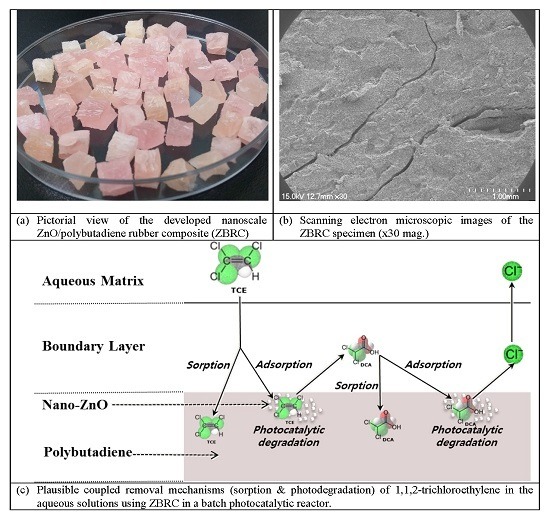
Enhanced Removal of Trichloroethylene in Water Using Nano-ZnO/Polybutadiene Rubber Composites
Abstract
:1. Introduction
2. Results and Discussion
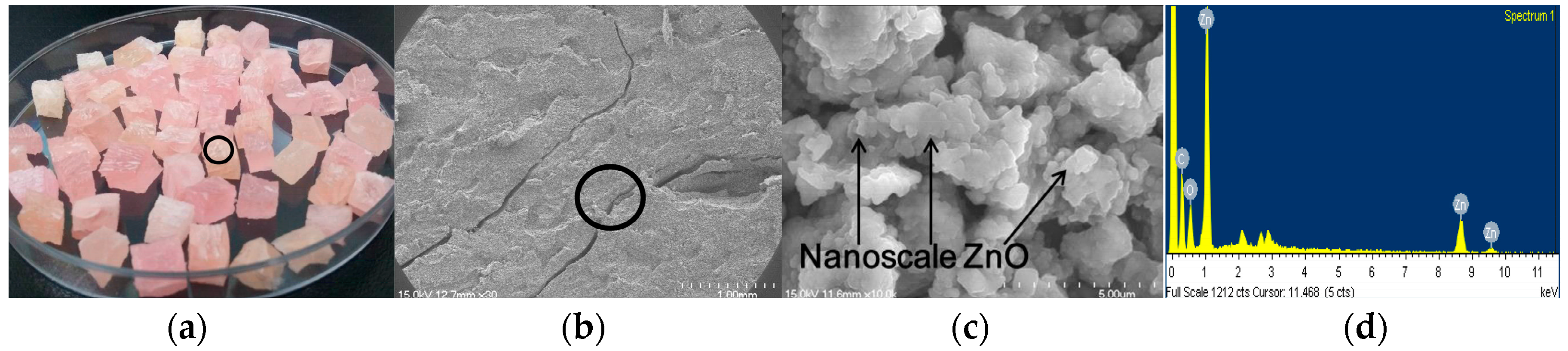
2.1. Characterization of the Nanoscale ZnO/Polybutadiene Rubber Composite (ZBRC)
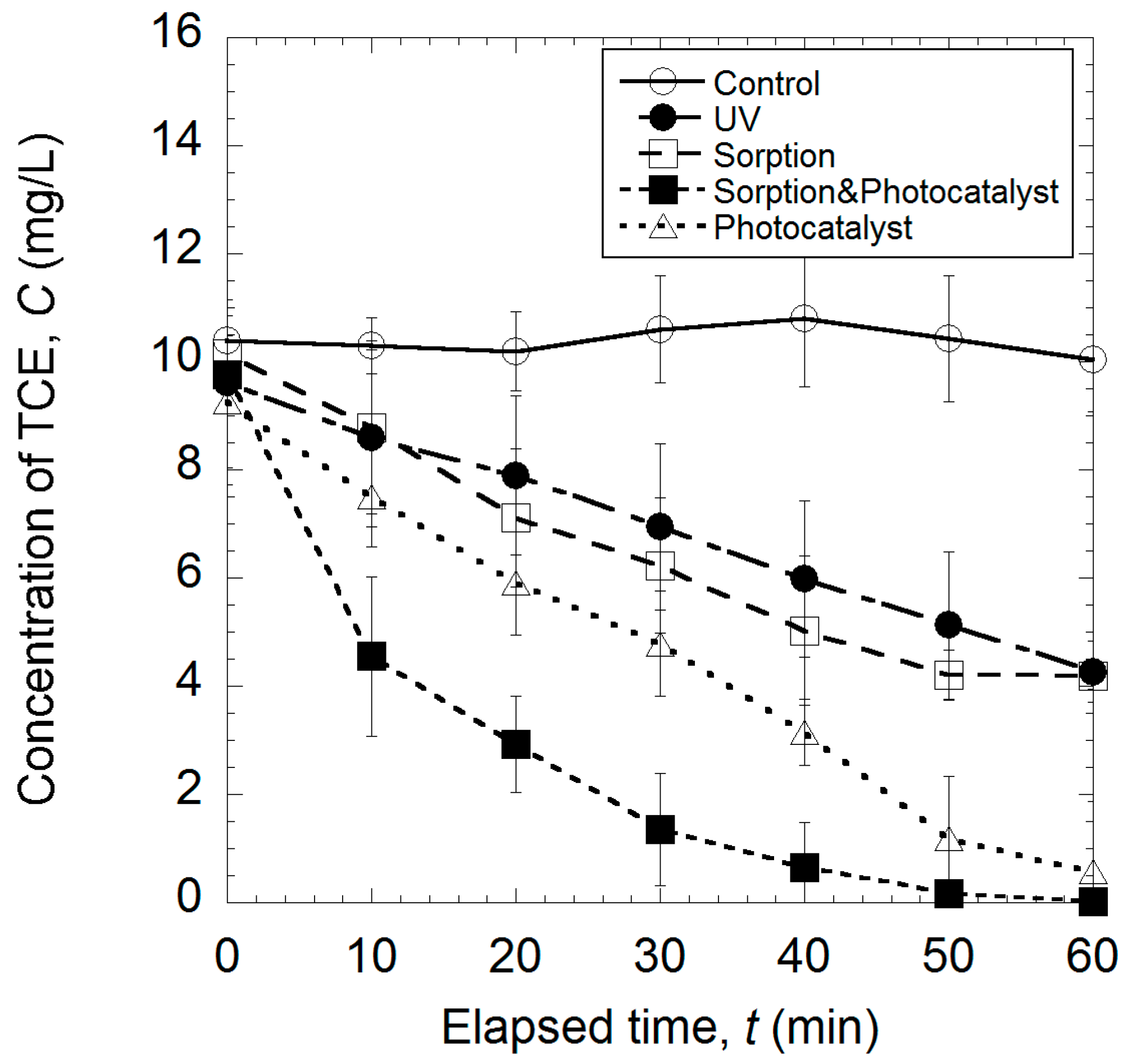
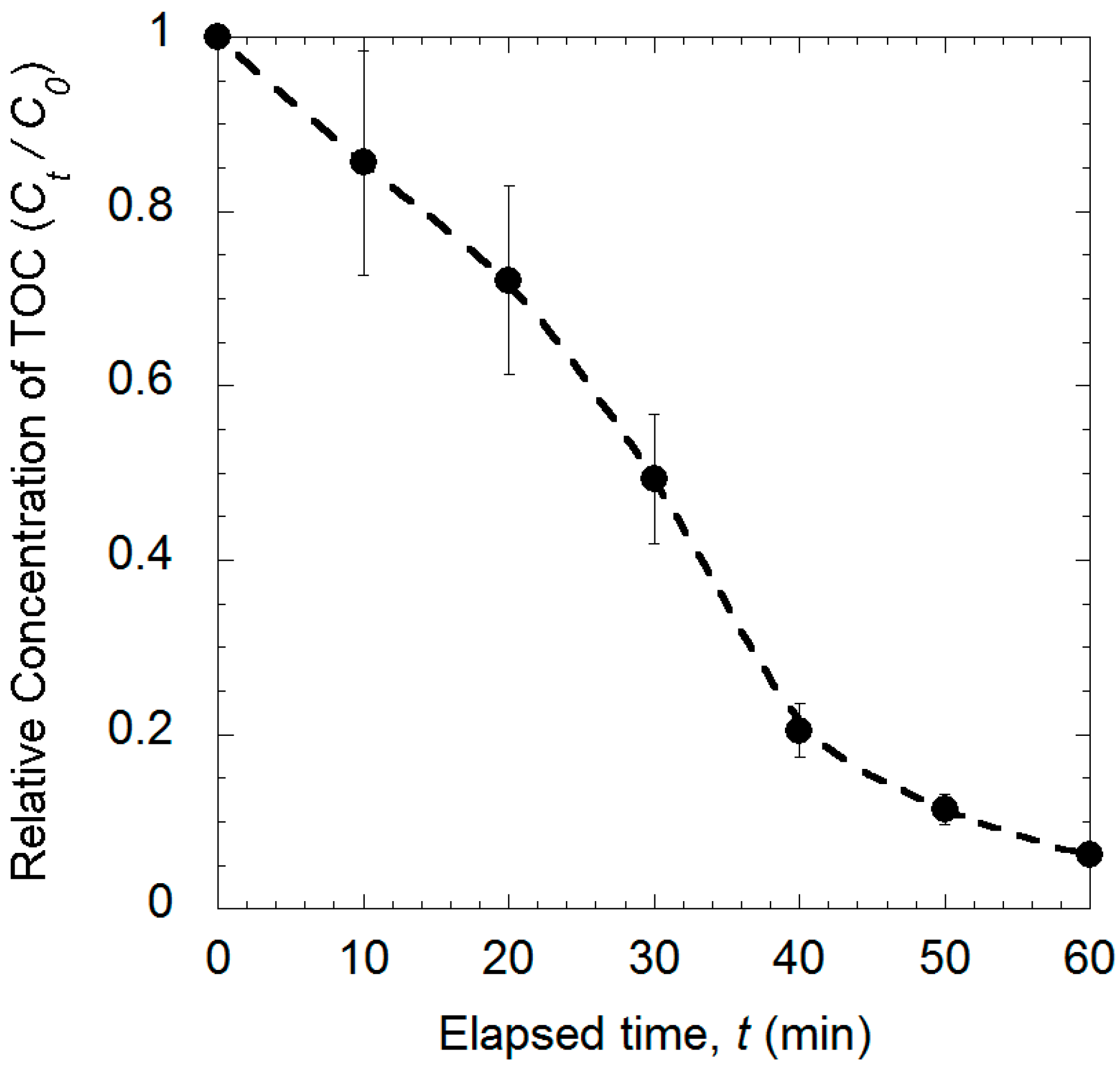
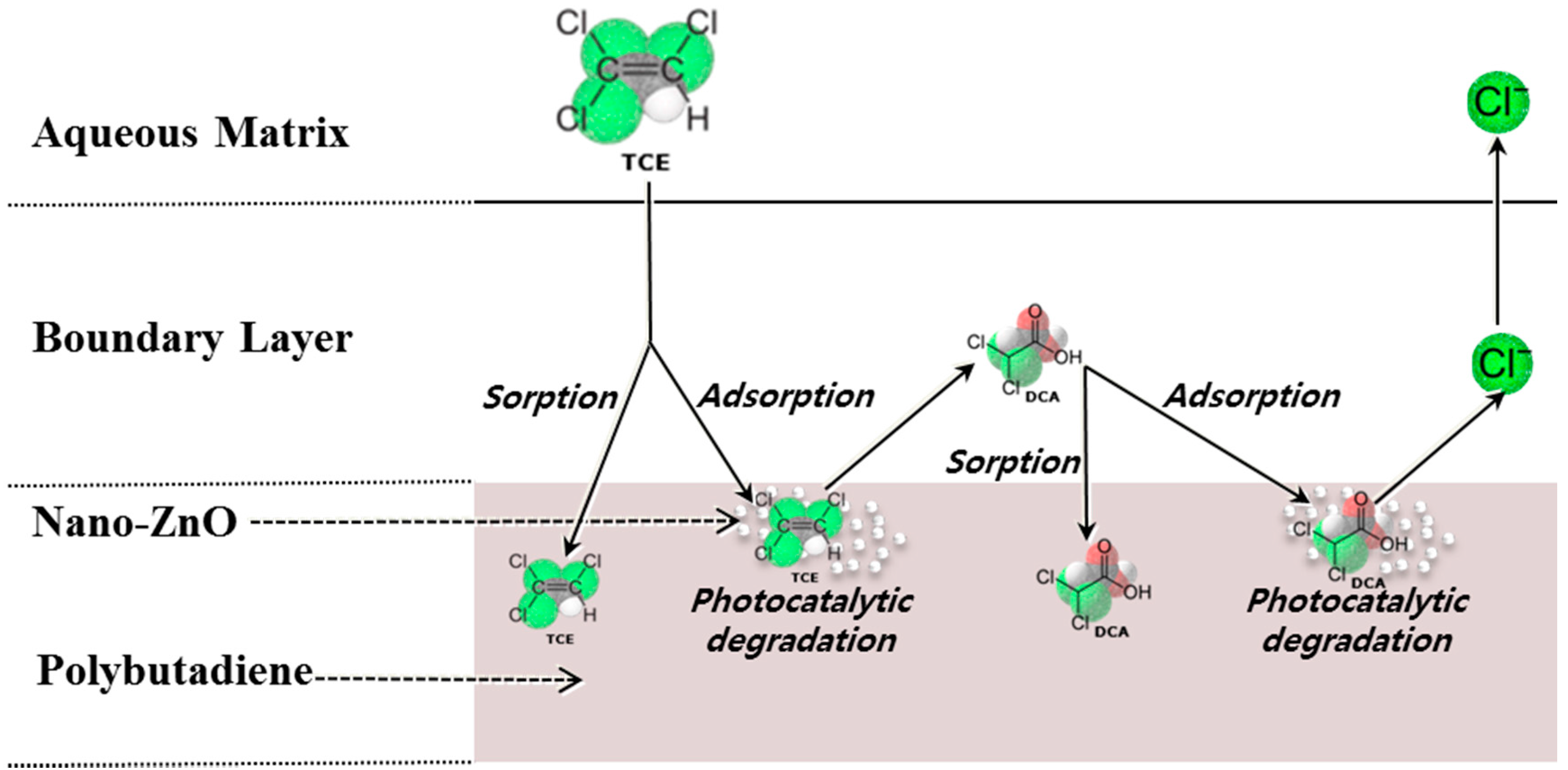
2.2. Coupled Removal of TCE Using the Nanoscale ZnO/Polybutadiene Rubber Composite (ZBRC)
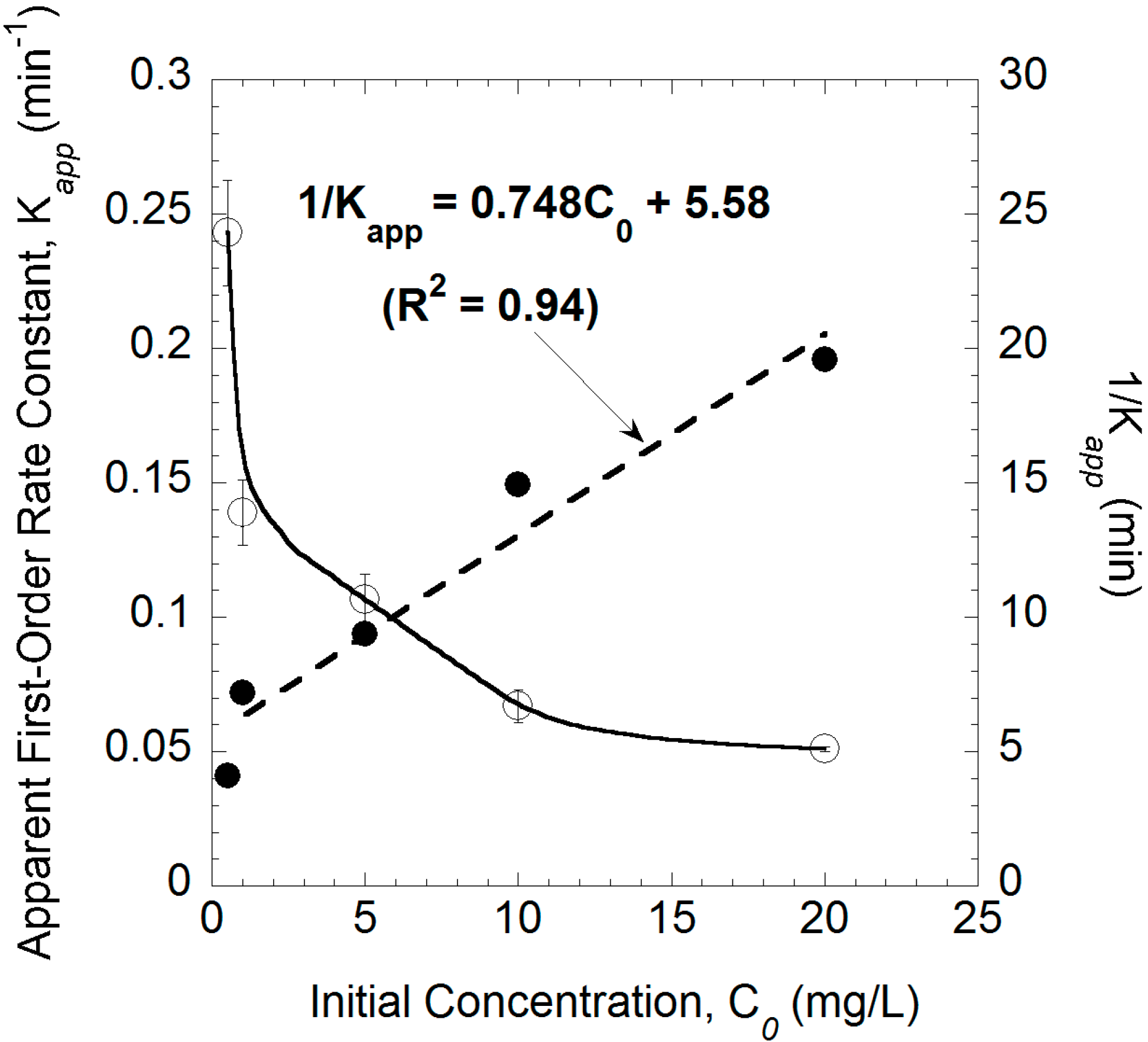
2.3. Effect of the Initial TCE Concentration
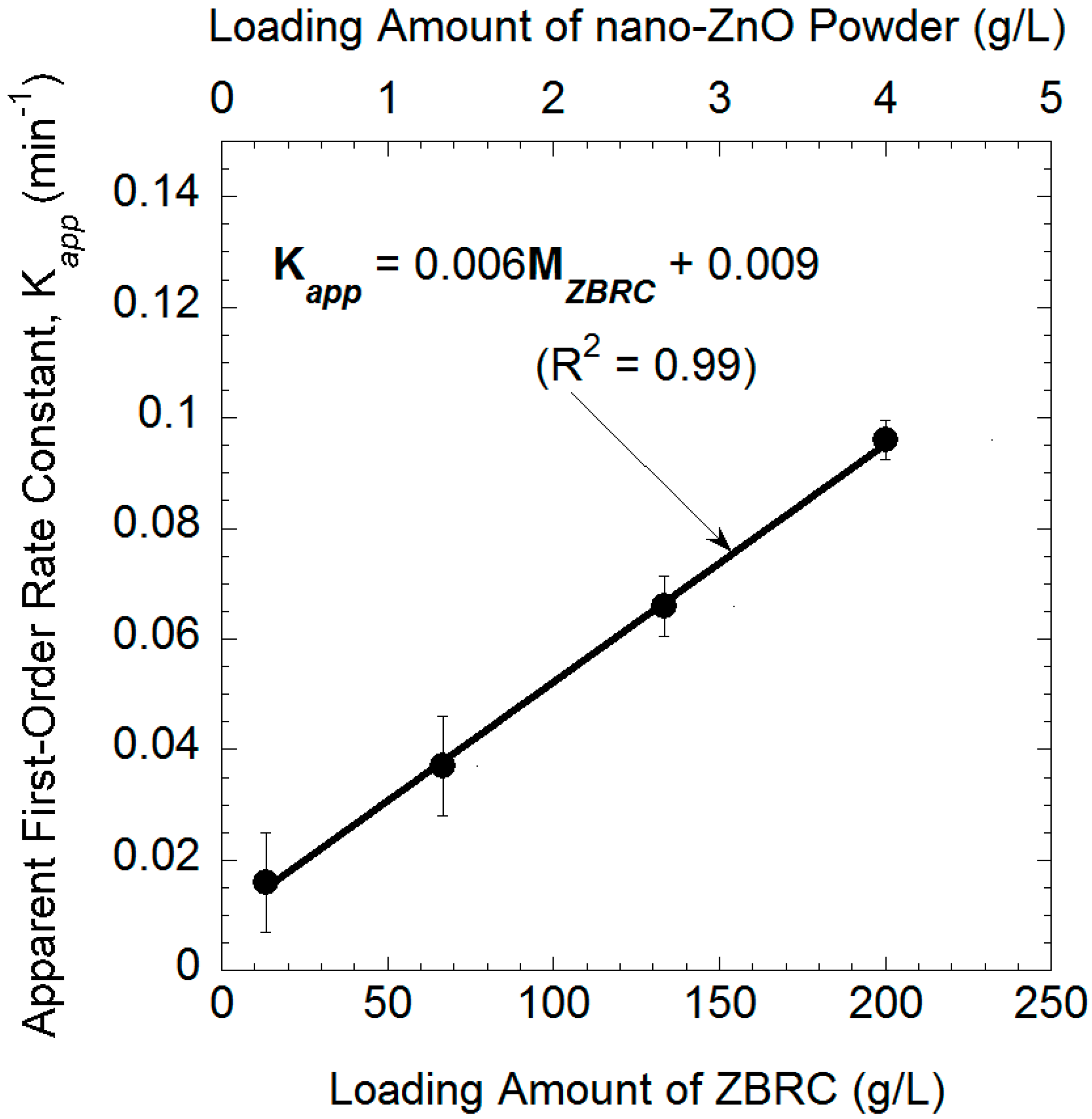
2.4. Effect of the ZBRC Loading Amounts
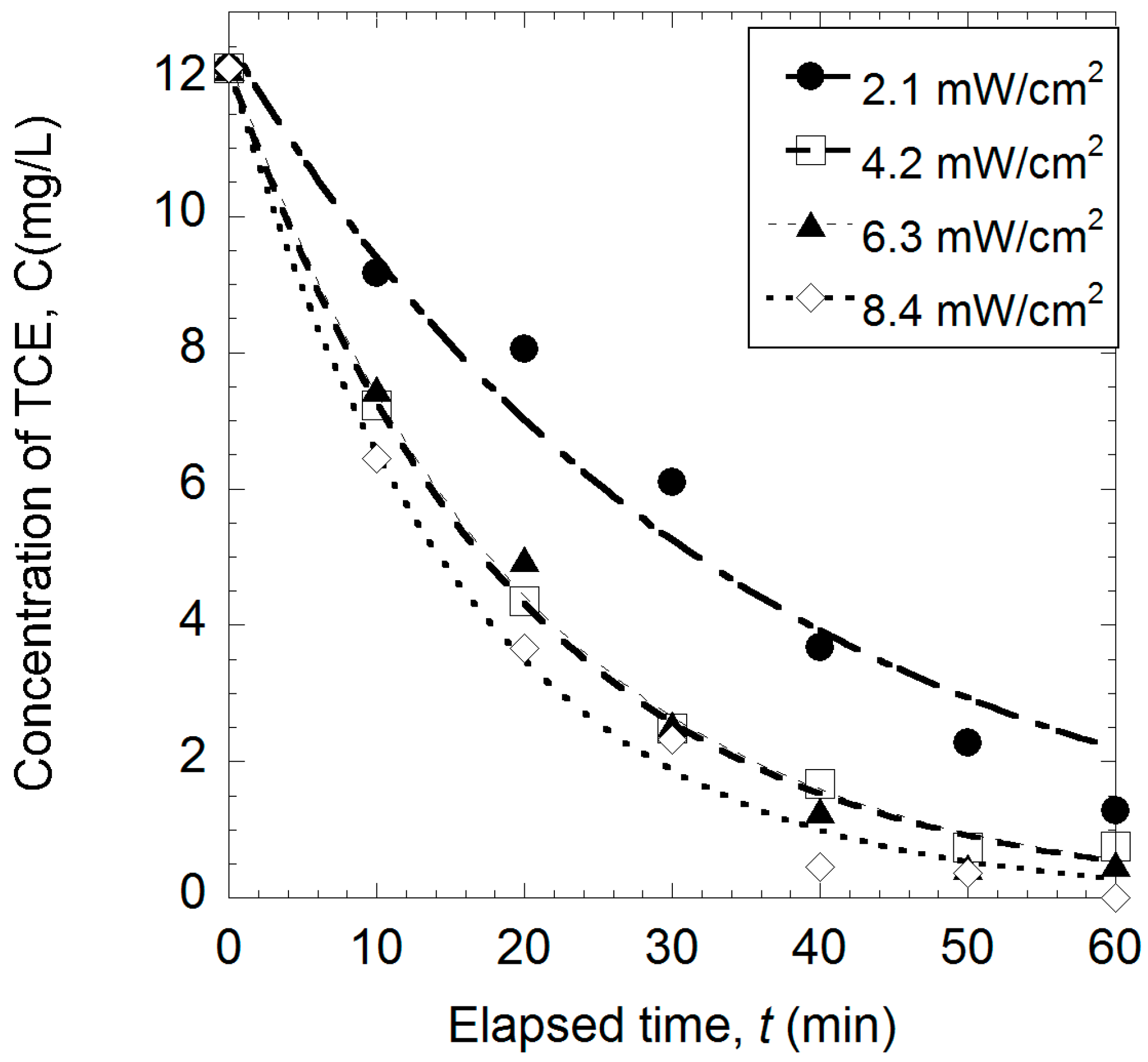
2.5. Effect of Light Intensity
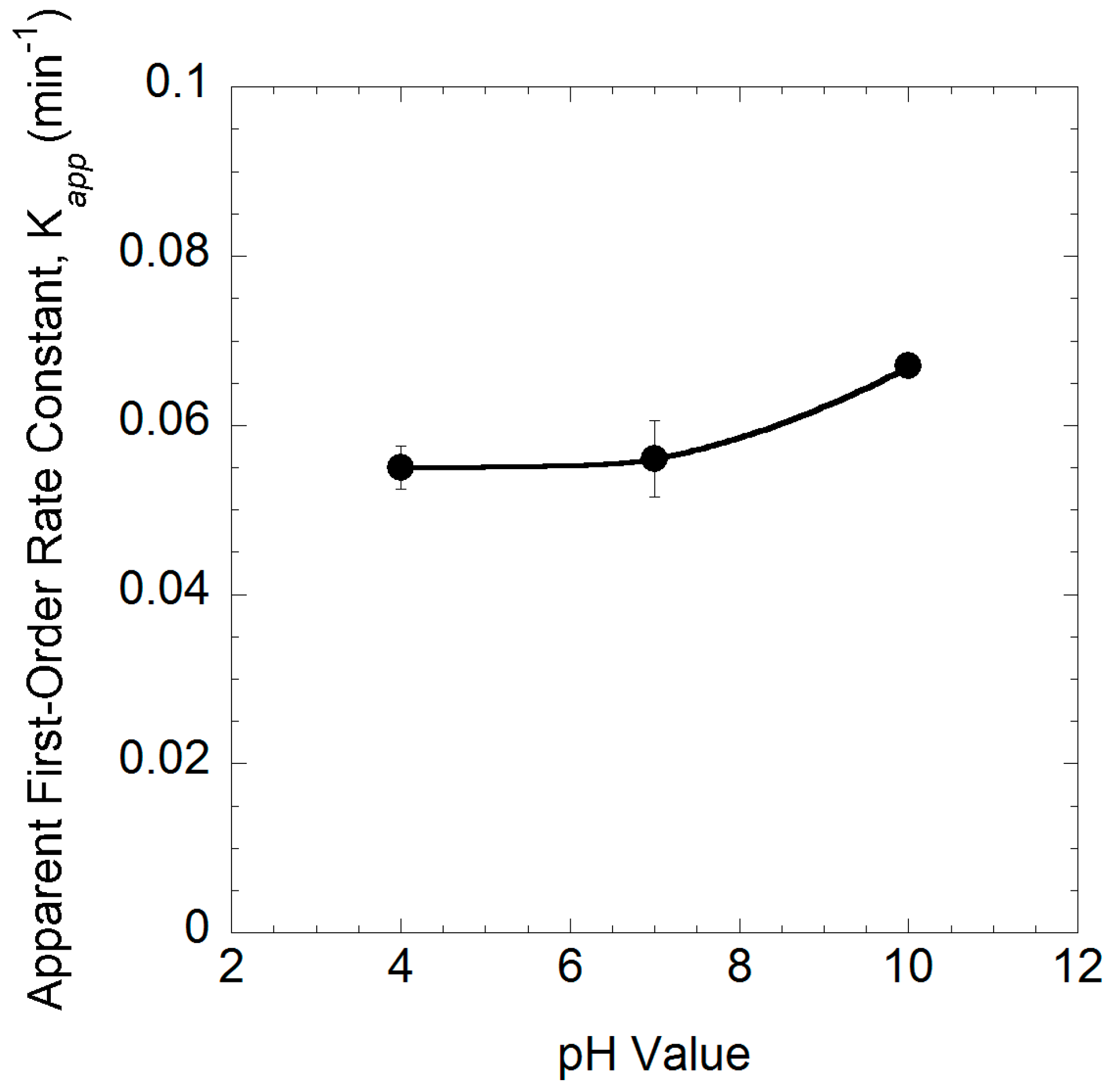
2.6. Effect of pH
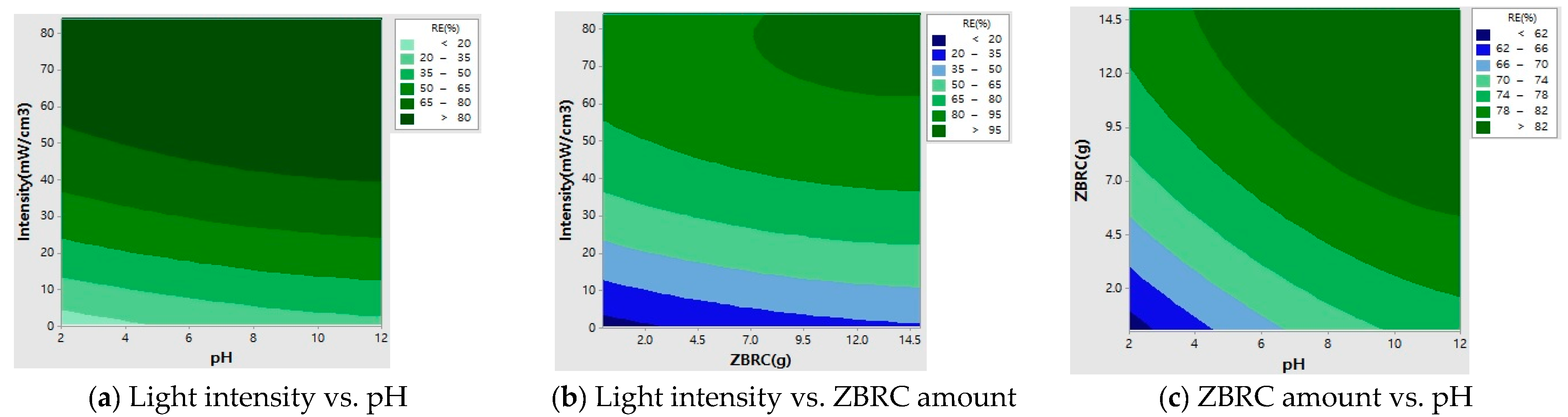
2.7. Response Surface Analysis
3. Materials and Methods
3.1. Nanoscale ZnO/Polybutadiene Rubber Composite (ZBRC)
3.2. 1,1,2-Trichloroethylene (TCE) and Analytical Methods
3.3. Batch Photocatalytic Reactor and Experimental Design
3.4. Kinetics of Photocatalytic Degradation
3.5. Response Surface Analysis
4. Summary and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crittenden, J.C.; Liu, J.; Hand, D.W.; Perram, D.L. Photocatalytic oxidation of chlorinated hydrocarbons in water. Water Res. 1997, 31, 429–438. [Google Scholar] [CrossRef]
- Yamazaki, S.; Matsunaga, S.; Hori, K. Photocatalytic degradation of trichloroethylene in water using TiO2 pellets. Water Res. 2001, 35, 1022–1028. [Google Scholar] [CrossRef]
- Daneshvar, N.; Ayazloo, M.; Khataee, A.R.; Pourhassan, M. Biological decolorization of dye solution containing Malachite Green by microalgae Cosmarium sp. Bioresource Technol. 2007, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H.; Zainal, Z.; Hussein, M.Z. Photocatalytic treatment of 4-chlorophenol in aqueous ZnO suspensions: Intermediates, influence of dosage and inorganic anions. J. Hazard. Mater. 2009, 168, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, Y.; Oki, K.; Nishikiori, H.; Tatsumi, Y.; Tanaka, N.; Fujii, T. Photocatalytic degradation of trichloroethylene using N-doped TiO2 prepared by a simple sol–gel process. Res. Chem. Intermed. 2009, 35, 43–53. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; García-López, E.; Marcì, G.; Palmisano, L. A survey of photocatalytic materials for environmental remediation. J. Hazard. Mater. 2012, 211–212, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Doria, F.C.; Borges, A.C.; Kim, J.K.; Nathan, A.; Joo, J.C.; Campos, L.C. Removal of metaldehyde through photocatalytic reactions using nano-sized zinc oxide composites. Water Air Soil Pollut. 2013, 224, 1434–1442. [Google Scholar] [CrossRef]
- Joo, J.C.; Ahn, C.H.; Jang, D.G.; Yoon, H.H.; Kim, J.K.; Campos, L.; Ahn, H.S. Photocatalytic degradation of trichloroethylene in aqueous phase using nano-ZNO/Laponite composites. J. Hazard. Mater. 2013, 263, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, C. Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: Revisited. Appl. Catal. A 2006, 304, 55–61. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment: A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membraine Sci. 2014, 472, 167–194. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, W.; He, B.; Zhang, J.; Anpo, M. Characterization of Fe-TiO2 photocatalysts synthesized by hydrothermal method and their photocatalytic reactivity for photodegradation of XRG dye diluted in water. J. Mol. Catal. A 2004, 216, 35–43. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem. Photobiol. A 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Tian, M.; Hu, Q.; Wu, H.; Zhang, L.; Fong, H.; Zhang, L. Formation and morphological stability of polybutadiene rubber fibers prepared through combination of electrospinning and in-situ photo-crosslinking. Mater. Lett. 2011, 65, 3076–3079. [Google Scholar] [CrossRef]
- Joo, J.C.; Kim, J.Y.; Nam, K. Mass transfer of organic compounds in dilute aqueous solutions into high density polyethylene geomembranes. J. Environ. Eng. 2004, 130, 175–183. [Google Scholar] [CrossRef]
- Joo, J.C.; Nam, K.; Kim, J.Y. Estimation of mass transport parameters of organic compounds through high density polyethylene geomembranes using a modifield double-compartment apparatus. J. Environ. Eng. 2005, 131, 790–799. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Daneshvar, N.; Rabbani, M. Photocatalytic degradation of an azo dye in a tubular continuous-flow photoreactor with immobilized TiO2 on glass plates. Chem. Eng. J. 2006, 127, 167–176. [Google Scholar] [CrossRef]
- Yang, H.; Ana, T.; Li, G.; Song, W.; Cooper, W.J.; Luo, H.; Guo, X. Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: A case of blockers. J. Hazard. Mater. 2010, 179, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Munesh, S.; Meena, R.C. Photocatalytic degradation of textile dye through an alternative photocatalyst methylene blue immobilized resin dowex 11 in presence of solar light. Arch. Appl. Sci. Res. 2012, 4, 472–479. [Google Scholar]
- El-Kemary, M.; El-Shamy, H.; El-Mehasseb, I. Photocatalytic degradation of ciprofloxacin drug in water using ZnO nanoparticles. J. Lumin. 2010, 130, 2327–2331. [Google Scholar] [CrossRef]
| Initial Conc. (mg/L) | Loading Amount (g/76 mL) | Intensity of Lamp (μW/cm2) | Equivalent Chloride Conc. (mg/L) | Measured Chloride Conc. (mg/L) | Average Conversion Rate (%) |
|---|---|---|---|---|---|
| 0.5 | 10 | 84 | 0.42 | N.D. a | N.A. b |
| 1 | 10 | 84 | 0.83 | 0.53 ± 0.09 | 63.6 |
| 5 | 10 | 84 | 4.2 | 2.8 ± 0.85 | 67.2 |
| 10 | 10 | 84 | 8.3 | 6.6 ± 1.20 | 79.2 |
| 20 | 10 | 84 | 16.7 | 13.9 ± 2.8 | 83.4 |
| Test (Effect) | Initial Conc. (mg/L) | Loading Amount (g/76 mL) | Intensity of Lamp (μW/cm2) | pH | Kapp (min−1) | R2 |
|---|---|---|---|---|---|---|
| UV only (photolysis) | 10 | N.A. a | 84 | 7.0 | 0.012 | 0.993 |
| UV + nano-ZnO particles | 10 | 0.05 | 84 | 7.0 | 0.030 ± 0.006 | 0.966 |
| 1. Effect of Loading Amount | ||||||
| 1.1 | 10 | 1 | 84 | 7.0 | 0.016 ± 0.004 | 0.993 |
| 1.2 | 10 | 5 | 84 | 7.0 | 0.037 ± 0.004 | 0.979 |
| 1.3 | 10 | 10 | 84 | 7.0 | 0.066 ± 0.002 | 0.999 |
| 1.4 | 10 | 15 | 84 | 7.0 | 0.096 ± 0.001 | 0.998 |
| 2. Effect of Initial Concentration | ||||||
| 2.1 | 0.5 | 10 | 84 | 7.0 | 0.243 ± 0.045 | 0.999 |
| 2.2 | 1 | 10 | 84 | 7.0 | 0.139 ± 0.011 | 0.996 |
| 2.3 | 5 | 10 | 84 | 7.0 | 0.107 ± 0.004 | 0.996 |
| 2.4 | 10 | 10 | 84 | 7.0 | 0.067 ± 0.003 | 0.997 |
| 2.5 | 20 | 10 | 84 | 7.0 | 0.051 ± 0.001 | 0.999 |
| 3. Effect of Lamp Intensity | ||||||
| 3.1 | 10 | 10 | 21 | 7.0 | 0.029 ± 0.004 | 0.982 |
| 3.2 | 10 | 10 | 42 | 7.0 | 0.051 ± 0.005 | 0.999 |
| 3.3 | 10 | 10 | 63 | 7.0 | 0.051 ± 0.009 | 0.997 |
| 3.4 | 10 | 10 | 84 | 7.0 | 0.067 ± 0.011 | 0.997 |
| 4. Effect of pH | ||||||
| 4.1 | 10 | 10 | 84 | 4.0 | 0.055 ± 0.001 | 0.995 |
| 4.2 | 10 | 10 | 84 | 7.0 | 0.056 ± 0.002 | 0.992 |
| 4.3 | 10 | 10 | 84 | 10.0 | 0.067 ± 0.001 | 0.999 |
| Run | pH | ZBRC (g) | Light Intensity (min) | Removal Efficiency, RE (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Coded Value | Actual Value | Coded Value | Actual Value | Coded Value | Actual Value | Experimental | Predicted | |
| R1 | (1) | 10 | (1) | 10 | (1) | 63 | 87.9 | 95.0 |
| R2 | (α) | 12 | (0) | 5 | (−1) | 42 | 85.8 | 81.7 |
| R3 | (1) | 10 | (1) | 10 | (1) | 21 | 60.1 | 63.9 |
| R4 | (−1) | 4 | (−1) | 1 | (0) | 21 | 49.1 | 42.7 |
| R5 | (0) | 7 | (0) | 5 | (0) | 42 | 80.5 | 78.0 |
| R6 | (0) | 7 | (0) | 5 | (0) | 42 | 81.0 | 78.0 |
| R7 | (0) | 7 | (0) | 5 | (α) | 84 | 98.9 | 93.0 |
| R8 | (0) | 7 | (−α) | 0 | (0) | 42 | 56.8 | 70.3 |
| R9 | (0) | 7 | (0) | 5 | (0) | 42 | 80.0 | 78.0 |
| R10 | (0) | 7 | (0) | 5 | (0) | 42 | 80.1 | 78.0 |
| R11 | (0) | 7 | (0) | 5 | (−α) | 0 | 21.0 | 23.3 |
| R12 | (1) | 10 | (−1) | 1 | (−1) | 21 | 58.0 | 53.7 |
| R13 | (−1) | 4 | (1) | 10 | (−α) | 63 | 86.4 | 91.8 |
| R14 | (1) | 10 | (−1) | 1 | (−1) | 63 | 86.1 | 87.9 |
| R15 | (−α) | 2 | (0) | 5 | (0) | 42 | 67.7 | 69.4 |
| R16 | (0) | 7 | (α) | 15 | (1) | 42 | 92.2 | 84.5 |
| R17 | (−1) | 4 | (−1) | 1 | (−1) | 63 | 83.3 | 80.8 |
| R18 | (0) | 7 | (0) | 5 | (0) | 42 | 81.0 | 78.0 |
| R19 | (0) | 7 | (0) | 5 | (0) | 42 | 80.1 | 78.0 |
| R20 | (−1) | 4 | (1) | 10 | (α) | 21 | 55.2 | 56.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, D.G.; Ahn, C.H.; Choi, J.S.; Kim, J.H.; Kim, J.K.; Joo, J.C. Enhanced Removal of Trichloroethylene in Water Using Nano-ZnO/Polybutadiene Rubber Composites. Catalysts 2016, 6, 152. https://doi.org/10.3390/catal6100152
Jang DG, Ahn CH, Choi JS, Kim JH, Kim JK, Joo JC. Enhanced Removal of Trichloroethylene in Water Using Nano-ZnO/Polybutadiene Rubber Composites. Catalysts. 2016; 6(10):152. https://doi.org/10.3390/catal6100152
Chicago/Turabian StyleJang, Dae Gyu, Chang Hyuk Ahn, June Seok Choi, Jong Ho Kim, Jong Kyu Kim, and Jin Chul Joo. 2016. "Enhanced Removal of Trichloroethylene in Water Using Nano-ZnO/Polybutadiene Rubber Composites" Catalysts 6, no. 10: 152. https://doi.org/10.3390/catal6100152
APA StyleJang, D. G., Ahn, C. H., Choi, J. S., Kim, J. H., Kim, J. K., & Joo, J. C. (2016). Enhanced Removal of Trichloroethylene in Water Using Nano-ZnO/Polybutadiene Rubber Composites. Catalysts, 6(10), 152. https://doi.org/10.3390/catal6100152