Abstract
The study is devoted to developing a rather high-efficiency NH3-SCR (selective catalytic reduction) catalyst for NOx removal using TiO2-SiO2 support made from blast furnace slag. Through adjusting hydrolytic pH value of TiOSO4 solution obtained from acidolysis of slag with 70 wt. % H2SO4, a series of TiO2-SiO2 mixed oxides was prepared to have different mass ratios of TiO2 to SiO2. The supports are further impregnated with V2O5 and WO3 to make the SCR catalysts for NOx removal. Characterizing the catalysts show that silica and unavoidable impurities in support prepared from slag were responsible for maintaining their mesoporous structure and the enhancements in the acidity and reducible form of active species on the catalyst surface, which thus rendered the catalysts to have higher NOx reduction capability than catalyst using commercial TiO2. Furthermore, the low-cost catalyst prepared from slag-based TiO2 support possesses good stability, and strong resistance to SO2 and H2O poisoning, which are beneficial to practical deNOx applications.
1. Introduction
Nitrogen oxides (NOx) are the key pollutant for overall improvement of ambient air quality and multi-objective environmental management. In Asian countries such as China, the NOx emission is predicted to increase further as a result from its economic development that causes fast growth of coal consumption [1,2,3,4]. Various technologies have been developed to control emissions of NOx (deNOx), mainly including the Selective Non-Catalytic Reduction by NH3 (NH3-SNCR), Selective Catalytic Reduction by NH3 (NH3-SCR) and oxidation absorption with liquid. Among them, NH3-SCR is the most common technology for deep removal of NOx from various kinds of flue gas. In this process, the NOx in flue gas selectively reacts with gaseous ammonia over the catalyst to produce nitrogen and water without generating any secondary pollutants [5,6,7]. Currently, the global demand for SCR systems is increasing rapidly and China has the biggest market. The NH3-SCR catalyst costs 40% of the entire investment and most widespread commercial SCR catalyst is based on V2O5/TiO2 formulations, such as V2O5-WO3/TiO2 and V2O5-MoO3/TiO2 [7,8]. Technical development continues to seek “better” deNOx catalysts that possess high SCR performance, good resistance to SO2 poisoning and low preparation cost [9,10,11,12]. A major way is to upgrade the porous TiO2 support by incorporating with other metal oxides. Commercial titania fail to provide high surface area; however, to be used as a support for deNOx catalyst, titania should be characterized by high surface area and high onset temperature to rutilation. Thus, numerous metal oxides doped with TiO2 have been studied as mechanical promoters including Al2O3, SiO2, Fe2O3, and CeO2 for the improvement of deNOx catalytic activity [12,13,14,15]. Among various mixed oxides, TiO2-SiO2 has drawn special attention owing to its highly active in the SCR reaction and simultaneously, less active in the SO2 oxidation reaction. A commercial V2O5-WO3/TiO2 usually has 0–10 wt. % silica introduced in catalyst molding [10,12]. Many reports in the literature [5,16,17,18] have reported that TiO2-SiO2 support can improve catalytic activity and SO2 durability. Such superior low-temperature SCR activity was mainly attributed to the presence of polymeric vanadate species and high redox properties besides high surface area. The SO2 oxidation activity was found to be significantly suppressed with increasing SiO2 content in the support, which leads to a remarkable improvement of the sulfur tolerance of the catalyst and thus is favorable for industrial applications of SCR process. Raising the SiO2 content beyond 10 wt. % decreased the realized NO conversion, due to the active-component transfer and NH3 adsorption on strong acid sites [17,18,19].
Southwestern China is rich in magnetite bearing vanadium and titanium; its blast furnace slag (BFS) from iron-steel making process contain 20–22 wt. % TiO2 [20]. This titanium-bearing BFS is quite different from ilmenite, which contains 40–50 wt. % TiO2. The latter are currently used to produce TiO2 support for SCR deNOx catalyst through sulfate method [21], where the ilmenite ore is dissolved in concentrated sulfuric acid solution to prepare a titanium sulfate solution (TiOSO4). This solution is further purified and hydrolyzed to produce pure TiO2 [21,22]. In China, only 25% of Ti-bearing BFS is used to make dam concrete or road-paving material. The accumulated Ti-slag has exceeded 70 million tons, and it still increases by a rate of three million tons per year in China [20,23]. This not only wastes its containing Ti resource but also causes serious environmental pollution [23]. The slag contains both TiO2 and SiO2, and it can potentially be converted into the TiO2-SiO2 support for deNOx catalyst. The use of TiO2 made from slag as a support for NH3-SCR catalyst is important in terms of lowering the catalyst cost and exploiting a new way to fully utilize the Ti-BFS.
The feasibility of making NH3-SCR catalyst using Ti-bearing BFS based on sulfate method has been reported in our previous work [11]. The obtained catalyst showed even higher deNOx activity than that based on commercial TiO2. This work continues to upgrade such a V2O5-WO3/TiO2 catalyst by using TiO2-SiO2 supports made from Ti-bearing BFS under varied hydrolytic conditions. The TiO2-SiO2 supports with different TiO2/SiO2 ratios are prepared to clarify how the deNOx activity of catalyst varies with the composition, structure and acidity of the prepared support. However, besides containing certain SiO2, all samples made with slag had some unavoidable impurities such as Al2O3 and Fe2O3. The purifications of these impurities have been performed according to [24,25,26], but the process was too complicated and increased the preparation cost. As mentioned above, the incorporation of Al2O3 and Fe2O3 into TiO2 as mechanical promoters helped to improve the SCR activity. An earlier study [13] also reported that the co-introduction of SiO2 and Al2O3 into TiO2 increased the resistance to SO2 and H2O poisoning in SCR of NO by NH3. Thus, aim at minimizing the preparation cost, the obtained support from different hydrolytic conditions will be used directly without any other purification steps.
2. Results and Discussion
2.1. Composition Varying with Hydrolytic pH Value
The control of pH value is very important for hydrolysis of the TiOSO4 solution obtained from acidolysis of BFS because the precipitation of SiO2 is incomplete at pH values below 2.8 [27,28]. To achieve and maintain the pH value during hydrolysis, aqueous ammonia (NH3.xH2O 25%) was slowly added into the formed TiOSO4 solution. Four parallel of syntheses were formed and named as BFS-TiO2 N with N (pH = 1, 2, 3), denoting the pH value, and further NA for the case without pH adjustment. During leaching, TiO2, SiO2, Al2O3, and MgO become soluble, whereas CaO remains as CaSO4 residue [29]. The composition of precipitated products, as shown in Table 1, obviously varied with hydrolytic pH value, indicating the dependence on isoelectric points (IEPs) of different components in the solution.

Table 1.
Yield and main chemical composition of BFS-TiO2 supports obtained under different hydrolytic pH values (mass %).
For SiO2, its electrical mobility is zero at pH between 2 and 3, and the rate of aggregation for silica sol particles gets its minimum in this range of pH. Thus, the content of SiO2 in precipitate increased to 14.5 wt. % when pH value was raised to 2 and then slightly decreased with higher pH values. Meanwhile, iron oxides and alumina sol started to precipitate from the solution and their amounts slowly increased with raising pH of the TiOSO4 solution for hydrolysis. This trend is consistent with the result in Seggiani et al. [27] who reported that with the rise in pH value the positive charge of anions decreased until a certain point was reached to cause negative adsorption of anions, at pH values of 6–7 for Al2O3 and 8.4–8.6 for Fe2O3 [27,28,30]. Besides, the positively-charged species cannot effectively be adsorbed onto the positively-charged anatase TiO2 surface (IEP = 3.5). Therefore, only little iron oxides and alumina gel were deposited on TiO2. Furthermore, the recovery ratio of TiO2 also varied with pH value for the four prepared samples. At pH of 3, the realized highest yield of total precipitate and TiO2 recovery ratio were 92.7% and 78.6%, respectively. The total product yield was found to increase with raising pH value of hydrolysis solution since more impurities were co-precipitated at higher pH values.
2.2. Structure of Prepared Supports
Figure 1a compares the shapes of adsorption isotherms for a commercial TiO2 sample and several synthetic BFS-TiO2 samples. According to the IUPAC (International Union of Pure and Applied Chemistry) classification, the isotherms of all BFS-TiO2 are almost type IV to show obvious mesoporous structure in the samples [31]. Meanwhile, the TiO2 support belongs to the type H1, implying a uniform cylindrical pore geometry in the material. In all the synthetic BFS-TiO2 samples, the shape and location of their hysteresis loops vary with the hydrolytic conditions, and the physical properties of the produced TiO2 are strongly subject to the incorporated Si. The isotherm for the BFS-TiO2 NA sample is actually a mixture of the types H2 and H3 hysteresis loops, but that for the BFS-TiO2 samples at pH = 1–3 belongs to the type H2. Thus, the mesopores in all BFS-TiO2 supports are irregular in size and shape [31]. From Figure 1b we can see that the pore size distribution (PSDs) moves towards smaller sizes when increasing the SiO2 content to cause collapse of inter-aggregated pores, as represented by the hysteresis loop at high P/P0 [32]. The contribution of SiO2 to the formation of pores in BFS-TiO2 was also proven by the increased surface area and decreased mean pore sizes with the rise of SiO2 content. Interestingly, the PSDs change from a bimodal structure for the commercial TiO2 and BFS-TiO2 NA to a unimodal mesopore structure for the BFS-TiO2 samples made at pH = 1–3 which contain silica and oxide dopants like Al2O3 and Fe2O3 in high amounts. This indicates that both silica and impurities in the supports help to maintain their high mesopore fraction, resulting the greater surface area shown in Table 2 [33]. Generally, a large pore volume is due to a high fraction of mesopores with high and non-uniform surface area (H2 and H3 hysteresis loops), which improves the catalytic activity by obtaining better dispersion of vanadium species on support and facilitating the spread of reactant molecules (NO) to active sites existing in the meso-structure framework [7,34]. All of this suggests that both silica and impurities in the slag-based deNOx catalyst facilitate the catalytic NH3-SCR reactions, especially for the support made at pH 1 that has the largest mesopore volume.
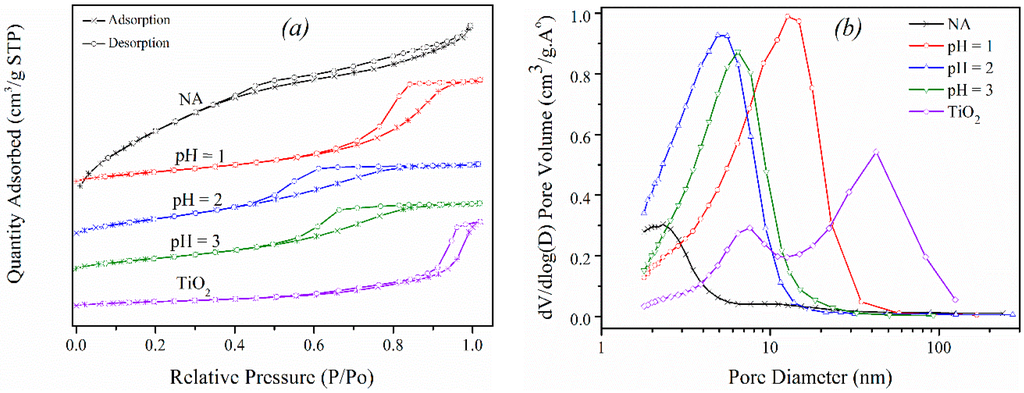
Figure 1.
Characterization of BFS-TiO2 and TiO2 supports: (a) isotherms of N2 adsorption and desorption; and (b) pore size distribution.

Table 2.
Structural parameters of supports and loading amount of active components for their catalysts.
The microstructures of synthetic BFS-TiO2 samples have been studied using SEM (Scanning Electron Microscopy) and TEM (Transmission Electron Microscopy) analysis, presented in Figure 2. It could be observed from SEM images Figure 2(a,d) that the BFS-TiO2 supports possess a rough porous surface accompanied with many mesopores (TEM images Figure 2b,e) to imply high surface area and high adsorptive capacity [35]. The micrographs also revealed that the secondary particles, as observed in Figure 2(a,d), comprise agglomerated irregular primary particles, of which the size was dependent on hydrolytic pH value, as observed in Figure 2(b,e) [35]. Meanwhile, SEM image showed that the BFS-TiO2 NA support had a broad size distribution and two types of aggregates, namely elongated shape and mainly spherical shape with loosely agglomerated. The micrographs of BFS-TiO2 samples with pH adjustment were very similar and represented by sample prepared at pH 1; their SEM showed the asymmetric plate-like morphology with larger, more consolidated agglomerates, thus exposing new textural properties (see Figure 1) in comparison to sample without pH adjustment in hydrolysis. The severe agglomeration observed in samples having pH adjustment is probably because the strong acidity of the initial hydrolysis solution caused slow hydrolyzation; the enhancement in the hydrolytic pH value might have helped to increase in reaction rate that led to increase the number of particles and, consequently, the number of collisions leading to agglomeration [21,36,37].
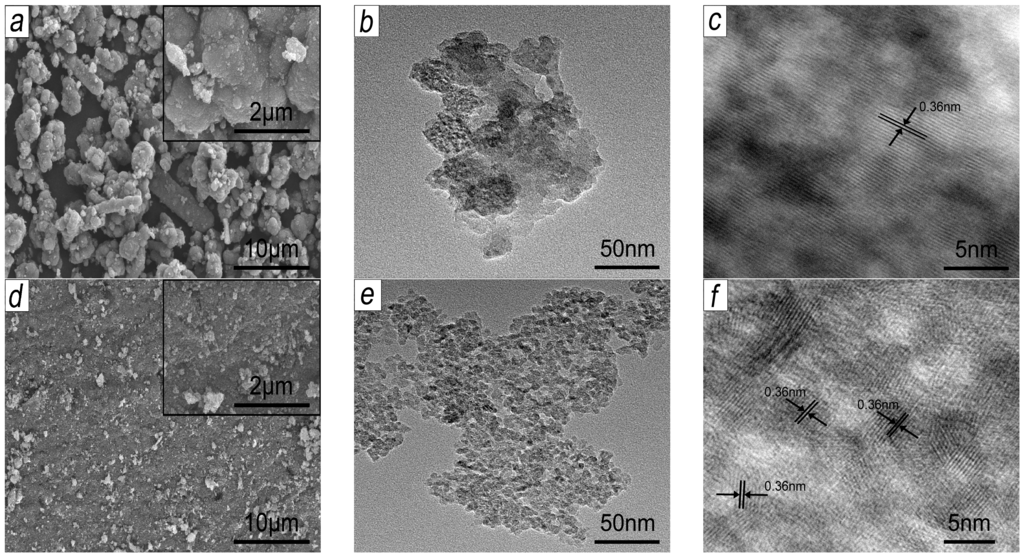
Figure 2.
SEM, TEM and HRTEM micrographs of BFS-TiO2 samples made at different pH values of hydrolysis: (a–c) NA; and (d–f) pH 1.
Further identification of the configuration of morphology was achieved by TEM and HRTEM images. TEM analysis revealed that the synthetic BFS-TiO2 supports had obviously spherical primary particles without any coating on the surface [21]. A high resolution transmission electron microscopic (HRTEM) study was performed to clarify the distribution of crystalline titania within the BFS-TiO2 samples. All the observed lattice fringes of BFS-TiO2 nanoparticles show a d-spacing of 0.360 nm, which can be well assigned to the (101) lattice fringes of anatase TiO2 (d = 0.352 nm, JCPDS No. 21-1272), confirming the presence of anatase TiO2 in all the BFS-TiO2 supports [38].
In order to further understand the structure of BFS-TiO2 from hydrolysis under different pH values, FT-IR (Fourier Transform Infrared Spectroscopy) spectra were taken for all tested supports (Figure 3). For this characterization, each sample was dried at 200 °C for 2 h to remove free water. Usually, the IR broad band at 400–850 cm−1 is accepted to be the stretching vibration of Ti–O bonds in Ti–O–Ti [39]. Meanwhile, the broad band at 3200–3600 cm−1 is attributed to the stretching vibration of –OH of adsorbed water as well as surface hydroxyl, and that at 1635 cm−1 is assigned to the bending vibration of –OH. For all synthetic BFS-TiO2 samples, the new absorption bands at 1100 cm−1 and 960 cm−1 are induced by the stretching vibration of Si–O–Si and Ti–O–Si bonds, respectively. The IR results suggest that BFS-TiO2 is a composite of TiO2 and SiO2, and that Ti–O–Si bond is formed between TiO2 and SiO2 particles [40].
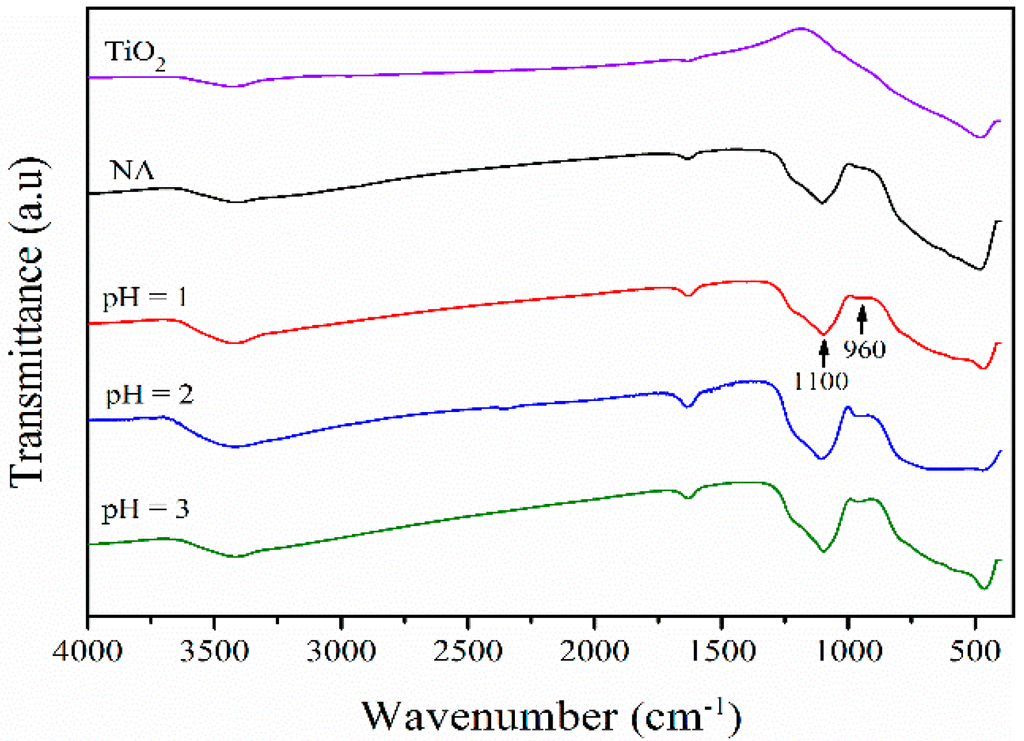
Figure 3.
FT-IR spectra of different synthetic BFS-TiO2 samples and commercial TiO2.
The crystallographic phase shown in Figure 4 can be identified as anatase TiO2, which is in accordance with TEM results. For all BFS-TiO2 samples, no pronounced change was observed in the XRD pattern, except for a lower intensity than that for pure TiO2. The lattice aberrances turn bigger for all BFS-TiO2 containing higher silica content, which is consistent with the literatures [39,40]. According to the Scherrer’s formula, the sizes of the anatase crystallites was between 14.9–7.4 nm, as listed in Table 2. The suppression in crystalline size together with the formation of Ti–O–Si bond proved by FT-IR spectra indicated that SiO2 is uniformly incorporated into titania lattice and no peaks corresponding to SiO2 were observed, suggesting the formation of amorphous SiO2 [40]. Furthermore, the presence of SiO2 in BFS-TiO2 does not allow structural changes except for a slight shift towards higher 2θ values due to shrinkage of the pores, thereby stabilizing the meso-structures [41]. Neither impurities, Al2O3 nor Fe2O3, are detected, probably because of their weakness compared to the strong scattering from anatase, suggesting that impurities well dispersed over the surface of titania or existed as an amorphous phase [13,14]. The difference in crystallinity is not proportional to reflect the enabled NOx conversion because the support also has different acidities and acid sites. The acidity of support is associated with NH3 adsorption on catalyst, which should increase with raising acidity [12,17].
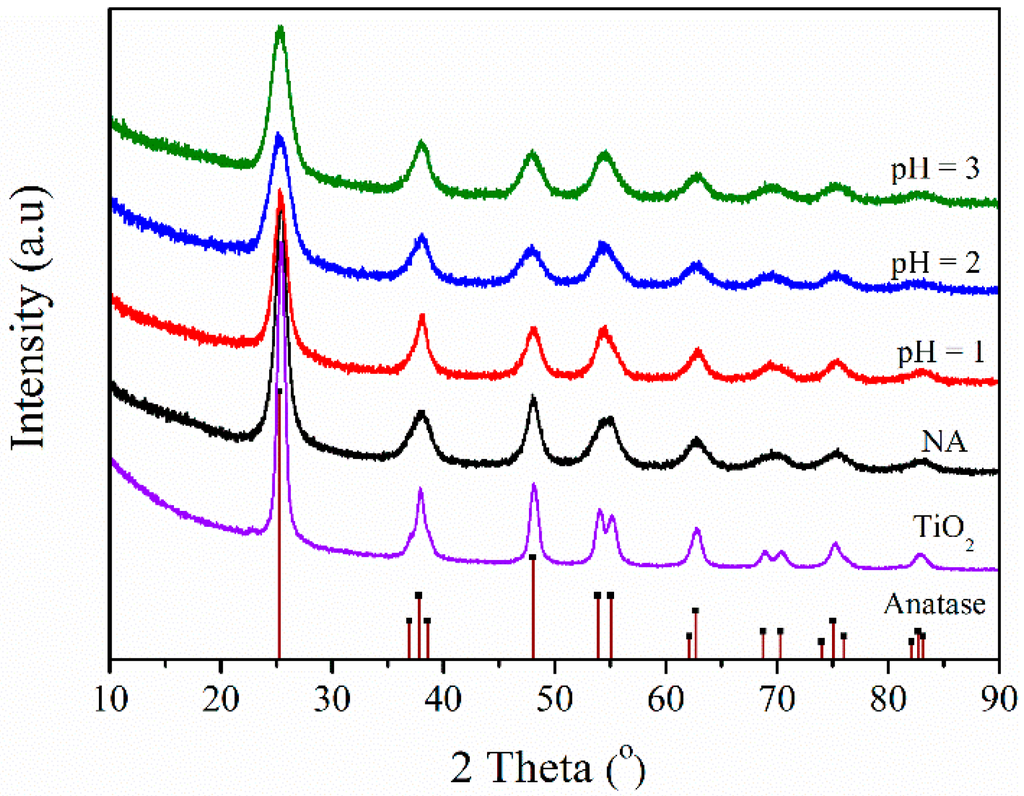
Figure 4.
XRD patterns of different synthetic BFS-TiO2 samples and commercial TiO2.
The temperature programmed desorption (TPD) of NH3 is a well-known method for determination of acidic properties of solid heterogeneous catalysts. In the NH3–TPD curves, peaks are generally distributed into two regions: below and above 400 °C, referred to as low-temperature (LT) and high-temperature (HT) regions, respectively. The peaks in the HT region can be attributed to the desorption of NH3 from strong acid sites, and the peak in the LT region is assigned as the desorption of NH3 from some relatively weak acid sites [42]. NH3-TPD profiles of synthetic BFS-TiO2 and TiO2 supports, presented in Figure 5, showed broadly distributed acid sites in the LT region. In general, their TPD curves are similar in shape but clearly different in magnitude. The signal of all BFS-TiO2 samples greatly increased as compared to pure TiO2, which meant the density of acid sites was enhanced with the incorporation of SiO2. The formation of Ti–O–Si band was believed to develop more acid sites [42,43]. This result implies that the presence of SiO2 in BFS-TiO2 samples brought more active NH3 adsorbed species, which is reported to be significantly beneficial to SCR reaction [9,13,17]. The amount of NH3 desorbed on the samples has increased with the increase of SiO2 content [9], indicating that more acidic sites occurred in the catalyst having higher SiO2 content and the weak acid sites are predominant [39].
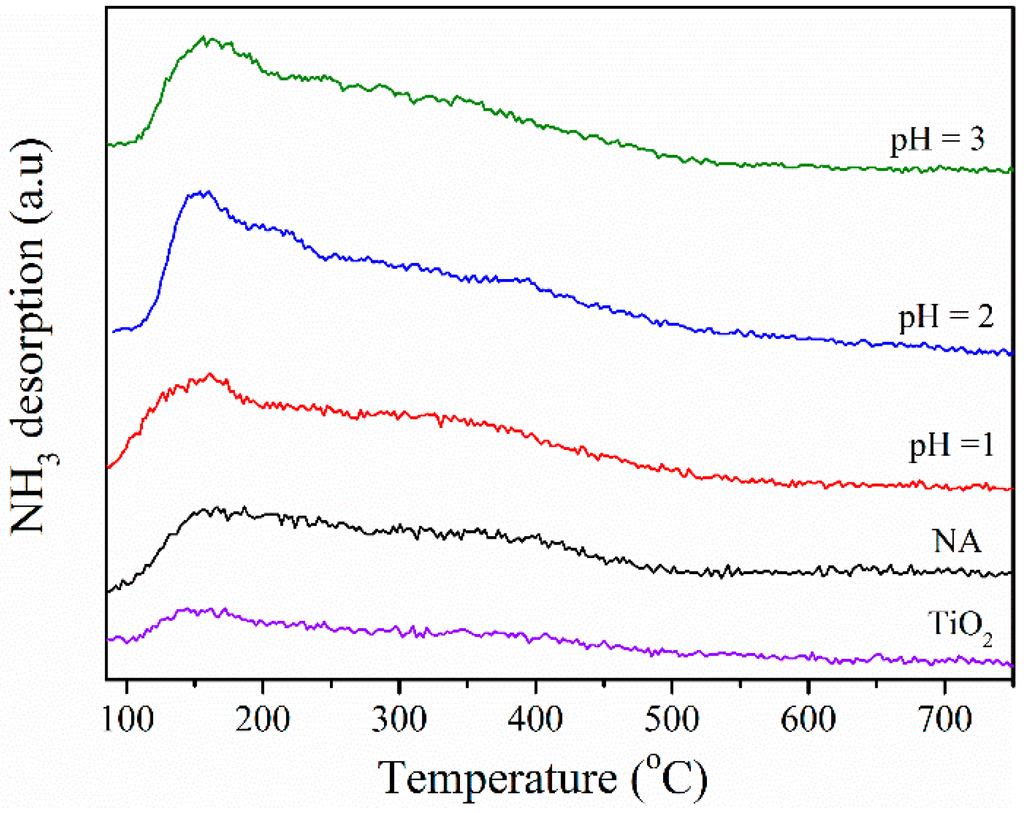
Figure 5.
NH3-TPD patterns of BFS-TiO2 samples made at different pH values of hydrolysis and commercial TiO2.
H2-Temperature programmed reduction (H2-TPR) measurements were conducted to characterize the reduction performances and the interactions of dispersed active species with support [12]. As shown in Figure 6, H2-TPR profiles of different catalysts from supports with varied TiO2/SiO2 ratios are characterized by two broad peaks with their Tmax around 431–483 °C and 742–848 °C, indicating the reductions of V2O5 and WO3, respectively. The different reduction properties of V2O5 and WO3 on BFS-TiO2 supports was observed, illustrating their different interactions with support [44]. It can be seen in Figure 6 that the catalysts from BFS-TiO2 showed larger peak areas than that from commercial TiO2. As we all know, a catalyst possessing a larger reduction peak means the more reducible form of active species on the catalyst surface, which has a positive effect on NO catalytic reduction with NH3 [45]. These results demonstrated that the incorporation of SiO2 to BFS-TiO2 support is beneficial to disperse VOx and WOx species on the surface than pure TiO2 support did [12]. Additionally, Tmax of reduction peak for mainly active VOx species also reflects the difficulty of its valence change during deNOx reaction, which is directly related to the deNOx activity of a catalyst. The reduction peaks of VOx species shift to lower temperature region, thus the reduction reaction takes place more easily [12,44]. One can see that the VOx reduction peaks firstly shifted to low temperature with raising the amount of SiO2 in support, suggesting that the reducibility of some surface vanadium oxide species increases with increasing silica [17,46]. Surprisingly, the Tmax value of VOx species in the catalyst having the highest SiO2 content (BFS-TiO2 pH 2) is significantly higher than that of both other BFS-TiO2 samples and commercial TiO2, suggesting that the modification of titania by high content of SiO2 seems to decrease the reducibility of surface vanadium oxide species and that a strong interaction exists between the surface vanadium oxide and surface silica species, resulting in the difficult reduction. Such observation has also been reported by Gao et al. [46], which verifies the TiO2/SiO2 ratio in 1 wt.% V2O5/TiO2-SiO2 to study reducibility properties of catalyst and type of the surface VOx species on the support. Tmax of VOx reduction was found to be the lowest for the catalyst with the support at pH = 1, suggesting stronger redox properties for this sample.
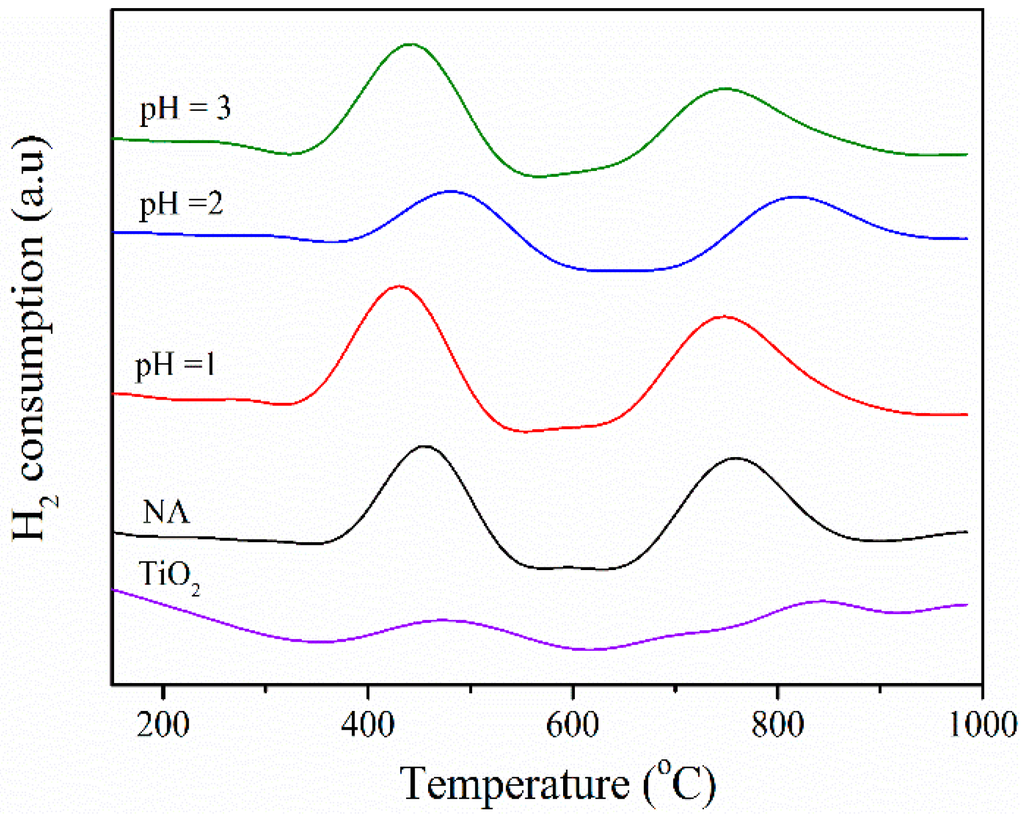
Figure 6.
H2-TPR profiles of catalysts on commercial TiO2 and BFS-TiO2 made at different pH values of hydrolysis (2 wt. % V2O5, 5 wt. % WO3).
2.3. Catalytic Performance of Catalysts
The activity test in the reduction of NO by NH3 was carried out over catalysts prepared by impregnating 2 wt. % V2O5 and 5 wt. % WO3 on the prepared BFS-TiO2 and commercial TiO2 as support. Evaluation of catalysts was conducted at 150–500 °C in simulated flue gas and the results are compared in Figure 7. The catalytic activity increased with raising temperature from 150 °C to 400 °C and then sharply decreased at rather higher temperatures, which was caused by the parallel oxidation of NH3 by O2, resulting a decrease in the amount of NH3 participating in the SCR of NO [44]. In 200–400 °C, the best deNOx efficiency was shown for V2O5-WO3/BFS-TiO2 (pH = 1) with 9.2 wt. % SiO2 in its support, whereas the lowest activity was for the catalyst on BFS-TiO2 (pH = 2) with the highest SiO2 content of 14.5 wt. %. The catalyst made with the commercial TiO2 without any SiO2 exhibited higher catalytic activity than that with BFS-TiO2 (pH = 2) did. Meanwhile, the catalysts on the supports made with pH = 3 and NA (no pH adjustment) had a similar catalytic activity at all temperatures tested. These obvious differences in catalytic activity among the catalysts should be subject to different compositions, pore structures and acidic properties of used supports.
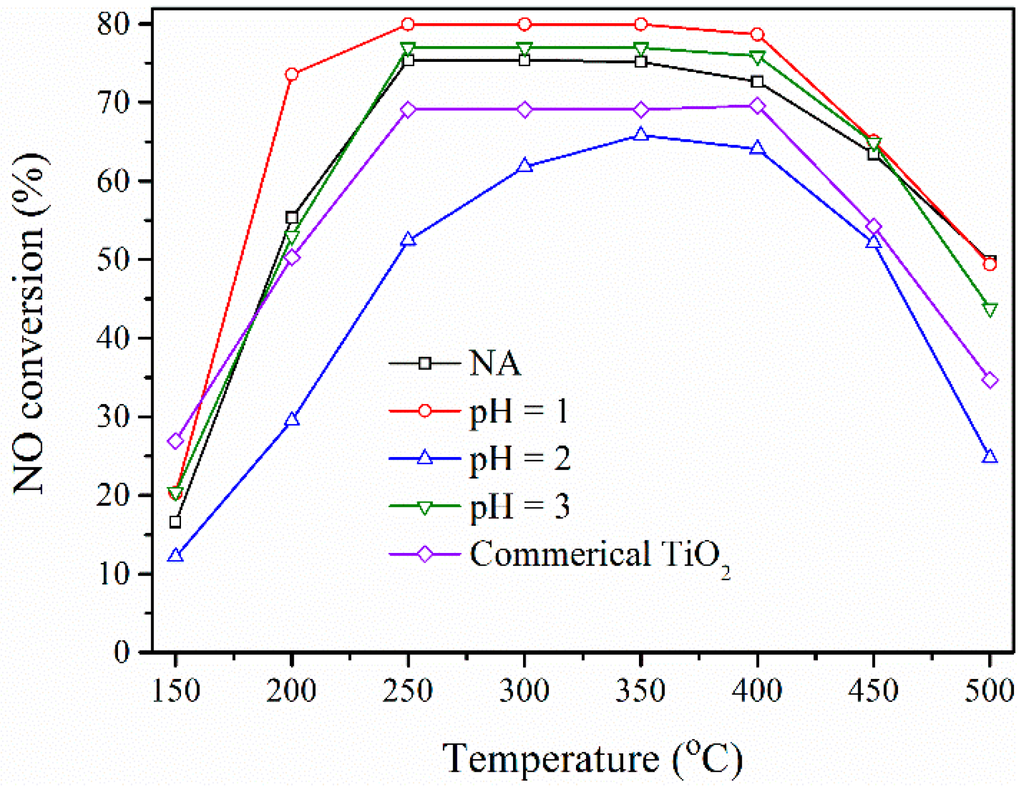
Figure 7.
NO conversion versus temperature for catalysts with 2 wt. % V2O5 and 5 wt. % WO3 on BFS-TiO2 and commercial TiO2 supports.
The high surface area, surface acidity coupled with the more reducible form of VOx and WOx species on the surface of catalysts prepared from BFS-TiO2 supports allowed an enhancement of the catalytic efficiency in the SCR of NO by NH3 as compare to catalyst prepared from pure TiO2 [12,45]. The deNOx activity of slag-based catalysts increased with the increase of SiO2 content up to 9.2 wt. % (BFS-TiO2 pH 1) and then decreased. It was obvious that the total acidic strength of BFS-TiO2 pH 2 having the highest SiO2 content was greater than that of the other catalysts, but the NO conversion over this catalyst was the lowest. An earlier study of V2O5/TiO2–SiO2 by Kobayashi et al. [17] reported that this behavior was attributed to the difference in the NH3 adsorption capacity. It is considered that NH3 is not utilized effectively for the transformation of NO to N2 since NH3 is too strongly adsorbed on the large number of acidic sites in a large concentration of SiO2, which is reversely disadvantageous for SCR reaction. Moreover, a strong interaction exists between the surface vanadium oxide and surface silica species caused the difficult reducibility of VOx species, resulting its low catalytic activity [46]. Table 3 listed the distribution of VOx reduction peak from H2-TPR results and the reaction rate constant (kmass) for comparison of catalytic activity irrespective of the small loading deviations occurred during impregnation process. The results revealed that the reaction rate constant of the prepared catalyst complies with their H2-TPR profile. The best redox performance and the highest reaction rate constant shown for the support made at pH = 1 had the highest mesopore volume. Pinnavaia et al. [34] reported that sample having high mesopore volume can facilitate the diffusion of reactant molecules (NO) or reaction intermediates to active sites on the mesoporous support. These results verify that the amount of SiO2 content catalyst support does have great effect on the redox properties of surface VOx species, surface acidity and textural properties, and, consequently, affects the NO catalytic reduction with NH3 [12,17,46].

Table 3.
Position of VOx peak maxima (Tmax) obtained from H2-TPR analysis and the mass rate constant (kmass) at 300 °C of NO-NH3 reaction.
In practical use, the SCR reaction atmosphere usually contains some fraction of H2O and SO2. Therefore, the effect of 10 vol. % H2O and 600 ppm of SO2 on the SCR reaction over the catalyst on BFS-TiO2 at pH = 1 and commercial TiO2 supports was investigated. The experiments were performed at 300 °C with an NH3/NO ratio of 0.8 and GSHV of 24,000 h−1. Figure 8 revealed that the presence of steam and SO2 in the feed inhibited significantly the NH3-SCR reaction over these catalysts, especially V2O5-WO3/TiO2 catalyst. For catalyst prepared from BFS-TiO2 support, the presence of steam lowered NO conversion by about 5%, but still retained a stable NO conversion to N2 in an 8-h test, and stopping the feed of H2O soon restored its catalytic activity. However, with the presence of both SO2 and steam, the NO conversion was stabilized at a value about 10% lower than that for the gas without SO2 and steam; furthermore stopping SO2 and steam (~24 h), the NO conversion again restored quickly to a high value. The same trends were also observed over catalyst made with commercial TiO2, a significant reduction from 66% to 45% in NO conversion was observed when gas feed contained both SO2 and steam. Nonetheless, its catalytic activity recovered gradually and did not recover to the original level after stopping the feed of SO2 and steam. Literature studies found that the most significant reason for maintaining a high activity of catalyst on TiO2-SiO2 support in the presence of SO2 and steam is due to the special network structure of the Ti-Si that can suppress the sulfate formation the surface of the Ti-Si binary oxide support [12,13,17,19]. These results suggest that the catalyst made with the slag-based TiO2 support (by pH = 1) can enhance the catalytic activity and possesses good stability, strong resistance to SO2 and H2O poisoning, which are beneficial to practical deNOx applications as compared to catalyst prepared from commercial TiO2 support.
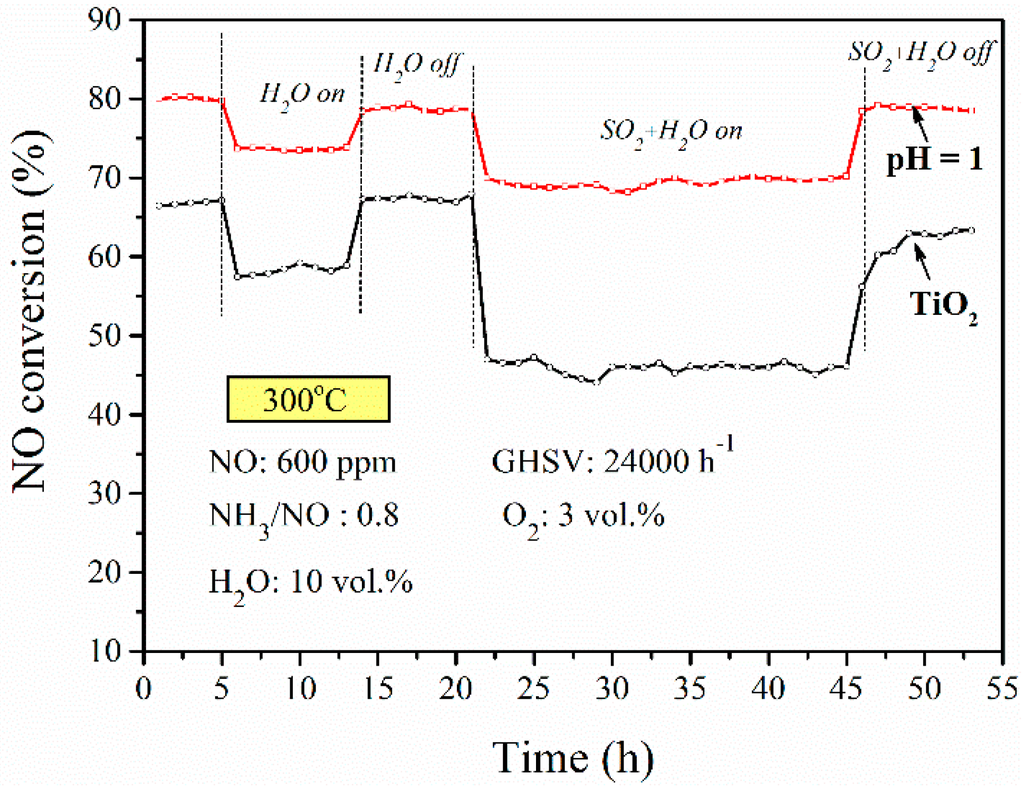
Figure 8.
Resistance to poisoning of SO2 and steam for the catalyst prepared with commercial TiO2 support and BFS-TiO2 support made at pH = 1 of hydrolysis (2 wt. % V2O5, 5 wt. % WO3).
3. Experimental Section
3.1. Slag Treatment and Catalyst Preparation
The raw Ti-bearing blast furnace slag (BFS) was provided by Panzihua Iron & Steel Group Co., Ltd. in China. Prior to use, the slag was dried and crushed into particle sizes below 0.2 mm. Then, the XRF analysis was applied to determine slag composition, as shown in Table 1. Figure 9 shows the XRD pattern of the slag. A broad diffraction peak around 30° was identified to be CaO-SiO2-Al2O3-TiO2 glass structure [47], and there was no anatase TiO2 diffraction peak. Thus, the BFS needs to be treated for getting anatase TiO2 used as the support of SCR catalyst [17,19].
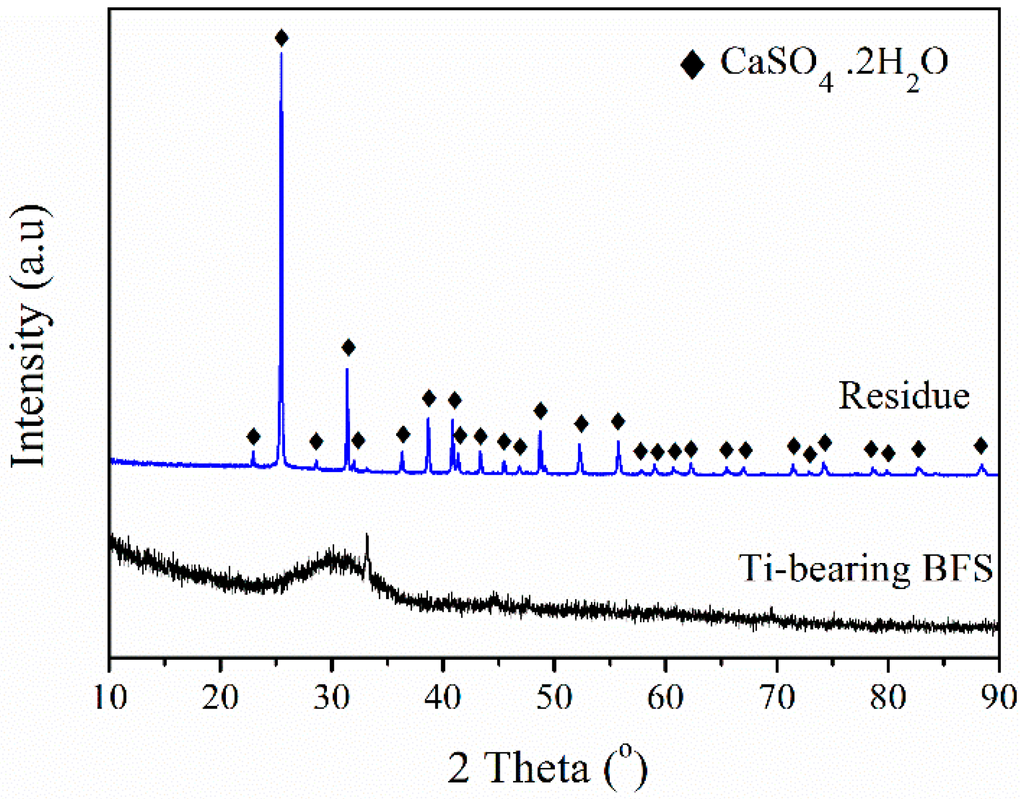
Figure 9.
XRD patterns of treated blast furnace slag (BFS) and leaching residue.
Studies on ilmenite leaching with sulfuric acid clarified that the maximal dissolution rate of ilmenite occurs at 70–76 wt. % H2SO4 at 88–100 °C [21,22]. The leaching was thus conducted using 70 wt. % sulfuric acid at 90 °C and lasted for 3 h. The mass ratio of H2SO4 over BFS was maintained at 1.5. Figure 9 also shows that the residue of leaching mainly consists of anhydrite (CaSO4·2H2O, JCPDS 01-072-0503) and 1.2 wt. % TiO2 (XRF analysis). This confirms that nearly all TiO2 was dissolved by sulfuric acid [29]. The black TiOSO4 solution formed by this acidolysis was further hydrolyzed to make precipitates through adjusting the hydrolytic pH to a value of 1, 2 or 3 using 25% aqueous ammonia. For hydrolysis, the TiOSO4 solution was first hydrolyzed at 80 °C for 5 h and then reacted at 110 °C for 5 h to form a H2TiO3 slurry. By filtration and washing further, the obtained filter cake was finally dried at 110 °C and calcined at 600 °C for 4 h to transform H2TiO3 into anatase TiO2. The total product yield and recovery percent of TiO2 are calculated (Equations (1) and (2)):
In order to carry out catalytic activity comparison between slag-based catalysts and the most widespread commercial V2O5-WO3/TiO2-based SCR catalyst at the same amount of active species, the anatase TiO2 employed as a deNOx catalyst support was prepared by calcination of metatitanic acid (H2TiO3 99%, Chengdu XiYa Chemical Technology Co., Chengdu, China) at 600 °C for 4 h.
In turn, the deNOx catalyst V2O5-WO3/BFS-TiO2 and V2O5-WO3/TiO2 was prepared according to the successive impregnation method using ammonium paratungstate ([NH4]6W7O24·6H2O), Sinopharm, Shanghai, China) and ammonium metavanadate (NH4VO3, Sinopharm, Shanghai, China) as precursors for W and V, respectively. Firstly, the supports were added into the [NH4]6W7O24·6H2O solution and the slurry was dryness under continuous stirring at 60 °C until the solution became a paste, then drying at 110 °C for 10 h took place. After that, NH4VO3 was dissolved in oxalic acid (10 wt. %) to form the blue complex vanadyl oxalate VO(C2O4)2. The above obtained powder was introduced into this solution and the slurry was brought to dryness under continuous stirring at 60 °C until the solution became a paste. Finally, the paste was dried at 110 °C for 10 h and calcined at 600 °C for 4 h. All the catalysts were made to have the same composition of metal oxides, 2 wt. % V2O5, 5 wt. % WO3 and balanced TiO2. Besides, all the used materials and solvents for catalyst preparation and experiments were commercially bought.
3.2. Characterization and Evaluation
XRD measurement was carried out in 2θ angles of 10–90° on a D/Max-RB diffractometer (Rigaku Corp., Tokyo, Japan) with Cu Kα radiation. Nitrogen adsorption/desorption isotherms were recorded on an ASAP 2020 (Micromeritics Instrument Corp., Norcross, GA, USA) at 77 K. All the products were degassed in vacuum at 150 °C for 6 h prior to BET measurements. The Brunauer–Emmett–Teller (BET) equation was used to calculate the specific surface area (SBET). Pore size distributions were calculated from the adsorption branch using the Barret–Joyner–Halenda (BJH) model, while the nitrogen adsorption volume at the relative pressure (P/Po) of 0.994 was used to determine the pore volume and average pore size. XRF analysis in the Axios X-ray fluorescence (XRF) spectrometer (PANalytical X’pert, Almelo, The Netherlands) was performed to determine the sample composition. Scanning electron microscopy (SEM) images were recorded at 10 kV on JSM-7001F electron microscopy (JEOL Ltd., Tokyo, Japan), samples were coated with Au prior to analysis and imaged directly. Transmission electron microscopy (TEM) observation was carried out on a JEM-2100 of JEOL transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 200 kV, TEM samples were mounted on a copper-supported carbon polymer grid by placing a few droplets of a suspension of the ground sample in ethanol on the grid, followed by drying at ambient conditions. Transmission FT-IR spectra were recorded from Bruker Tensor 27 instrument (Bruker, Rheinstetten, Germany) in the 400–4000 cm−1 resolution, 1 mg dry powder was dispersed into 100 mg an IR transmissive material (KBr) and pressed to obtain transparent disks.
NH3-TPD and H2-TPR measurements were carried out on the ChemBET Pulsar TPR/TPD equipment from Quantachrome Instruments (FL, USA). Firstly, 0.1 g of sample was loaded into a quartz U-tube and heated from room temperature to 300 °C at 10 °C.min−1 and then maintained there for 150 min in a Helium atmosphere. In turn, the sample was cooled to 90 °C and was further followed by heating to 700 °C at 5 °C·min−1 for NH3-TPD or to 1000 °C at 5 °C·min−1 for H2-TPR in a gas flow of 30 mL·min−1. The consumed NH3 or H2 in the process of temperature rise was continuously monitored on-line using a mass spectrometry (Proline Mass Spectrometer, Ametek, PA, USA).
Catalytic activity measurement for SCR of NO by NH3 was conducted in a quartz fixed bed reactor of 15 mm in internal diameter under atmospheric pressure. The tested catalyst was powder with particle sizes below 0.2 mm, and the simulated flue gas consisted of 0.06 vol. % NO, 0.048 vol. % NH3, 3 vol. % O2, 10 vol. % H2O, 0.06 vol. % SO2 and balanced N2. The total flow rate through the reactor was 400 mL·min−1 (STP) to give a Gas Hourly Space Velocity (GHSV) of 24,000 h−1 for activity test in two case studies, absence and presence of SO2 and steam in the reaction stream. Taking this high space velocity was for evidently comparing the activities of different catalysts. The molar concentrations of gases entering and exiting the reactor were continually monitored using a using a PG-300 portable gas analyzer from Horiba Ltd. (Kyoto, Japan). The reaction usually reached a steady state after about 1 h at a given temperature, and the realized NO conversion [7] was calculated according to the measured inlet and outlet NO concentrations (Equation (3)):
The reaction rate constant (kmass) for the maximal deNOx efficiency was calculated by assuming a pseudo-first order SCR reaction with respect to NO and zero order to NH3 [7] (Equation (4)):
where V* is the total flow rate under reaction conditions, W the loaded amount of active component and XNO the NO conversion.
4. Conclusions
NOx removal catalysts with low cost and good SCR performance were prepared using BFS-TiO2 support made from blast furnace slag (BFS). In hydrolysis of TiOSO4 solution obtained from acidolysis of slag, the hydrolytic pH value was varied to control the precipitation of metal components and thus the properties of the obtained BFS-TiO2 support such as composition, structure and acidic properties. All synthetic BFS-TiO2, which contained certain SiO2 and unavoidable impurities Al2O3 and Fe2O3, had mesoporous structure with high BET surface area of about 172–427 m2/g. The incorporated SiO2 in BFS-TiO2 samples was responsible for the enhancement in the amount of NH3 adsorbed and the reducible species formed on the catalyst surface, which enabled them to have good activity for catalyzing NO-NH3 reaction in comparison to the commercial TiO2 support. However, deNOx efficiency over V2O5-WO3 supported on BFS-TiO2 pH 2 (having the highest SiO2 content) is significantly lower than that of both other BFS-TiO2 samples and commercial TiO2. This suggested that the modification of titania by high content of SiO2 caused difficulty in reducibility of VOx in its catalyst due to the strong interaction between the surface VOx and surface SiO2 species; and strongly NH3 adsorbed on its catalyst surface is subjected to the suppression of NH3 participating in the SCR of NO to N2. The best deNOx efficiency, nearly 80% NO conversion at an NH3/NO ratio of 0.8 in 200–400 °C for the catalyst prepared from BFS-TiO2 pH 1, which also showed fairly good ability of resistance to the SO2 and steam poisoning. All of these show in fact that V2O5 and WO3 loaded on BFS-TiO2 pH 1 having 9.2 wt. % of SiO2 is suitable as SCR catalyst for practical deNOx application due to high activity in SCR, good stability, strong resistance to SO2 and steam poisoning, and, especially, low preparation catalyst cost.
Acknowledgments
The authors are grateful to the financial supports of International Science and Technology Cooperation Program of China (2013DFA51530), Strategic Priority Research Program of Chinese Academy of Sciences (XDA07030300), and Japan Society for the Promotion of Science (JSPS) for the postdoctoral fellowship grant (P15758).
Author Contributions
The experimental work was conceived and designed by T.T., J.Y. and G.X. T.T. performed the experiments; T.T, J.Y. and G.X. analyzed the data; L.G. and F.G. contributed reagents/materials/analysis tools; and T.T, J.Y., D.P. and G.X. drafted the paper. The manuscript was amended through the comments of all authors. All authors have given approval for the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.X.; Zhao, B.; Cai, S.Y.; Klimont, Z.; Nielsen, C.P.; Morikawa, T.; Woo, J.H.; Kim, Y.; Fu, X.; Xu, J.Y.; et al. Emission trends and mitigation options for air pollutants in East Asia. Atmos. Chem. Phys. 2014, 14, 6571–6603. [Google Scholar] [CrossRef]
- Felix, J.D.; Elliott, E.M.; Shaw, S.L. Nitrogen isotopic composition of coal-fired power plant NOx: Influence of emission controls and implications for global emission inventories. Environ. Sci. Technol. 2012, 46, 3528–3535. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, K.; Hao, J.; Wang, Y.; Gao, J.; Qiu, P.; Zhu, C. Nitrogen oxides emissions from thermal power plants in China: Current status and future predictions. Environ. Sci. Technol. 2013, 47, 11350–11357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, S.X.; Liu, H.; Xu, J.Y.; Fu, K.; Klimont, Z.; Hao, J.M.; He, K.B.; Cofala, J.; Amann, M. Nox emissions in China: Historical trends and future perspectives. Atmos. Chem. Phys. 2013, 13, 9869–9897. [Google Scholar] [CrossRef]
- Shan, W.; Song, H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wang, J. NOx sensor reading correction in diesel engine selective catalytic reduction system applications. IEEE/ASME Trans. Mech. 2016, 21, 460–471. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Kröcher, O. VOx surface coverage optimization of V2O5/WO3-TiO2 SCR catalysts by variation of the V loading and by aging. Catalysts 2015, 5, 1704–1720. [Google Scholar] [CrossRef]
- China SCR Denitration Catalyst Industry Report, 2013–2016. PRNewswire, 8 January 2014.
- Peng, Y.; Liu, C.; Zhang, X.; Li, J. The effect of SiO2 on a novel CeO2–WO3/TiO2 catalyst for the selective catalytic reduction of NO with NH3. Appl. Catal. B 2013, 140–141, 276–282. [Google Scholar] [CrossRef]
- Casanova, M.; Schermanz, K.; Llorca, J.; Trovarelli, A. Improved high temperature stability of NH3-SCR catalysts based on rare earth vanadates supported on TiO2-WO3-SiO2. Catal. Today 2012, 184, 227–236. [Google Scholar] [CrossRef]
- Yang, J.; Lei, S.; Yu, J.; Xu, G.W. Low-cost V−W−Ti SCR catalyst from Titanium-bearing blast furnace slag. J. Environ. Chem. Eng. 2014, 2, 1007–1010. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, W.; Zhong, Q.; Cai, W.; Li, H. Promotional effect of Si-doped V2O5/TiO2 for selective catalytic reduction of NOx by NH3. J. Environ. Sci. 2013, 25, 1703–1711. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, Y.; Wan, Y.; Li, L.; Yao, S.; Li, X.; Gu, J.; Li, Y.; Shi, J. Promotion effects of SiO2 or/and Al2O3 doped CeO2/TiO2 catalysts for selective catalytic reduction of NO by NH3. J. Hazard. Mater. 2014, 278, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, C.; Ma, L.; Peng, Y.; Qu, Z.; Yan, N.; Chen, J.; Chang, H.; Li, J. Substitution of WO3 in V2O5/WO3-TiO2 by Fe2O3 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2013, 3, 161–168. [Google Scholar] [CrossRef]
- Matralis, H.K.; Ciardelli, M.; Ruwet, M.; Grange, P. Vanadia catalysts supported on mixed TiO2-Al2O3 supports: Effect of composition on the structure and acidity. J. Catal. 1995, 157, 368–379. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx abatement systems for mobile sources: From three-way to lean burn after-treatment technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kuma, R.; Masaki, S.; Sugishima, N. TiO2-SiO2 and V2O5/TiO2-SiO2 catalyst: Physico-chemical characteristics and catalytic behavior in selective catalytic reduction of NO by NH3. Appl. Catal. B: Environ. 2005, 60, 173–179. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hagi, M. V2O5-WO3/TiO2-SiO2-SO42− catalysts: Influence of active components and supports on activities in the selective catalytic reduction of NO by NH3 and in the oxidation of SO2. Appl. Catal. B: Environ. 2006, 63, 104–113. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Li, J.; Ma, L.; Arandiyan, H.; Du, Y.; Xu, J.; Hao, J. Enhancement of activity and sulfur resistance of CeO2 supported on TiO2–SiO2 for the selective catalytic reduction of NO by NH3. Environ. Sci. Technol. 2012, 46, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Recovery of titanium compounds from molten ti-bearing blast furnace slag under the dynamic oxidation condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Li, G. Preparation of Nano-Titanium Dioxide from ilmenite using sulfuric acid-decomposition by liquid phase method. Powder Technol. 2016, 287, 256–263. [Google Scholar] [CrossRef]
- Liang, B.; Li, C.; Zhang, C.; Zhang, Y. Leaching kinetics of panzhihua ilmenite in sulfuric acid. Hydrometallurgy 2005, 76, 173–179. [Google Scholar] [CrossRef]
- Chen, D.-S.; Zhao, L.-S.; Qi, T.; Hu, G.-P.; Zhao, H.-X.; Li, J.; Wang, L.-n. Desilication from titanium–vanadium slag by alkaline leaching. Trans. Nonferrous Met. Soc. China 2013, 23, 3076–3082. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, Z.; Wu, F.; Li, X.; Guo, H. Preparation of TiO2 from ilmenite using sulfuric acid decomposition of the titania residue combined with separation of Fe3+ with edta during hydrolysis. Adv. Powder Technol. 2013, 24, 60–67. [Google Scholar] [CrossRef]
- Liu, S.-S.; Guo, Y.-F.; Qiu, G.-Z.; Jiang, T.; Chen, F. Preparation of Ti-rich material from titanium slag by activation roasting followed by acid leaching. Trans. Nonferrous Met. Soc. China 2013, 23, 1174–1178. [Google Scholar] [CrossRef]
- Lasheen, T.A. Sulfate digestion process for high purity TiO2 from titania slag. Front. Chem. Eng. China 2009, 3, 155–160. [Google Scholar] [CrossRef]
- Seggiani, M.; Vitolo, S. Recovery of silica gel from blast furnace slag. Resour. Conserv. Recycl. 2003, 40, 71–80. [Google Scholar] [CrossRef]
- Rouseková, I.; Bajza, A.; Živica, V. Silica fume-basic blast furnace slag systems activated by an alkali silica fume activator. Cem. Concr. Res. 1997, 27, 1825–1828. [Google Scholar] [CrossRef]
- Valighazvini, F.; Rashchi, F.; Nekouei, R.K. Recovery of titanium from blast furnace slag. Ind. Eng. Chem. Res. 2013, 52, 1723–1730. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Zero-point-of-charge prediction from crystal chemistry and solvation theory. Geochim. Cosmochim. Acta 1994, 58, 3123–3129. [Google Scholar] [CrossRef]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.C.; Leung, M.K.P.; Ho, W.; Cheng, B.; Zhao, X.; Zhao, J. Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous titania. J. Catal. 2003, 217, 69–78. [Google Scholar] [CrossRef]
- Kim, J.; Song, K.C.; Foncillas, S.; Pratsinis, S.E. Dopants for synthesis of stable bimodally porous titania. J. Eur. Ceram. Soc. 2001, 21, 2863–2872. [Google Scholar] [CrossRef]
- Pinnavaia, T.J.; Pauly, T.R.; Kim, S.S. Mesoporous molecular sieve catalysis: Relationships between reactivity and long range structural order/disorder. In Supported Catalysts and Their Applications; Sherrington, D.C., Kybett, A.P., Eds.; The Royal Society of Chemistry: London, UK, 2001; pp. 19–26. [Google Scholar]
- Ngamta, S.; Boonprakob, N.; Wetchakun, N.; Ounnunkad, K.; Phanichphant, S.; Inceesungvorn, B. A facile synthesis of nanocrystalline anatase TiO2 from TiOSO4 aqueous solution. Mater. Lett. 2013, 105, 76–79. [Google Scholar] [CrossRef]
- Park, H.K.; Park, K.Y.; Jung, K.Y. Alumina-precursor nanoparticles prepared by partial hydrolysis of AlCl3 vapor in tubular flow reactor: Effect of hydrolysis conditions on particle size distribution. Ind. Eng. Chem. Res. 2014, 53, 10372–10379. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-S.; Jang, S.-R.; Hong, J.S.; Vittal, R.; Kim, K.-J. Preparation of composite anatase TiO2 nanostructure by precipitation from hydrolyzed TiCl4 solution using anodic alumina membrane. Chem. Mater. 2003, 15, 4633–4636. [Google Scholar] [CrossRef]
- Ren, C.; Qiu, W.; Chen, Y. Physicochemical properties and photocatalytic activity of the TiO2/SiO2 prepared by precipitation method. Sep. Purif. Technol. 2013, 107, 264–272. [Google Scholar] [CrossRef]
- Dong, R.-L.; Na, C.; Zhang, H.-P.; Chen, Z.-D.; Jin, C.-C. TiO2/SiO2 mesoporous microspheres with intelligently controlled texture. Mater. Des. 2016, 89, 830–838. [Google Scholar] [CrossRef]
- Saha, J.; Mitra, A.; Dandapat, A.; De, G. TiO2 nanoparticles doped SiO2 films with ordered mesopore channels: A catalytic nanoreactor. Dalton Trans. 2014, 43, 5221–5229. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-M.; Mei, P.; Xiong, L.; Gao, L.; Yang, Q.; Gong, L. Mesoporous titania-silica-polyoxometalate nanocomposite materials for catalytic oxidation desulfurization of fuel oil. Catal. Sci. Technol. 2013, 3, 1985–1992. [Google Scholar] [CrossRef]
- Jin, R.; Wu, Z.; Liu, Y.; Jiang, B.; Wang, H. Photocatalytic reduction of NO with NH3 using Si-doped TiO2 prepared by hydrothermal method. J. Hazard. Mater. 2009, 161, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Chang, H.; Peng, Y.; Li, J. Dispersion of tungsten oxide on SCR performance of V2O5-WO3/TiO2: Acidity, surface species and catalytic activity. Chem. Eng. J. 2013, 225, 520–527. [Google Scholar] [CrossRef]
- Youn, S.; Song, I.; Kim, D.H. Promotional effect on selective catalytic reduction of NOx with NH3 over overloaded W and Ce on V2O5/TiO2 catalysts. J. Nanomater. 2015, 1–7. [Google Scholar] [CrossRef]
- Gao, X.; Bare, S.R.; Fierro, J.L.G.; Wachs, I.E. Structural characteristics and reactivity/reducibility properties of dispersed and bilayered V2O5/TiO2/SiO2 catalysts. J. Phys. Chem. B 1999, 103, 618–629. [Google Scholar] [CrossRef]
- Ren, S.; Zhao, Q.; Yao, L.; Liu, Q. Precipitation behavior of perovskite and anosovite crystals from high ti-bearing blast furnace slag with small amount of B2O3. Cryst. Eng. Comm. 2016, 18, 1393–1402. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).