Oxygen Reduction Electrocatalysts Based on Coupled Iron Nitride Nanoparticles with Nitrogen-Doped Carbon
Abstract
:1. Introduction
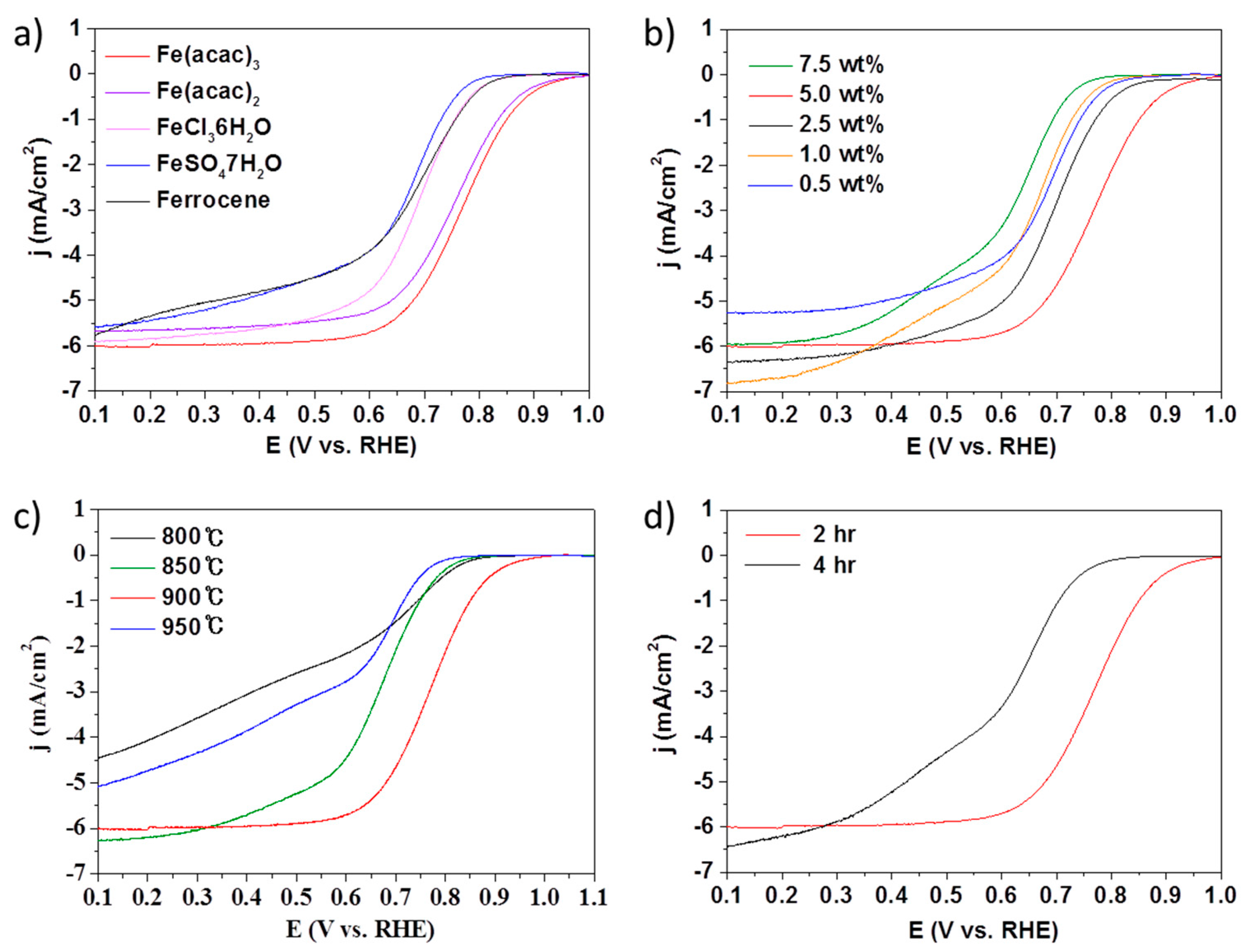
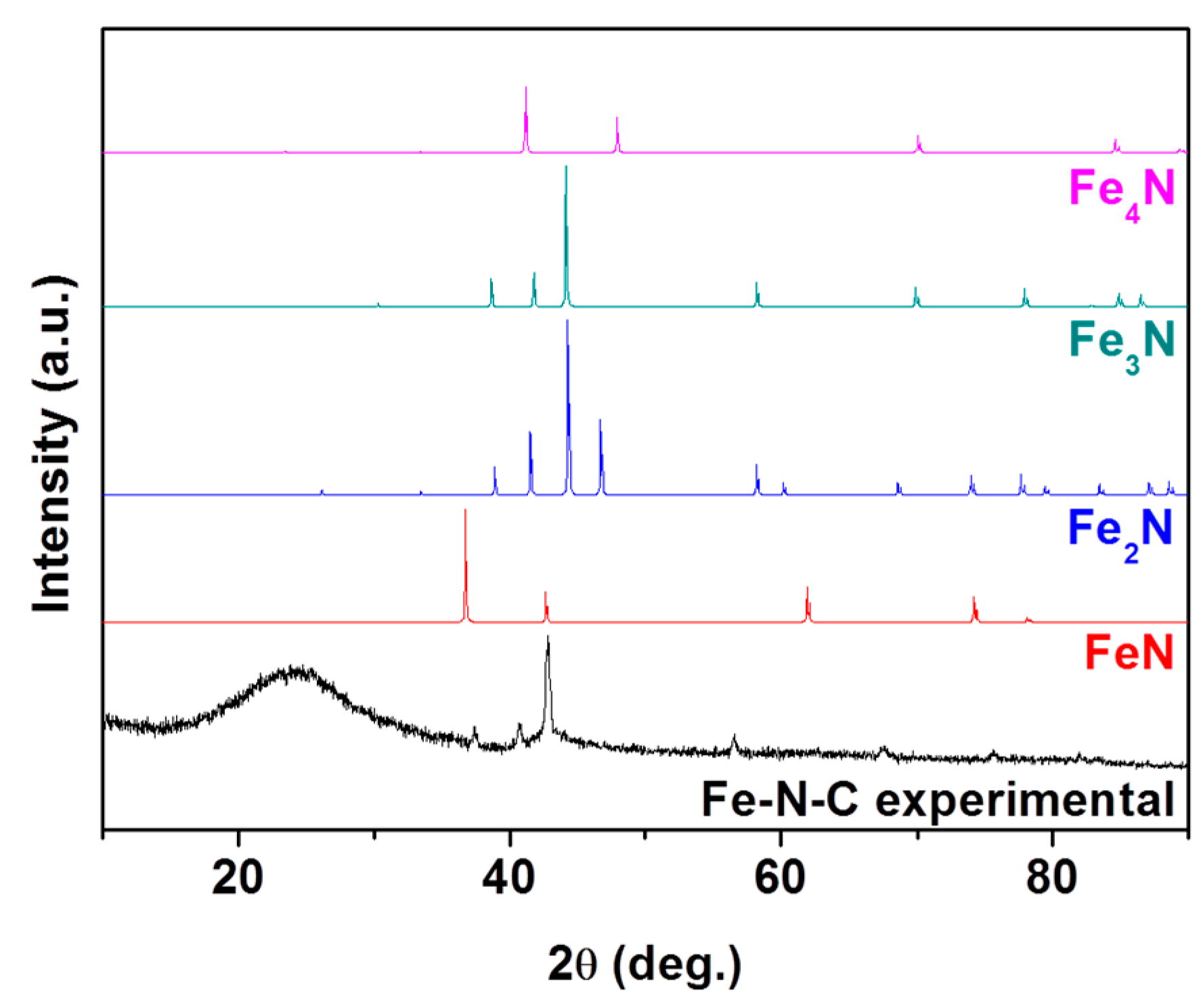
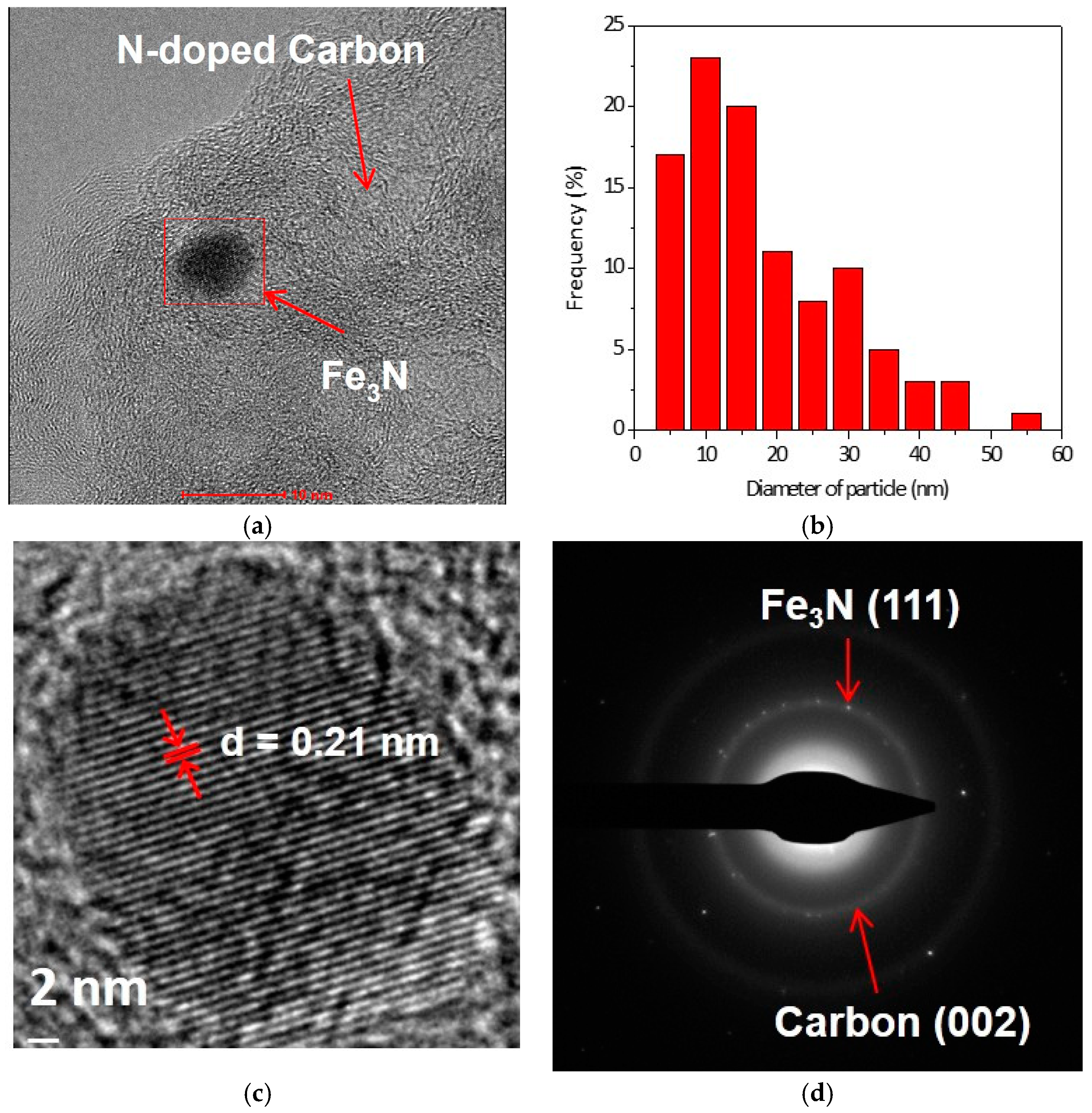
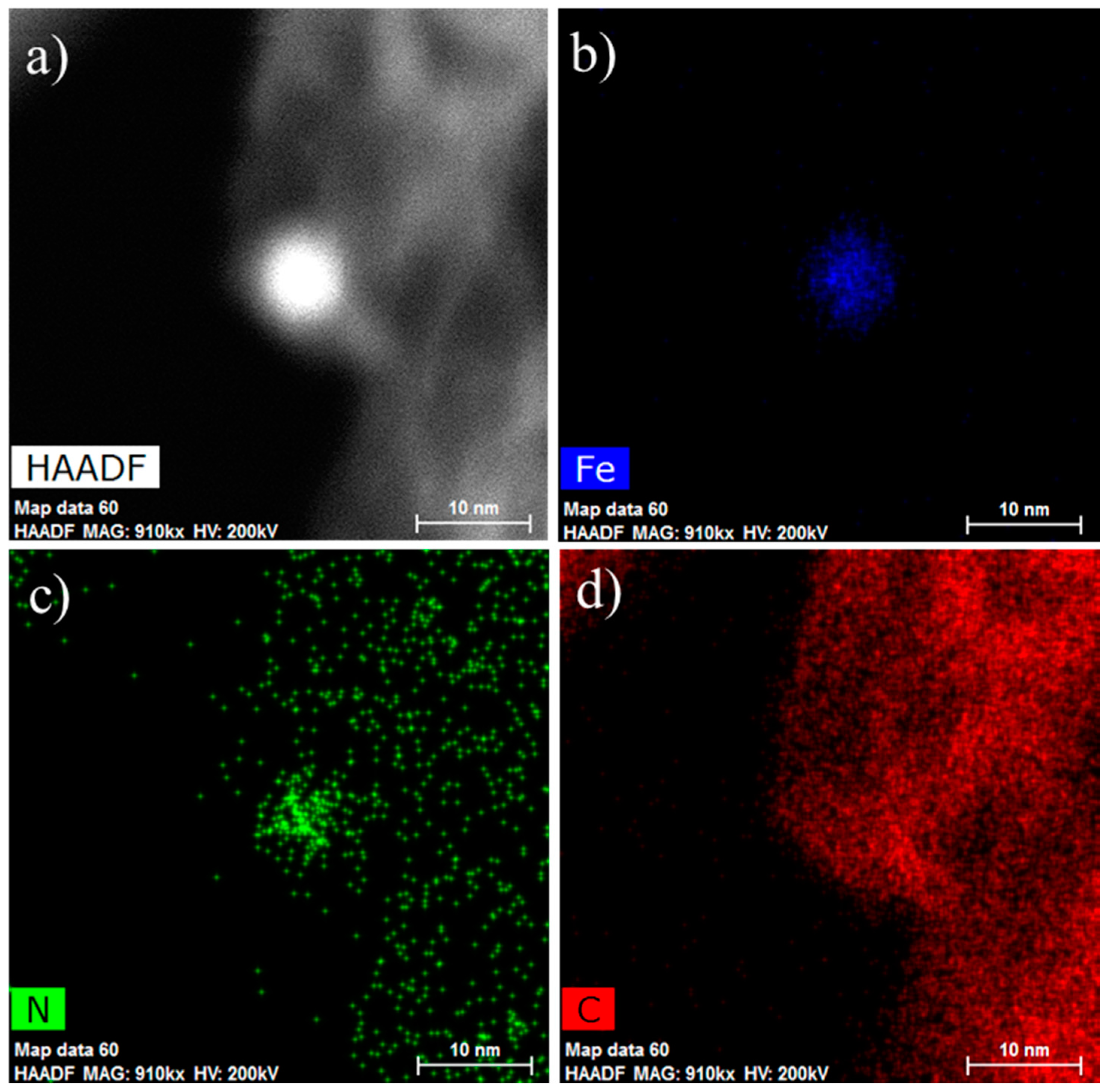
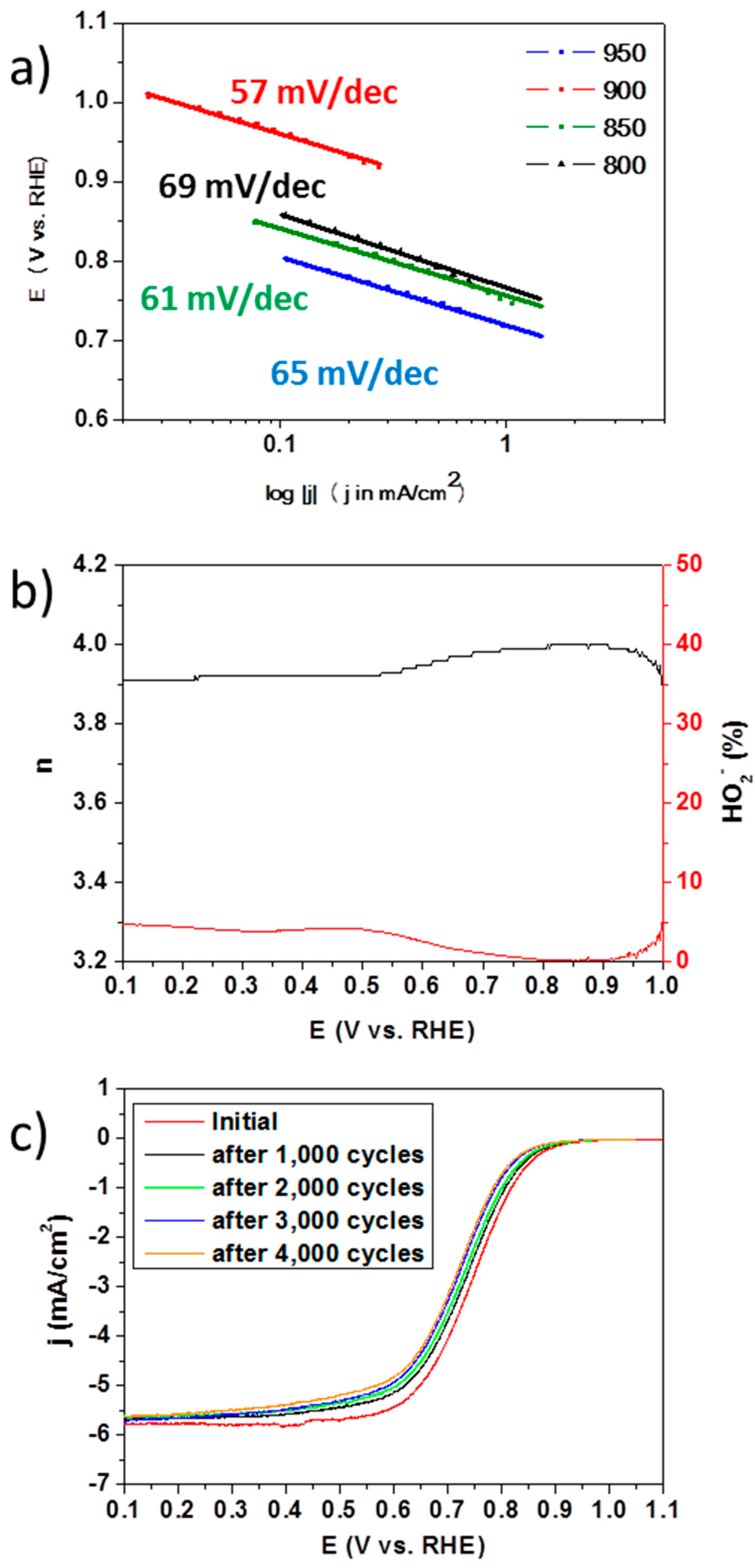
2. Results and Discussions
3. Experimental
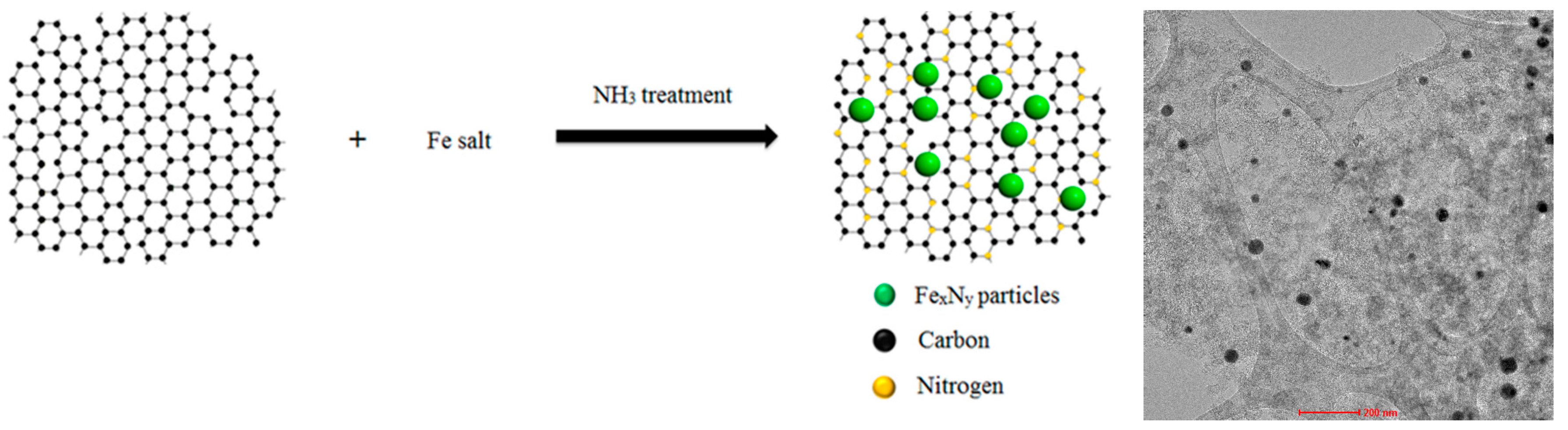
3.1. Synthesis of FexNy/NC Catalyst
3.2. Characterization of Catalysts
3.3. Electrochemical Measurements
4. Conclusion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brian, C.H.S.; Angelika, H. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar]
- Perry, M.L.; Fuller, T.F. A historical perspective of fuel cell technology in the 20th century. J. Electrochem. Soc. 2002, 149, S59–S67. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Jaouen, F.; Lefevre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Jang-Soo, L.; Gi, S.P.; Sun, T.K.; Meilin, L.; Jaephil, C. A highly efficient electrocatalyst for the oxygen reduction reaction; n-doped ketjenblack incorporated into Fe/Fe3C-functionalized melamine foam. Angew. Chem. Int. Ed. 2013, 52, 1026–1030. [Google Scholar]
- Wen, Z.; Ci, S.; Zhang, F.; Feng, X.; Cui, S.; Mao, S.; Luo, S.; He, Z.; Chen, J. Nitrogen-enriched core-shell structured Fe/Fe3C-C nanorods as advanced electrocatalysts for oxygen reduction reaction. Adv. Mater. 2012, 24, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhu, J.; Feng, L.; Liu, C.; Xing, W. Meso/macroporous nitrogen-doped carbon architectures with iron carbide encapsulated in graphitic layers as an efficient and robust catalyst for the oxygen reduction reaction in both acidic and alkaline solutions. Adv. Mater. 2015, 27, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Bong, S.; Woo, S.; Park, S.K.; Ha, J.; Choi, E.; Piao, Y. Facile synthesis of one-dimensional iron-oxide/carbon hybrid nanostructures as electrocatalysts for oxygen reduction reaction in alkaline media. J. Nanosci. Nanotechnolo. 2014, 14, 8852–8857. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Key, J.; Linkov, V.; Ji, S.; Mao, X.; Wang, Q.; Wang, R. Ultrafine iron oxide nanoparticles supported on n-doped carbon black as an oxygen reduction reaction catalyst. Int. J. Hydrogen Energy 2014, 39, 14777–14782. [Google Scholar] [CrossRef]
- Tylus, U.; Jia, Q.; Strickland, K.; Ramaswamy, N.; Serov, A.; Atanassov, P.; Mukerjee, S. Elucidating oxygen reduction active sites in pyrolyzed metal-nitrogen coordinated non-precious-metal electrocatalyst systems. J. Phys. Chem. C 2014, 118, 8999–9008. [Google Scholar] [CrossRef] [PubMed]
- Palaniselvam, T.; Kannan, R.; Kurungot, S. Facile construction of non-precious iron nitride-doped carbon nanofibers as cathode electrocatalysts for proton exchange membrane fuel cells. Chem. Commun. 2011, 47, 2910–2912. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, C.; Liu, F.; Hou, Y. Hybrid of iron nitride and nitrogen-doped graphene aerogel as synergistic catalyst for oxygen reduction reaction. Adv. Funct. Mater. 2014, 24, 2930–2937. [Google Scholar] [CrossRef]
- Zhang, J.; He, D.; Su, H.; Chen, X.; Pan, M.; Mu, S. Porous polyaniline-derived FeNxC/C catalysts with high activity and stability towards oxygen reduction reaction using ferric chloride both as an oxidant and iron source. J. Mater. Chem. A 2014, 2, 1242–1246. [Google Scholar] [CrossRef]
- Michel, L.; Eric, P.; Frédéric, J.; Jean-Pol, D. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Leonard, N.; Barton, S.C. Impact of transition metal on nitrogen retention and activity of iron-nitrogen-carbon oxygen reduction catalysts. Phys. Chem. Chem. Phys. 2014, 16, 4576–4585. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.F.; Tu, M.H.; Tsai, C.W.; Chen, C.J.; Liu, R.S.; Liu, W.R.; Lo, M.Y. Influence of pyrolysis temperature on oxygen reduction reaction activity of carbon-incorporating iron nitride/nitrogen-doped graphene nanosheets catalyst. Int. J. Hydrogen Energy 2013, 38, 3956–3962. [Google Scholar] [CrossRef]
- Peng, H.; Mo, Z.; Liao, S.; Liang, H.; Yang, L.; Luo, F.; Song, H.; Zhong, Y.; Zhang, B. High performance Fe- and n-doped carbon catalyst with graphene structure for oxygen reduction. Sci. Rep. 2013. [Google Scholar] [CrossRef]
- Schnepp, Z.; Thomas, M.; Glatzel, S.; Schlichte, K.; Palkovits, R.; Giordano, C. One pot route to sponge-like Fe3N nanostructures. J. Mater. Chem. 2011, 21, 17760–17764. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The materials project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Kattel, S.; Atanassov, P.; Kiefer, B. A density functional theory study of oxygen reduction reaction on non-PGM Fe-Nx-C electrocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 13800–13806. [Google Scholar] [CrossRef] [PubMed]
- Kramm, U.I.; Herranz, J.; Larouche, N.; Arruda, T.M.; Lefevre, M.; Jaouen, F.; Bogdanoff, P.; Fiechter, S.; Abs-Wurmbach, I.; Mukerjee, S.; et al. Structure of the catalytic sites in Fe/N/C-catalysts for O2-reduction in PEM fuel cells. Phys. Chem. Chem. Phys. 2012, 14, 11673–11688. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, C.E.; Lefevre, M.; Kramm, U.I.; Dodelet, J.P.; Vidal, F. A density functional theory study of catalytic sites for oxygen reduction in Fe/N/C catalysts used in H2/O2 fuel cells. Phys. Chem. Chem. Phys. 2014, 16, 13654–13661. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Batchelor-McAuley, C.; Schuhmann, W.; Compton, R.G. Koutecky-levich analysis applied to nanoparticle modified rotating disk electrodes: Electrocatalysis or misinterpretation. Nano. Res. 2013, 7, 71–78. [Google Scholar] [CrossRef]
- Ward, K.R.; Gara, M.; Lawrence, N.S.; Hartshorne, R.S.; Compton, R.G. Nanoparticle modified electrodes can show an apparent increase in electrode kinetics due solely to altered surface geometry: The effective electrochemical rate constant for non-flat and non-uniform electrode surfaces. J. Electroanal. Chem. 2013, 695, 1–9. [Google Scholar] [CrossRef]
- Gara, M.; Ward, K.R.; Compton, R.G. Nanomaterial modified electrodes: Evaluating oxygen reduction catalysts. Nanoscale 2013, 5, 7304–7311. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.R.; Compton, R.G. Quantifying the apparent ‘catalytic’ effect of porous electrode surfaces. J. Electroanal. Chem. 2014, 724, 43–47. [Google Scholar] [CrossRef]
 denotes metallic Fe (JCPDS # 87-0722),
denotes metallic Fe (JCPDS # 87-0722),  denotes FeN (JCPDS # 50-1087),
denotes FeN (JCPDS # 50-1087),  denotes Fe3N (JCPDS # 86-0232), and
denotes Fe3N (JCPDS # 86-0232), and  denotes Fe4N (JCPDS # 86-0231).
denotes Fe4N (JCPDS # 86-0231).
 denotes metallic Fe (JCPDS # 87-0722),
denotes metallic Fe (JCPDS # 87-0722),  denotes FeN (JCPDS # 50-1087),
denotes FeN (JCPDS # 50-1087),  denotes Fe3N (JCPDS # 86-0232), and
denotes Fe3N (JCPDS # 86-0232), and  denotes Fe4N (JCPDS # 86-0231).
denotes Fe4N (JCPDS # 86-0231).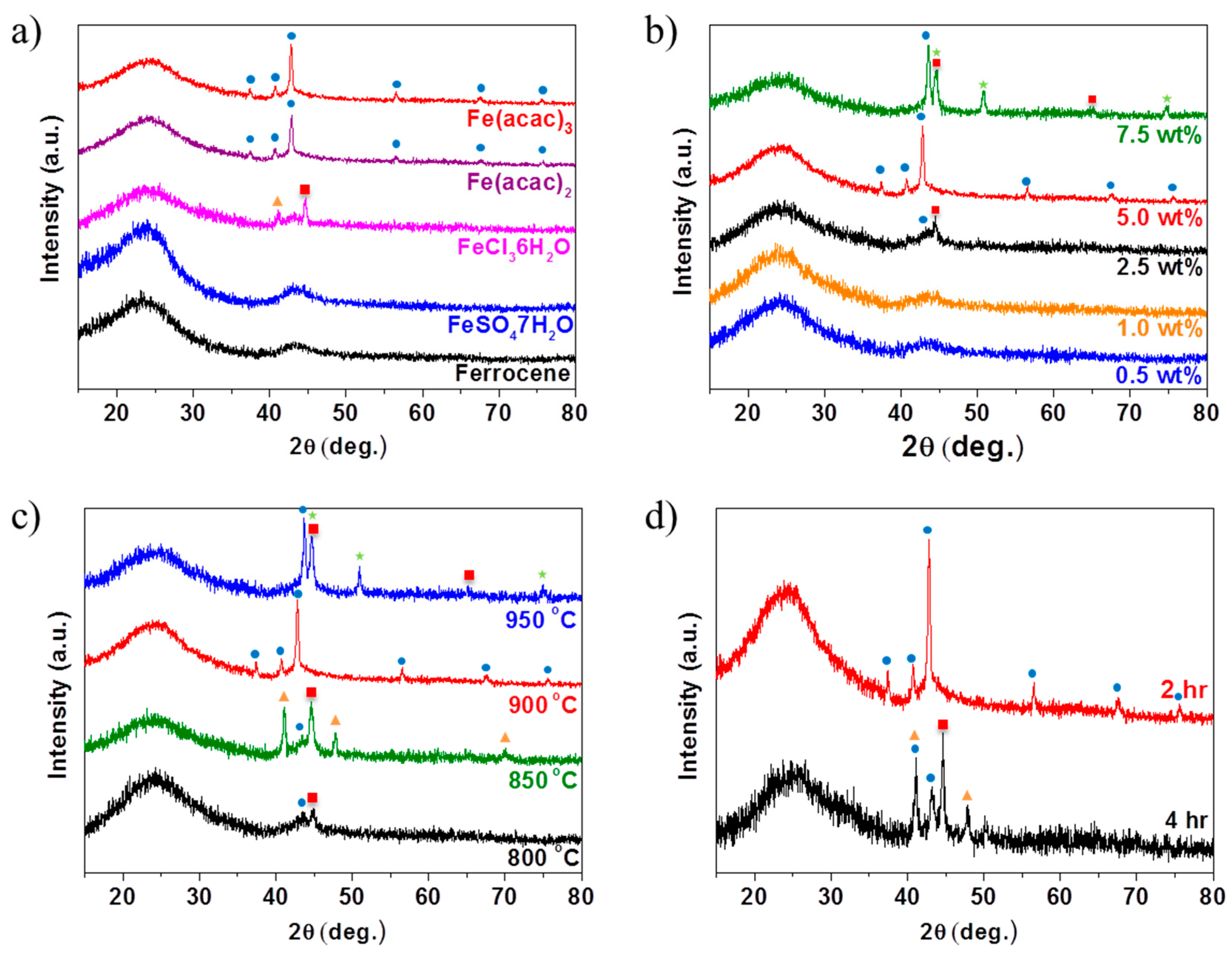
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.J.; Lee, J.H.; Hembram, K.P.S.S.; Lee, K.-R.; Han, S.S.; Yoon, C.W.; Nam, S.-W.; Kim, J.Y. Oxygen Reduction Electrocatalysts Based on Coupled Iron Nitride Nanoparticles with Nitrogen-Doped Carbon. Catalysts 2016, 6, 86. https://doi.org/10.3390/catal6060086
Park MJ, Lee JH, Hembram KPSS, Lee K-R, Han SS, Yoon CW, Nam S-W, Kim JY. Oxygen Reduction Electrocatalysts Based on Coupled Iron Nitride Nanoparticles with Nitrogen-Doped Carbon. Catalysts. 2016; 6(6):86. https://doi.org/10.3390/catal6060086
Chicago/Turabian StylePark, Min Jung, Jin Hee Lee, K. P. S. S. Hembram, Kwang-Ryeol Lee, Sang Soo Han, Chang Won Yoon, Suk-Woo Nam, and Jin Young Kim. 2016. "Oxygen Reduction Electrocatalysts Based on Coupled Iron Nitride Nanoparticles with Nitrogen-Doped Carbon" Catalysts 6, no. 6: 86. https://doi.org/10.3390/catal6060086
APA StylePark, M. J., Lee, J. H., Hembram, K. P. S. S., Lee, K.-R., Han, S. S., Yoon, C. W., Nam, S.-W., & Kim, J. Y. (2016). Oxygen Reduction Electrocatalysts Based on Coupled Iron Nitride Nanoparticles with Nitrogen-Doped Carbon. Catalysts, 6(6), 86. https://doi.org/10.3390/catal6060086




