Dolomite-Derived Ni-Based Catalysts with Fe Modification for Hydrogen Production via Auto-Thermal Reforming of Acetic Acid
Abstract
:1. Introduction
2. Results
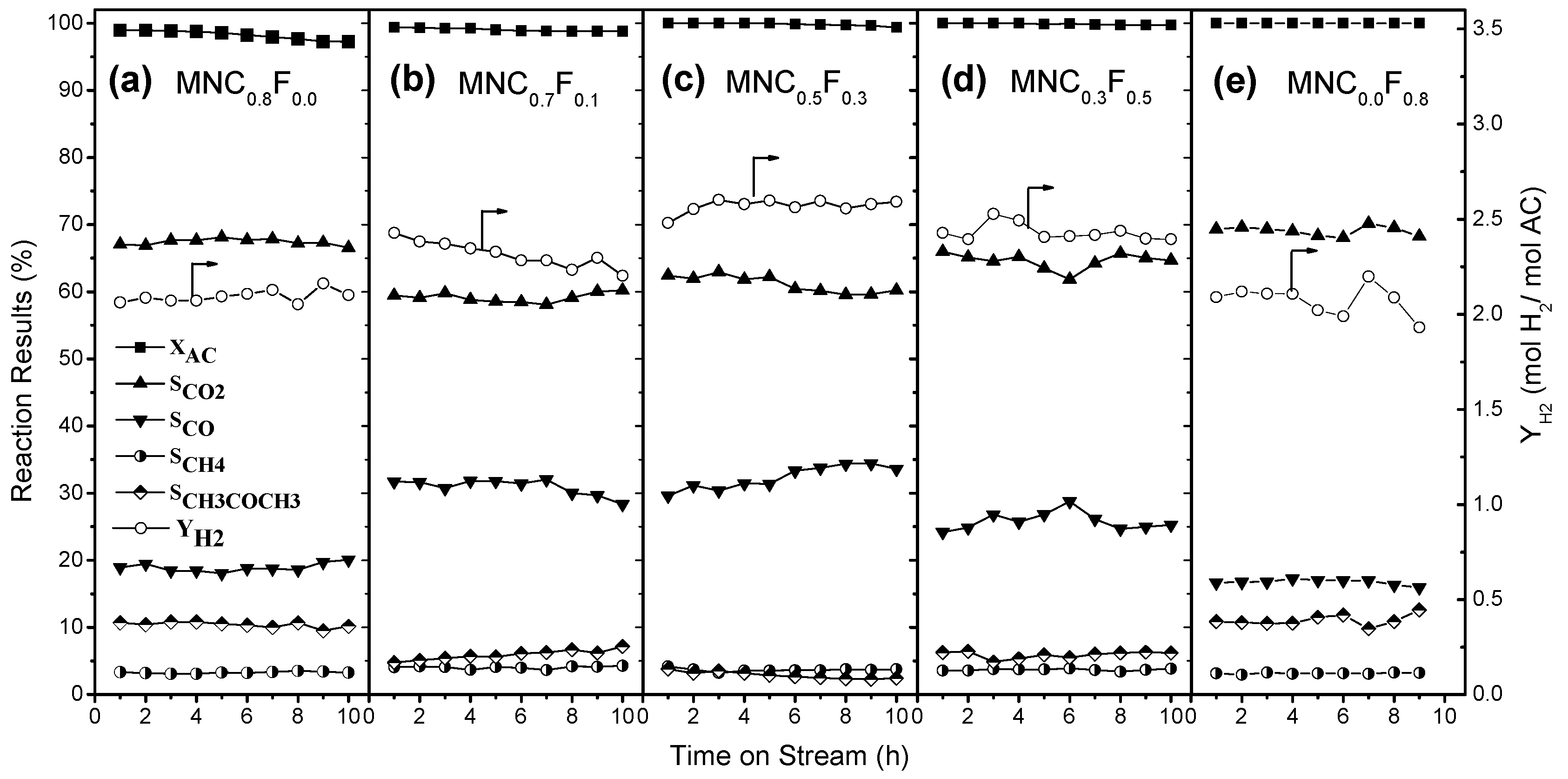
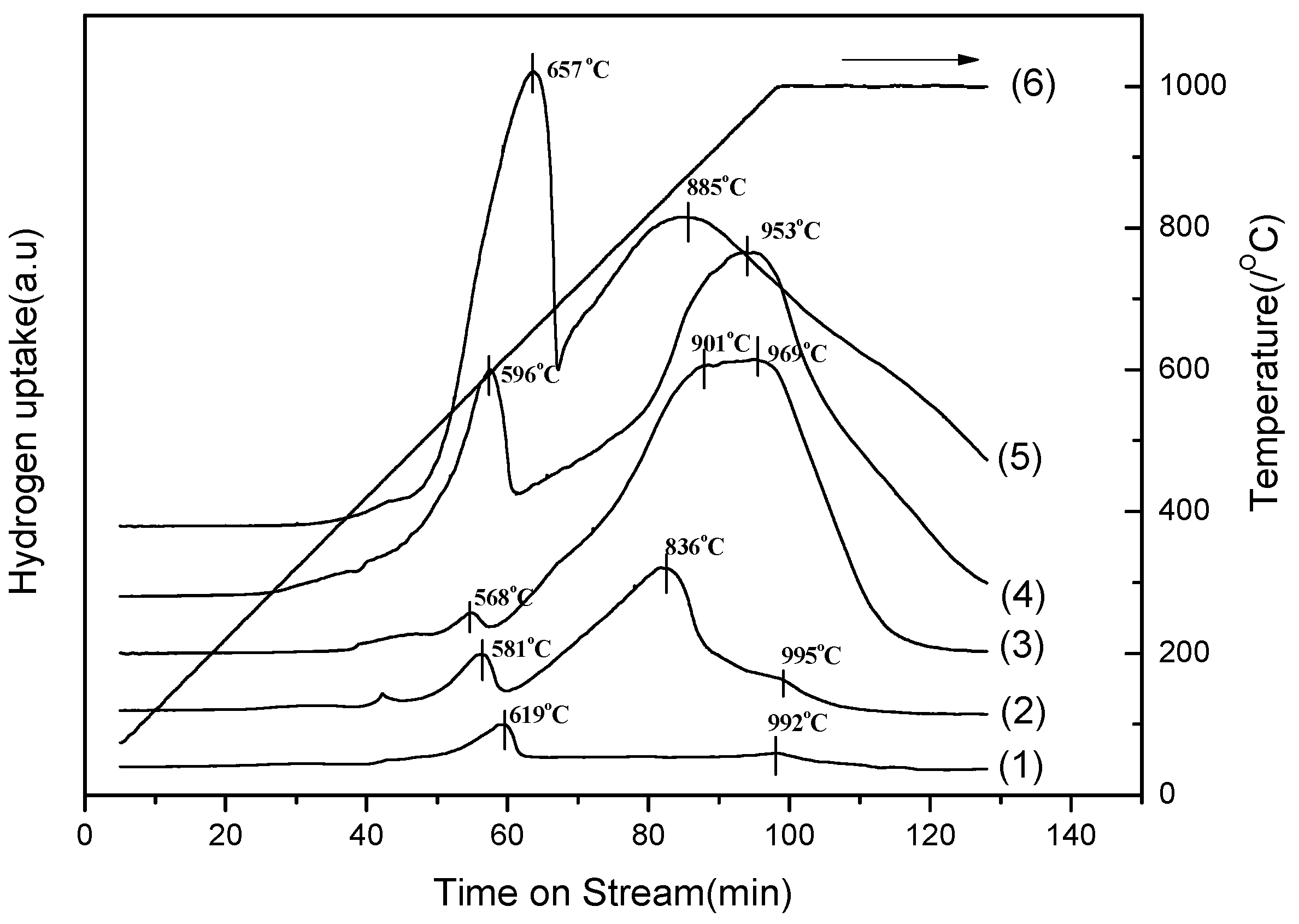
2.1. Auto-Thermal Reforming of Acetic Acid
2.2. Characterizations
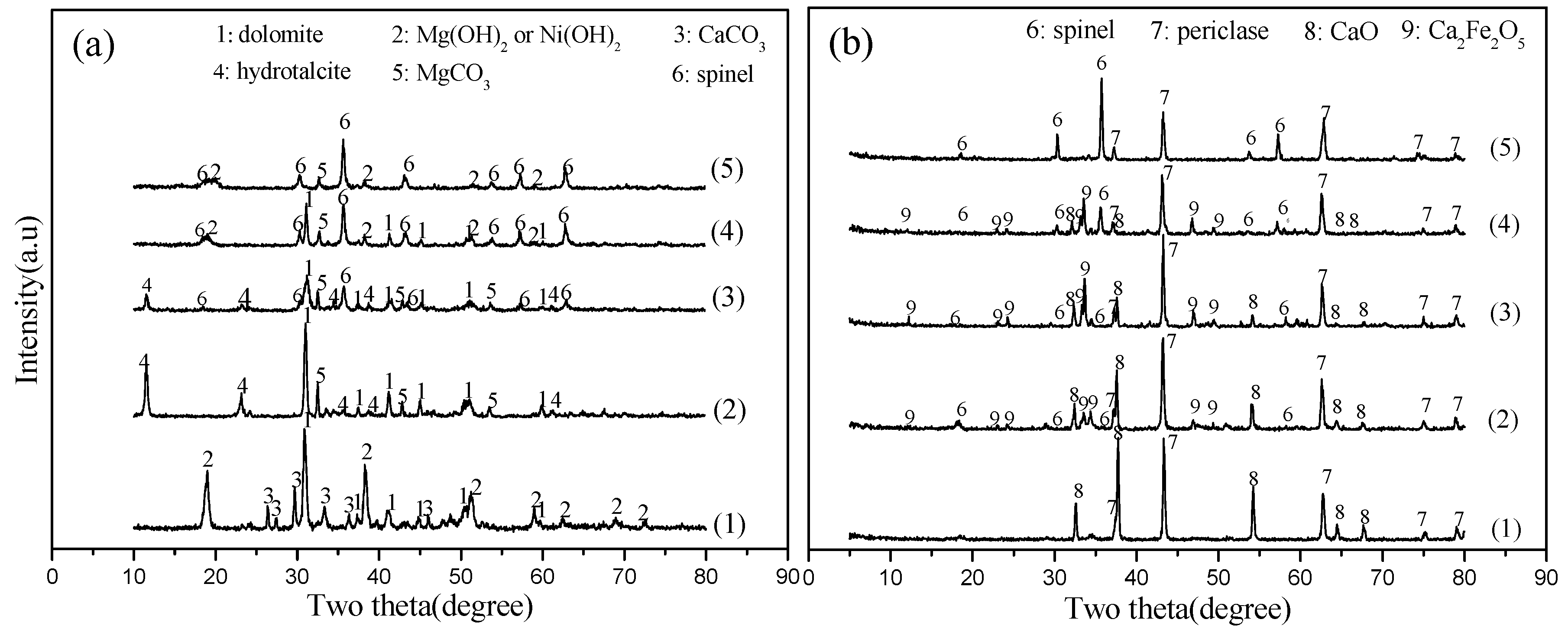
2.2.1. Precursors of Catalysts
2.2.2. Oxides of Catalysts
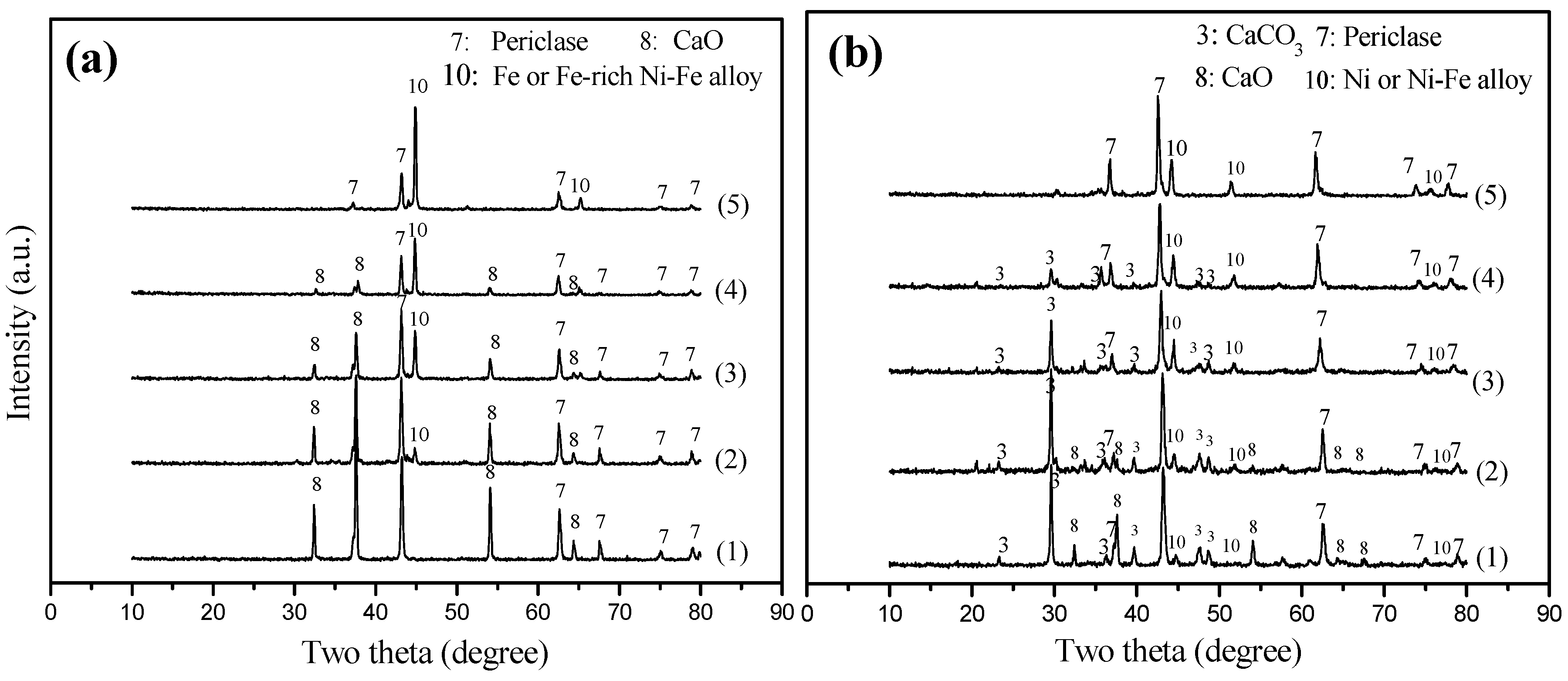
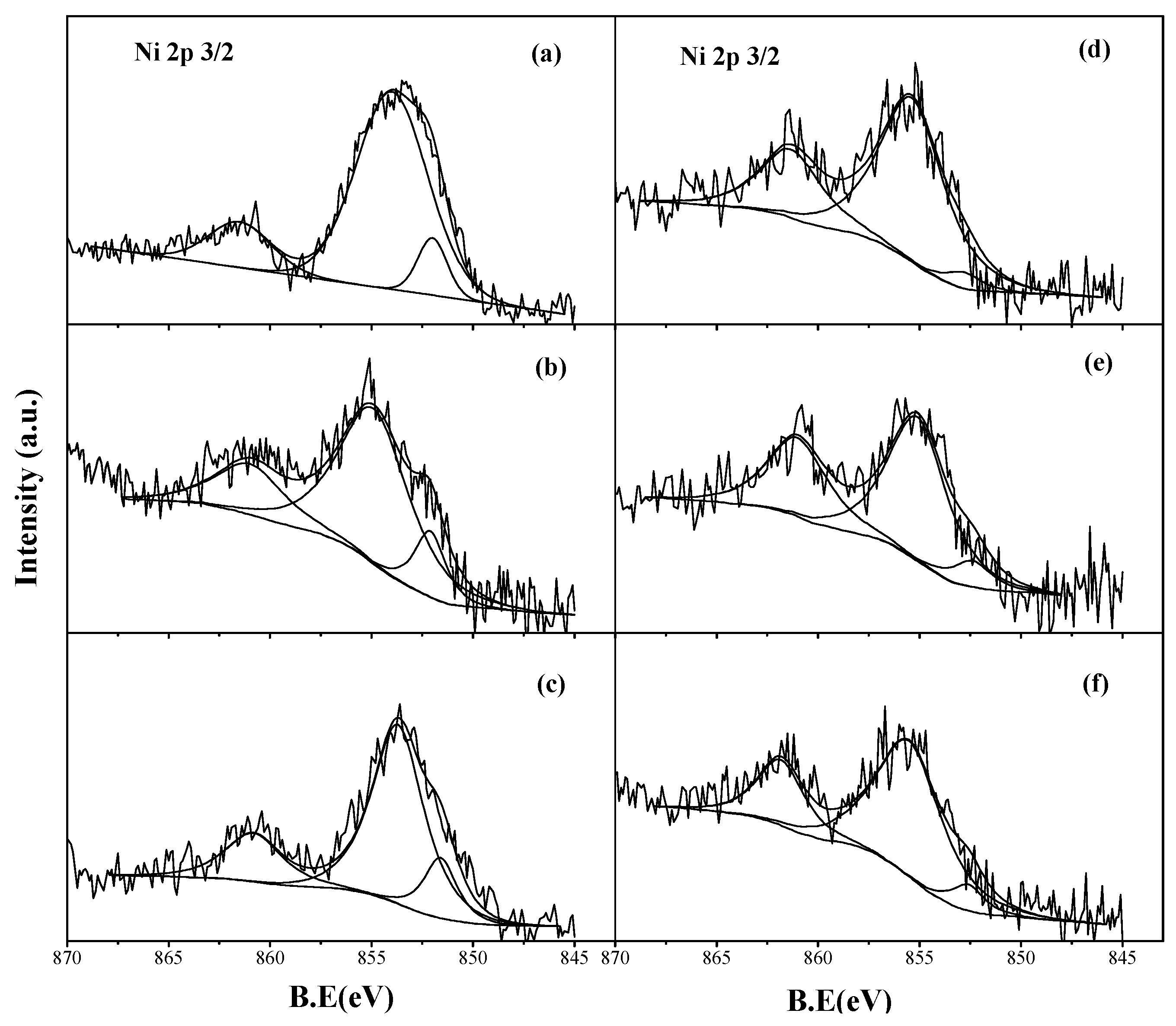
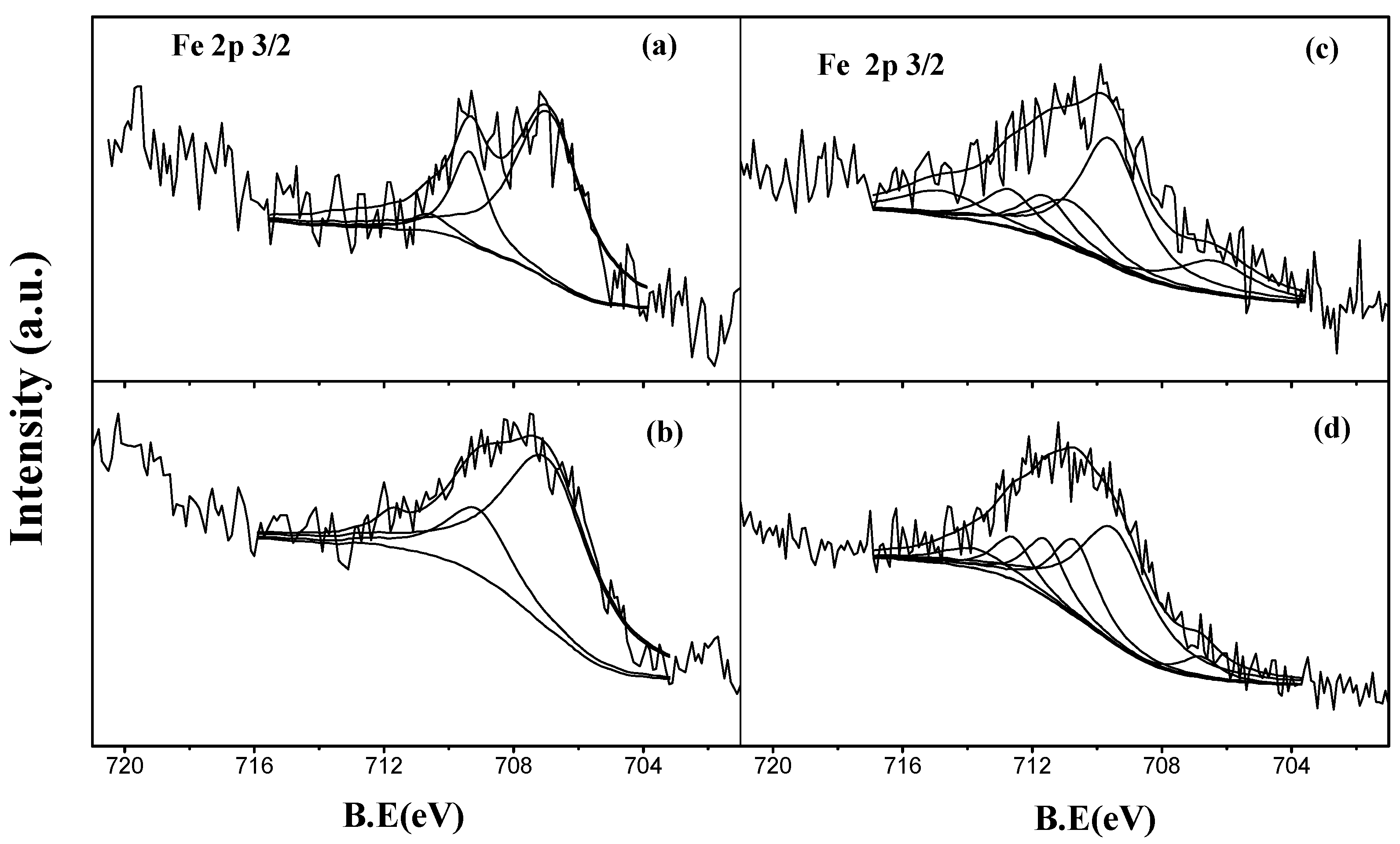
2.2.3. Reduced Catalysts
2.2.4. Spent Catalysts
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalytic Performance Test
4.3. Catalyst Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrog. Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Ayalur Chattanathan, S.; Adhikari, S.; Abdoulmoumine, N. A review on current status of hydrogen production from bio-oil. Renew. Sustain. Energy Rev. 2012, 16, 2366–2372. [Google Scholar] [CrossRef]
- Chen, T.; Wu, C.; Liu, R. Steam reforming of bio-oil from rice husks fast pyrolysis for hydrogen production. Bioresour. Technol. 2011, 102, 9236–9240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, X.; Zhang, L.; Liu, S.; Lu, G. Steam reforming of acetic acid over Ni/ZrO2 catalysts: Effects of nickel loading and particle size on product distribution and coke formation. Appl. Catal. A-Gen. 2012, 417–418, 281–289. [Google Scholar] [CrossRef]
- Mohanty, P.; Patel, M.; Pant, K.K. Hydrogen production from steam reforming of acetic acid over Cu–Zn supported calcium aluminate. Bioresour. Technol. 2012, 123, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G. Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts 2016, 6. [Google Scholar] [CrossRef]
- Feng, M.S.; Liu, J.D.; Zhang, F.B.; Huang, L.H. Ni-based olivine-type catalysts and their application in hydrogen production via auto-thermal reforming of acetic acid. Chem. Pap. 2015, 69, 1166–1175. [Google Scholar] [CrossRef]
- Seyedeyn Azad, F.; Abedi, J.; Salehi, E.; Harding, T. Production of hydrogen via steam reforming of bio-oil over Ni-based catalysts: Effect of support. Chem. Eng. J. 2012, 180, 145–150. [Google Scholar] [CrossRef]
- Jang, W.-J.; Jeong, D.-W.; Shim, J.-O.; Kim, H.-M.; Han, W.-B.; Bae, J.W.; Roh, H.-S. Metal oxide (MgO, CaO, and La2O3) promoted Ni-Ce0.8Zr0.2O2 catalysts for H2 and CO production from two major greenhouse gases. Renew. Energy 2015, 79, 91–95. [Google Scholar] [CrossRef]
- Nichele, V.; Signoretto, M.; Pinna, F.; Menegazzo, F.; Rossetti, I.; Cruciani, G.; Cerrato, G.; Di Michele, A. Ni/ZrO2 catalysts in ethanol steam reforming: Inhibition of coke formation by CaO-doping. Appl. Catal. B Environ. 2014, 150–151, 12–20. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effects of support modifiers on the catalytic performance of Ni/Al2O3 catalyst in CO2 reforming of methane. Fuel 2014, 129, 197–203. [Google Scholar] [CrossRef]
- Huang, L.H.; Liu, Q.; Chen, R.R.; Hsu, A.T. Hydrogen production via auto-thermal reforming of bio-ethanol: The role of iron in layered double hydroxide-derived Ni0.35Mg2.65AlO4.5 +/δ catalysts. Appl. Catal. A Gen. 2011, 393, 302–308. [Google Scholar] [CrossRef]
- Pighini, C.; Belin, T.; Mijoin, J.; Magnoux, P.; Costentin, G.; Lauron-Pernot, H. Microcalorimetric and thermodynamic studies of CO2 and methanol adsorption on magnesium oxide. Appl. Surf. Sci. 2011, 257, 6952–6962. [Google Scholar] [CrossRef]
- Djaidja, A.; Messaoudi, H.; Kaddeche, D.; Barama, A. Study of Ni–M/MgO and Ni–M–Mg/Al (M=Fe or Cu) catalysts in the CH4–CO2 and CH4–H2O reforming. Int. J. Hydrog. Energy 2015, 40, 4989–4995. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Shang, R.; Duan, Y.; Zhong, X.; Xie, W.; Luo, Y.; Huang, L. Formic Acid Modified Co3O4-CeO2 Catalysts for CO Oxidation. Catalysts 2016, 6. [Google Scholar] [CrossRef]
- Mekhemer, G.A.H.; Halawy, S.A.; Mohamed, M.A.; Zaki, M.I. Ketonization of acetic acid vapour over polycrystalline magnesia: in situ Fourier transform infrared spectroscopy and kinetic studies. J. Catal. 2005, 230, 109–122. [Google Scholar] [CrossRef]
- Guil-Lopez, R.; Navarro, R.M.; Ismail, A.A.; Al-Sayari, S.A.; Fierro, J.L.G. Influence of Ni environment on the reactivity of Ni–Mg–Al catalysts for the acetone steam reforming reaction. Int. J. Hydrog. Energy 2015, 40, 5289–5296. [Google Scholar] [CrossRef]
- Zhang, F.B.; Wang, N.; Yang, L.; Li, M.; Huang, L.H. Ni-Co bimetallic MgO-based catalysts for hydrogen production via steam reforming of acetic acid from bio-oil. Int. J. Hydrog. Energy 2014, 39, 18688–18694. [Google Scholar] [CrossRef]
- Adhlakha, N.; Yadav, K.L. Structural, dielectric, magnetic, and optical properties of Ni0.75Zn0.25Fe2O4-BiFeO3 composites. J. Mater. Sci. 2014, 49, 4423–4438. [Google Scholar] [CrossRef]
- Pintea, S.; Rednic, V.; Mărginean, P.; Aldea, N.; Tiandou, H.; Wu, Z.; Neumann, M.; Matei, F. Crystalline and electronic structure of Ni nanoclusters supported on Al2O3 and Cr2O3 investigated by XRD, XAS and XPS methods. Superlattice Microst. 2009, 46, 130–136. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Reddy, G.K.; Boolchand, P.; Smirniotis, P.G. Sulfur tolerant metal doped Fe/Ce catalysts for high temperature WGS reaction at low steam to CO ratios—XPS and Mössbauer spectroscopic study. J. Catal. 2011, 282, 258–269. [Google Scholar] [CrossRef]
- Brajpuriya, R.; Shripathi, T. Investigation of Fe/Al interface as a function of annealing temperature using XPS. Appl. Surf. Sci. 2009, 255, 6149–6154. [Google Scholar] [CrossRef]
- Lin, X.; Li, R.; Lu, M.; Chen, C.; Li, D.; Zhan, Y.; Jiang, L. Carbon dioxide reforming of methane over Ni catalysts prepared from Ni–Mg–Al layered double hydroxides: Influence of Ni loadings. Fuel 2015, 162, 271–280. [Google Scholar] [CrossRef]
- Ren, J.; Qin, X.; Yang, J.-Z.; Qin, Z.-F.; Guo, H.-L.; Lin, J.-Y.; Li, Z. Methanation of carbon dioxide over Ni–M/ZrO2 (M = Fe, Co, Cu) catalysts: Effect of addition of a second metal. Fuel Process. Technol. 2015, 137, 204–211. [Google Scholar] [CrossRef]

| Catalysts | Nominal Molar Composition | Weight Compositions Analyzed by ICP-AES/% | Surface Area/(m2/g) | Average Pore Size/nm | |||
|---|---|---|---|---|---|---|---|
| MgO | NiO | CaO | Fe2O3 | ||||
| MNC0.8F0.0 | MgNi0.2Ca0.8O2±δ | 39.12 | 15.11 | 45.77 | 0.00 | 4.27 | 7.44 |
| MNC0.7F0.1 | MgNi0.2Ca0.7Fe0.1O2±δ | 38.42 | 14.40 | 39.67 | 7.51 | 6.62 | 6.52 |
| MNC0.5F0.3 | MgNi0.2Ca0.5Fe0.3O2±δ | 37.74 | 13.91 | 26.33 | 22.02 | 8.71 | 6.16 |
| MNC0.3F0.5 | MgNi0.2Ca0.3Fe0.5O2±δ | 34.84 | 13.29 | 16.68 | 35.19 | 11.53 | 5.87 |
| MNC0.0F0.8 | MgNi0.2Fe0.8O2±δ | 33.01 | 12.31 | 0.00 | 54.68 | 4.69 | 5.37 |
| Catalysts | ||||||
|---|---|---|---|---|---|---|
| MNC0.8F0.0 | 98.2 | 67.4 | 18.9 | 3.3 | 10.4 | 2.09 |
| MNC0.7F0.1 | 99.0 | 59.1 | 30.9 | 4.0 | 5.9 | 2.31 |
| MNC0.5F0.3 | 99.9 | 61.1 | 32.3 | 3.7 | 2.9 | 2.59 |
| MNC0.3F0.5 | 99.9 | 64.6 | 25.8 | 3.7 | 5.9 | 2.43 |
| MNC0.0F0.8 | 100.0 | 69.1 | 16.7 | 3.2 | 11.0 | 2.07 |
| Species | MNC0.8F0.0 | MNC0.5F0.3 | MNC0.0F0.8 | |||
|---|---|---|---|---|---|---|
| Reduced | Spent | Reduced | Spent | Reduced | Spent | |
| Ni0/(Ni0v+ Ni2+)/% | 9.0 | 3.7 | 12.5 | 8.8 | 14.1 | 6.7 |
| Fe0/(Fe0 + Fe2+ + Fe3+)/% | N.A. | N.A. | 70.9 | 12.9 | 77.6 | 4.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Xie, W.; Wang, N.; Duan, Y.; Shang, R.; Huang, L. Dolomite-Derived Ni-Based Catalysts with Fe Modification for Hydrogen Production via Auto-Thermal Reforming of Acetic Acid. Catalysts 2016, 6, 85. https://doi.org/10.3390/catal6060085
Zhong X, Xie W, Wang N, Duan Y, Shang R, Huang L. Dolomite-Derived Ni-Based Catalysts with Fe Modification for Hydrogen Production via Auto-Thermal Reforming of Acetic Acid. Catalysts. 2016; 6(6):85. https://doi.org/10.3390/catal6060085
Chicago/Turabian StyleZhong, Xinyan, Wei Xie, Ning Wang, Yiping Duan, Ruishu Shang, and Lihong Huang. 2016. "Dolomite-Derived Ni-Based Catalysts with Fe Modification for Hydrogen Production via Auto-Thermal Reforming of Acetic Acid" Catalysts 6, no. 6: 85. https://doi.org/10.3390/catal6060085
APA StyleZhong, X., Xie, W., Wang, N., Duan, Y., Shang, R., & Huang, L. (2016). Dolomite-Derived Ni-Based Catalysts with Fe Modification for Hydrogen Production via Auto-Thermal Reforming of Acetic Acid. Catalysts, 6(6), 85. https://doi.org/10.3390/catal6060085







