016, 182, 94–101. [Google Scholar] [CrossRef]
- Yan, X.L.; Liu, Y.; Zhao, B.R.; Wang, Z.; Wang, Y.; Liu, C.J. Methanation over Ni/SiO2: Effect of the catalyst preparation methodologies. Appl. Catal. B 2013, 38, 2283–2291. [Google Scholar] [CrossRef]
- Miyao, T.; Tanaka, J.; Shen, W.; Hayashi, K.; Higashiyama, K.; Watanabe, M. Catalytic activity and durability of a mesoporous silica-coated Ni-alumina-based catalyst for selective CO methanation. Catal. Today 2015, 251, 81–87. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, P.; Zhang, Z.C.; Zhou, Y.; Zhang, J.K.; Zhang, H.; Shi, Z.J.; Han, R.P.S.; Huang, F.Q.; Ma, D. Nickel catalyst stabilization via graphene encapsulation for enhanced methanation reaction. J. Catal. 2016, 334, 42–51. [Google Scholar] [CrossRef]
- Takenaka, S.; Umebayashi, H.; Tanabe, E.; Matsune, H.; Kishida, M. Specific performance of silica-coated Ni catalysts for the partial oxidation of methane to synthesis gas. J. Catal. 2007, 245, 392–400. [Google Scholar] [CrossRef]
- Li, L.; He, S.C.; Song, Y.Y.; Zhao, J.; Ji, W.J.; Au, C.T. Fine-tunable Ni@porous silica core–shell nanocatalysts: Synthesis, characterization, and catalytic properties in partial oxidation of methane to syngas. J. Catal. 2012, 288, 54–64. [Google Scholar] [CrossRef]
- Takenaka, S.; Orita, Y.; Umebayashi, H.; Matsune, H.; Kishida, M. High resistance to carbon deposition of silica-coated Ni catalysts in propane stream reforming. Appl. Catal. A 2008, 351, 189–194. [Google Scholar] [CrossRef]
- Takenaka, S.; Shimizu, T.; Otsuka, K. Complete removal of carbon monoxide in hydrogen-rich gas stream through methanation over supported metal catalysts. Int. J. Hydrog. Energy 2004, 29, 1065–1073. [Google Scholar] [CrossRef]
- Li, S.; Tuel, A.; Laprune, D.; Meunier, F.; Farrusseng, D. Transition-metal nanoparticles in hollow zeolite single crystals as bifunctional and size-selective hydrogenation catalysts. Chem. Mater. 2015, 27, 276–282. [Google Scholar] [CrossRef]
- Park, J.C.; Bang, J.U.; Lee, J.; Ko, C.H.; Song, H. Ni@SiO2 yolk-shell nanoreactor catalysts: High temperature stability and recyclability. J. Mater. Chem. 2010, 20, 1217–1388. [Google Scholar]
- Wang, Z.Y.; Wu, R.F.; Zhao, Y.X. Effect of ZrO2 promoter on structure and catalytic activity of the Ni/SiO2 catalyst for CO methanation in hydrogen-rich gases. Catal. Today 2010, 158, 470–474. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Kim, M.S.; Park, E.D. A highly loaded Ni@SiO2 core–shell catalyst for CO methanation. Appl. Catal. A 2016, 513, 98–105. [Google Scholar] [CrossRef]
- Gao, J.J.; Wang, Y.L.; Ping, Y.; Hu, D.C.; Xu, G.W.; Gu, F.N.; Su, F.B. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Miyao, T.; Sakurabayashi, S.; Shen, W.; Higashiyama, K.; Watanabe, M. Preparation and catalytic activity of a mesoporous silica-coated Ni-alumina-based catalyst for selective CO methanation. Catal. Commun. 2015, 58, 93–96. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. CO methanation toward the production of synthetic natural gas over highly active Ni/TiO2 catalyst. AIChE J. 2014, 60, 1027–1035. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xin, Z.; Meng, X.; Lv, Y.H.; Tao, M. Effect of MoO3 on structures and properties of Ni-SiO2 methanation catalysts prepared by the hydrothermal synthesis method. Ind. Eng. Chem. Res. 2013, 52, 14533–14544. [Google Scholar] [CrossRef]
- Li, L.; Yao, Y.; Sun, B.; Fei, Z.Y.; Xia, H.; Zhao, J.; Ji, W.J.; Au, C.T. Highly active and stable lanthanum-doped core–shell structured Ni@SiO2 catalysts for the partial oxidation of methane to syngas. ChemCatChem 2013, 5, 3781–3787. [Google Scholar] [CrossRef]
- Guan, B.Y.; Wang, X.; Xiao, Y.; Liu, Y.L.; Huo, Q.S. A versatile cooperative template-directed coating method to construct uniform microporous carbon shells for multifunctional core–shell nanocomposites. Nanoscale 2013, 5, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Liu, C.J. Characterization of silica supported nickel catalyst for methanation with improved activity by room temperature plasma treatment. Catal. Lett. 2009, 133, 112–118. [Google Scholar] [CrossRef]


| Samples | BET Surface Area (m2/g) a | Pore Volume (cm3/g) b | Average Pore Size | Metal Crystallite |
|---|---|---|---|---|
| (nm) c | (nm) d | |||
| NiO@SiO2 | 263 | 0.79 | 12.1 | 8.6 |
| NiO/SiO2 | 162 | 0.14 | 3.5 | 19.8 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Wen, B.; Zhu, M. Core-Shell Structured Ni@SiO2 Catalysts Exhibiting Excellent Catalytic Performance for Syngas Methanation Reactions. Catalysts 2017, 7, 21. https://doi.org/10.3390/catal7010021
Han Y, Wen B, Zhu M. Core-Shell Structured Ni@SiO2 Catalysts Exhibiting Excellent Catalytic Performance for Syngas Methanation Reactions. Catalysts. 2017; 7(1):21. https://doi.org/10.3390/catal7010021
Chicago/Turabian StyleHan, Yang, Bo Wen, and Mingyuan Zhu. 2017. "Core-Shell Structured Ni@SiO2 Catalysts Exhibiting Excellent Catalytic Performance for Syngas Methanation Reactions" Catalysts 7, no. 1: 21. https://doi.org/10.3390/catal7010021
APA StyleHan, Y., Wen, B., & Zhu, M. (2017). Core-Shell Structured Ni@SiO2 Catalysts Exhibiting Excellent Catalytic Performance for Syngas Methanation Reactions. Catalysts, 7(1), 21. https://doi.org/10.3390/catal7010021